Abstract
Gene therapy is defined as the direct transfer of genetic material to tissues or cells for the treatment of inherited disorders and acquired diseases. For gene delivery, magnetic nanoparticles (MNPs) are typically combined with a delivery platform to encapsulate the gene, and promote cell uptake. Delivery technologies that have been used with MNPs contain polymeric, viral, as well as non-viral platforms. In this review, we focus on targeted gene delivery using MNPs.
Introduction
Nanotechnology defines the formation and application of materials, devices, and systems through the control of nanometer-sized materials, and their application in physics, chemistry, biology, engineering, applied science and other activities. In particular, rigorous efforts are in progress to develop nanomaterials for medical use as means that can be targeted to specific cells, tissues, and organs (CitationChouly et al. 1996, CitationSchlorf et al. 2010, CitationKami et al. 2011).
Many different types of nanoparticles, magnetic nanoparticles (MNPs) being just a class among them, present exciting opportunities for technologies at the interfaces between biology, physics, and chemistry. A number of MNPs have already been used in clinical practice as contrast-enhancing agents for magnetic resonance imaging (MRI) (CitationPrijic and Sersa 2011). Furthermore, several methods have been used to create these complexes, comprising hydrophobic interactions (CitationNamiki et al. 2009) and electrostatic interactions (CitationZheng et al. 2009, CitationLi et al. 2012).
The base of this therapeutic technique is to introduce a gene encoding a practical protein, altering the expression of an endogenous gene or possessing the capacity to cure or prevent the development of a disease (CitationRapti et al. 2011, CitationPouton and Seymour 2001, CitationRobbins and Ghivizzani 1998, CitationDizaj et al. 2014).
The procedure, based on the association of MNPs with gene vectors, is called magnetofection, which is used in order to enhance gene transfer in the presence of a magnetic field. It was developed by Christian Plank and coworkers for gene transfer in cell cultures and in vivo, using MNP-naked DNA complexes or MNP-viral vector complexes (CitationScherer et al. 2002). In this situation, the approach of magnetofection in cells was assumed to be simple: the MNP-DNA complex is added to a culture of adherent cells. The magnetic complexes are attracted to the bottom by a magnet, placed close below the bottom of the flask or plate, where they come in close contact with the cells and are physically internalized, without any particular influence of the magnetic force on the endocytic uptake mechanism () (CitationHuth et al. 2004, CitationSchwerdt et al. 2012).
Figure 1. Diagrammatic representation of the magnetofection principle in cells. MNPs are complexed to RAds and the complex is attracted to cells by a magnetic field. (Kindly provided by OZ Biosciences, Marseille, France, www.ozbiosciences.com) (CitationSchwerdt et al. 2012).
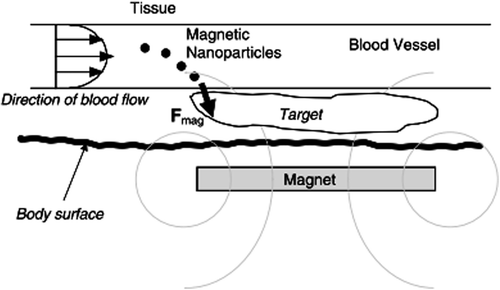
In vivo, magnetic fields focused over the target site have the potential to not only increase transfection but also target the therapeutic gene to a specific organ or location within the body (). Commonly, particles carrying the therapeutic gene are injected intravenously, and high-gradient external magnets are used to capture the particles as they flow through the bloodstream. Once captured by the field, the particles are detained at the target, where they are taken up by the tissue (CitationDobson 2006).
Figure 2. Schematic representation (side view section) of magnetic nanoparticle-based gene targeting in vivo. Dashed gray rings indicate the lines of magnetic flux due to the ex vivo permanent disk magnet. F mag is the magnetic force vector exerted on the particles as they flow through the bloodstream (CitationDobson 2006).
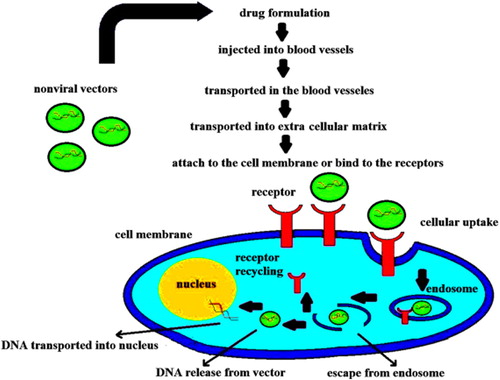
The delivery carriers are required to be small enough to be internalized into the cells and enter the nucleus, crossing through the cytoplasm and escaping the endosome/lysosome process following endocytosis (). The use of nanoparticles in gene delivery can benefit both the targeted and maintained gene delivery by protecting the gene against nuclease degradation and improving its stability (CitationDizaj et al. 2014, CitationDobson 2006, CitationZhou et al. 2011, CitationSinha et al. 2006, CitationMorachis et al. 2012).
Figure 3. Internalization of non-viral vectors into cell and passage to nucleus through the cytoplasm, following endocytosis (CitationDizaj et al. 2014).
Genetic medicine can prospectively benefit many diseases ranging from cancer (CitationRapti et al. 2011, CitationPouton and Seymour 2001) to hemophilia (CitationRobbins and Ghivizzani 1998). This therapy is not only used in genetic deficits, but also in other complicated illnesses, such as autoimmunity (rheumatoid arthritis), viral infection (human immunodeficiency virus), artery disease, diabetes, and coronary diseases (CitationHendriks et al. 2004). With the development of this method, gene therapy will become an effective therapeutic technique for neurodegenerative conditions, AIDS, asthma, and the myriad of other genetic and developed diseases that affect humanity (CitationPouton and Seymour 2001, CitationDizaj et al. 2014). A key hurdle to its clinical application is the deficiency of safe and operative delivery systems (CitationPark et al. 2006, CitationThomas et al. 2003). There are many barriers to gene delivery, comprising intracellular barriers such as intracellular uptake, DNA release, nuclear uptake, and endosomal escape, and extracellular barriers such as targeting to specific tissues and/or cells of interest, avoidance of particle clearance mechanisms, and protection of DNA from degradation (CitationPutnam 2006, CitationPack et al. 2005, CitationHarris et al. 2010).
In this study, we focus on targeted gene delivery via MNPs (called as magnetofection) and the application of these particles as therapeutic agents for some diseases. Moreover, gene delivery to cells and organs has been investigated (CitationFekri Aval et al. 2014, CitationZohre et al. 2014, CitationValizadeh et al. 2014, CitationMellatyar et al. 2014, CitationDadashzadeh et al. 2014, CitationRahimzadeh et al. 2014, CitationEbrahimi et al. 2014, CitationBadrzadeh et al. 2014).
Non-viral and viral vectors
In gene delivery, it is fairly common to follow biomimetic methods. Biological systems contain modified viruses and non-pathogenic bacteria. In the investigations of magnetic carriers for gene therapy, a viral vector which carries the therapeutic gene is coated onto the magnetic carrier's surface. By holding the carrier at the target location using external magnetic fields, the virus is kept in contact with the tissue for a longer period of time, increasing the efficiency of gene transfection and expression (CitationMah et al. 2000, CitationMah et al. 2002). New magnetic carriers are being developed specially for these applications (CitationHughes et al. 2001), and this is an area which shows great promise (CitationPankhurst et al. 2003). Viral vectors are more effective than non-viral vectors for DNA delivery, but may display a significant threat to patients, although non-viral carriers are inherently safer than viral carriers (CitationHigashi et al. 2009, CitationKostarelos and Miller 2005, CitationMastrobattista et al. 2006). In contrast to the viral gene delivery structures, the non-viral carriers are expected to be less immunogenic, with simple preparation and a possible adaptable surface modification (CitationPhilippi et al. 2010).
The non-viral vectors are usually made of lipids or polymers with or without using other inorganic substances, where they can also be prepared from a lipid-polymer or lipid-polymer-inorganic hybrid (CitationLu 2009). Naked DNA, generally in plasmid form, is the most basic form of non-viral transfer of a gene into a target cell (CitationConwell and Huang 2005, CitationNiidome and Huang 2002, CitationBigger et al. 2001, CitationMayrhofer et al. 2009). Non-viral delivery vectors can be classified as organic systems such as lipid complexes and conjugated polymers, and inorganic systems such as MNPs and gold nanoparticles (CitationLee et al. 2013).
In considering the viral gene delivery vector, with its safety concerns regarding the risk of extreme immune response (adenovirus) and supplement mutagenesis, the usage of non-viral vectors can overcome the safety problems mentioned (CitationBharali et al. 2005). Owing to the low transferring efficiency of a naked plasmid, several chemical (liposomes) and physical (electroporation) approaches have been exploited, to increase their transferring efficiency (CitationDizaj et al. 2014, CitationDeelman and Sharma 2009).
Targeted gene delivery in vivo
Gene delivery methods efficiently present a gene of interest in order to express its encoded protein in an appropriate host or host cell (CitationKami et al. 2011). In the case of magnetofection, the gene is attached directly to the carrier. These carriers commonly consist of a magnetic iron-oxide either dispersed within a polymer matrix – such as silica, polyvinyl alcohol (PVA), or dextran – or encapsulated within a polymer or metallic shell (CitationDobson 2006, CitationHarris et al. 2003, CitationNeuberger et al. 2005).
To obtain a large-sized nucleic acid molecule, the cytoplasm, or even the nucleus, an appropriate carrier system, such as virosomes, cationic liposomes, and nanoparticles, are required to deliver genes to cells, which improve cell internalization and protect the DNA molecule from nuclease enzymatic degradation. To achieve a suitable carrier structure, nanoparticles can be considered as good candidates for therapeutic applications because of several reasons, as follows (CitationAkbarzadeh et al. 2012b): They exist in the same size range as proteins (CitationWu et al. 2008), they have large surface areas and ability to attach to a large number of surface functional groups (CitationIndira and Lakshmi 2010), and they have controllable absorption and release properties, as also surface characteristics and particle size (CitationDizaj et al. 2014, CitationNitta and Numata 2013).
Inorganic nanoparticles, polymer-based nanoparticles, lipid-based nanoparticles and hybrid nanoparticles are four major groups exploited in gene delivery. MNPs are inorganic nanoparticles which are normally utilized as gene delivery carriers. The previous study reports have demonstrated that they are not subject to microbial attack and show also good storage stability (CitationDizaj et al. 2014, CitationJin et al. 2014).
Magnetism-based targeted delivery was first defined in 1978 (CitationWidder et al. 1978). However, techniques similar to those used for drug delivery have important potential to be used for gene therapy. For these applications, the approach must be adapted to account for the size and charge of nucleic acids (CitationLi et al. 2012).
Currently, there are three primary gene delivery methods that use viral vectors, nucleic acid electroporation, and nucleic acid transfection. These systems vary in efficiency (). It has been demonstrated that gene delivery by viral vectors can be highly effective, but may supplement viral vector nucleic acid sequences into the host genome, potentially causing undesirable effects, such as unsuitable expression of deleterious genes (CitationKami et al. 2011).
Table I. Gene delivery systems (CitationKami et al. 2011).
The use of MNPs to increase the effectiveness of the cell-fusion vector hemagglutinating virus Japan envelope (HVJ-E) was represented by Morishita and others. They found that by associating protamine sulfate (PS)-coated MNPs to HVJ-E, transfection was improved in vitro in BHK21 cells, even with a reduction in the amount of HVJ-E and no proof of toxicity (CitationDobson 2006, CitationMorishita et al. 2005). However, in order for MNPs to act as efficient carriers for DNA or pharmaceutical drugs, the external surface of the particles must first be modified to allow attachment of the target molecules. Molecules can be attached to the surface of the particles in some ways, such as employing cleavable linkers or utilizing electrostatic interactions between the particle surface and the therapeutic agent (CitationMcBain et al. 2008).
MNPs can be coated with compounds such as natural polymers (proteins and carbohydrates) (CitationAkbarzadeh et al. 2012c, CitationValizadeh et al. 2012, CitationAkbarzadeh et al. 2013, CitationAkbarzadeh et al. 2012a, CitationAkbarzadeh et al. 2012d, CitationAkbarzadeh et al. 2012e), synthetic organic polymers (polyethylene glycol, PVA, poly-L-lactic acid) (CitationAkbarzadeh et al. 2013, CitationMollazade et al. 2013, CitationNejati-Koshki et al. 2013, CitationRezaei-Sadabady et al. 2013), silica (CitationFallahzadeh et al. 2010), and gold (CitationKami et al. 2011, CitationEbrahimnezhad et al. 2013, CitationPourhassan-Moghaddam et al. 2013). In the case of in vitro magnetofection, the particles are generally coated with polyethylenimine (PEI), which attaches DNA to the particle's surface via charge interactions (CitationDobson 2006). In the first study to show targeted delivery of DNA using MNPs, Cathryn Mah, Barry Byrne, and coworkers coated the adeno-associated virus (AAV) encoding Green Fluorescent Protein (GFP) to the surface of MNPs using a cleavable heparin sulfate linker (CitationMah et al. 2000, CitationMcBain et al. 2008).
Although the use of target-specific linkers undoubtedly supplies an elegant approach to the attachment of target molecules, it is not always possible. An optional approach for binding DNA to the surface of particles is to employ the electrostatic interactions between the negatively charged phosphate backbone of DNA and the positively charged molecules connected to the particle surface. A current choice for this approach is the cationic polymer PEI (CitationAhmadi et al. 2014). It is now understood that particle DNA complexes normally enter the cell by endocytosis through clathrin-dependent pits (CitationDavaran et al. 2013), it is possible that this property of PEI may remain favorable for PEI-coated particles (CitationMcBain et al. 2008).
Another innovative and interesting approach to nanoparticle-mediated gene delivery is the use of nanotubes, which has been reported by (CitationGhasemali et al. 2013). This approach is based upon using nickel-embedded carbon nanotubes covered in DNA. When the nanotubes are hosted to cells in the presence of a specially oriented magnetic field, the nanotubes align with the magnetic flux lines as they are pulled towards the cells. This allows the nanotubes to spear the cells, pass through the membrane, and deliver the target DNA, and this technique has been successfully used to transfect a number of various cell types, while maintaining a high rate of cell viability after transduction (CitationMcBain et al. 2008).
Gene delivery using MNPs and magnetic force
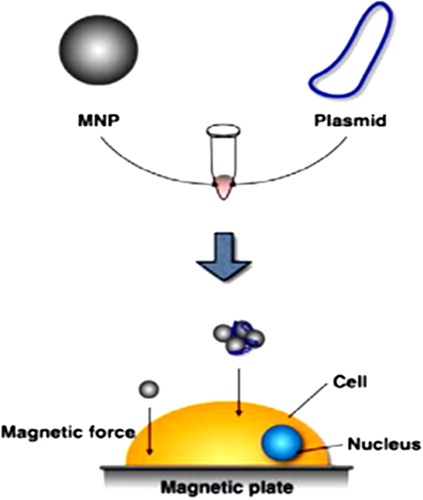
MNPs are already in use by some researchers to enhance transfection efficiencies of cultured cells. Therefore, MNP-nucleic acid complexes are added to cell culture media and then onto the cell surface by applying a magnetic force () (CitationMcBain et al. 2008).
Figure 4. MNP gene delivery system (Magnetofection). Plasmids are bound to MNPs, which are then moved from the media to the cell surface by applying a magnetic force (CitationSadat et al. 2014).
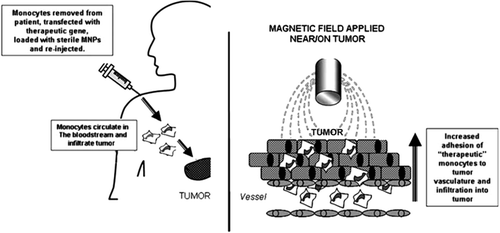
Today, research has made progress in finding a way to use MNPs which are ultra-small and biocompatible, to improve the overall uptake of genetically engineered cells like monocytes or macrophages by tumors, following their systemic administration (CitationDavaran et al. 2014). Muthana and coworkers suggested that this new magnetic targeting method could be used to target ‘therapeutically armed’ monocytes or other forms of cellular gene delivery vehicles to tumors, and thus overcome the difficulty of poor targeting in current cell-based gene therapy protocols () (CitationDavaran et al. 2014).
Figure 5. Schematic representation of the possible role of MNPs in enhancing monocyte-based gene delivery to tumors. MNP-loaded monocytes injected into the bloodstream of the patient circulate and are then drawn out of the blood vessels in the tumor under the influence of a local magnetic field (CitationDavaran et al. 2014).
As early as 1960, Freeman (CitationKouhi et al. 2014) proposed that such MNPs could be transported through the vascular system and concentrated in a special part of the body using an externally-applied magnetic field. Since then, MNPs have been conjugated to several therapeutic agents like the anti-cancer drugs, and a magnetic field applied to the target tissue (CitationAbbasi et al. 2014b, CitationPourhassan-Moghaddam et al. 2014). The hurdle with this approach has been that although the drug is concentrated within the target tissue by the magnet, comparatively little pierces beyond the perivascular areas, and thus the deeper regions of the tissue remain untreated (CitationAbbasi et al. 2014c, CitationEatemadi et al. 2014b).
Many researchers have reported magnetofection approaches (). They improved the surface of iron oxide-based MNPs to enhance transfection efficiency and decrease cytotoxicity (CitationKami et al. 2011).
Table II. Summary of magnetofection literature(CitationKami et al. 2011).
Topical and systemic delivery
Topical delivery
Magnetofection proposes two potential advantages for regional delivery to tumors. First, it can enhance the cellular uptake and retention of payloads at the injection region. A second advantage of magnetofection for topical delivery is tumor diffusion. Current delivery techniques cannot efficiently deliver therapeutic genes to all regions of tumors, specifically the hypoxic focus, due in part to the complex nature of the vasculature inside many tumors (CitationLi et al. 2012, CitationEatemadi et al. 2014b).
Systemic delivery
Efficient prolonged systemic gene therapy needs effective gene transfer, suitable expression, and long-standing survival of transduced cells. This technique might be used to prevent or treat cardiovascular diseases, such as thromboembolic disease, hypertension, hypercholesterolemia, or diabetes mellitus (DM). For the treatment of DM or hypertension, the ability to achieve physiologic regulation of expression will be essential prior to use these treatments in humans. Current gene therapy methods with retroviral and AAV vectors are restricted by their inability to achieve sufficient levels of expression for many disease, whereas adenoviral vectors are limited by short-term expression (CitationJalil et al. 2014).
Magnetofection in cells
Scherer et al. (CitationScherer et al. 2002) and Plank et al. (CitationAlizadeh et al. 2014) reported that using MNPs to carry gene vectors to various cells showed significantly increased uptake of these vectors, followed by high target protein expression. The magnetic field applied on the gene vector-magnetic particle complex may raise the accumulation of these complexes on the surface of several cells. Specifically, cell lines that have only imperfect efficiencies regarding target gene expression, such as human endothelial cells, can be well adapted to magnetofection (CitationNejati-Koshki et al. 2014, CitationAlizadeh et al. 2014, CitationAbbasi et al. 2014a, CitationEbrahimi et al. 2014a).
Endothelial and epithelial cells
Magnetofection has been described to potentiate gene delivery to cultured primary endothelial cells and to human umbilical vein endothelial cells (HUVEC). Therefore, up to a 360-fold increase in luciferase gene transfer was achieved by magnetofection, as compared to various conventional transfection procedures (CitationSchwerdt et al. 2012, CitationAbbasi et al. 2014d).
It has been observed in various cell lines that coupling of MNPs to gene vectors of any kind results in an effect of higher uptake of these vectors and consequently, target protein expression (CitationMcBain et al. 2008). Furthermore, it was seen that magnetofection allowed locally enhanced expression of ß-galactosidase activity in some cell lines (CitationMcBain et al. 2008).
Recently, the progress of MNPs coated with PEG and with covalently linked branched PEI (bPEI), has been reported. In HUVEC cultures, nonviral vector-hybrid MNP complexes demonstrated highly efficient magnetofection, even in serum-conditioned media (CitationGhalhar et al. 2014). In another study, MNPs complexed to Lipofectamine 2000 or cationic lipid 67/plasmid DNA (pDNA) liposome complexes were reported to be highly efficient for gene delivery in airway epithelial cell cultures, but less efficient than pDNA alone when applied in the murine nasal epithelium in vivo. The latter result is likely to be a consequence of the significant precipitation of the complexes achieved in vivo (CitationSchwerdt et al. 2012, CitationDaraee et al. 2014a).
Tumor and embryonic cells
Magnetofection of cDNA constructs and shRNA into mouse genital ridge tissue was applied as a means of gain-of-function and loss-of-function analysis, correspondingly. Ectopic expression of Sry convinced female-to-male sex-reversal, although knockdown of Sox9 expression caused male-to-female sex-reversal, consistent with the known functions of these genes. Also, the ectopic form of Tmem184a, a gene of unknown function in female genital ridges, resulted in the failure of gonocytes to arrive at meiosis. These results suggest that magnetofection may constitute a proper tool for the study of gene function in a broad range of developing tissues and organs (CitationSchwerdt et al. 2012, CitationTabatabaei Mirakabad et al. 2014).
Delivery to internal organs
Several studies investigating magnetic gene targeting of internal organs have used reporter genes. Namiki et al. have represented systems that have effectively delivered reporter genes that can also be used to successfully deliver therapeutic genes once they have been improved (CitationDaraee et al. 2014b). Gene therapy has shown promising results in treating hepatocellular carcinoma, both in vitro and in vivo. These methods consist of p53 gene replacement (CitationNasrabadi et al. 2014) and RNAi-mediated gene silencing (CitationChung et al. 2014). In both tests, gene therapy only worked when genes were directly applied to the liver (CitationSchwerdt et al. 2012).
In the spinal cord, magnetic nanoparticle/PEI complexes have been demonstrated to be targetable following intrathecal injection (CitationGhalhar et al. 2014). For spinal tumors, this method offers a unique technique for targeting several regions of the spine, by increasing the effect of a therapy at the tumor site and reducing exposure at other sites (CitationSchwerdt et al. 2012).
Conclusion
Magnetically-guided drug or gene targeting using MNPs is a favorable method for cancer gene therapy and cancer chemotherapy. The rationale behind these two treatment modalities is based on binding either chemotherapeutics or nucleic acids onto the surface of MNPs, which are then directed to the tumor by using an external magnetic field. Recently, binding of nucleic acids to MNPs has been confirmed as a successful non-viral transfection system of special cell lines in vitro. With the optimization of this technique, called magnetofection, we are confident that it will become another form of gene delivery for the treatment of cancer (CitationHerizchi et al. 2014, CitationKafshdooz et al. 2014, CitationBadrzadeh et al. 2014, CitationSohrabi et al. 2014, CitationTozihi et al.2014, CitationAfsaneh et al. 2014, CitationKordi et al. 2014, CitationAnganeh et al. 2014, CitationBarkhordari et al. 2014, CitationDadashzadeh et al. 2014).
Authors’ contributions
AA conceived of the study and participated in its design and coordination. SM, FZS and MS participated in the sequence alignment and drafted the manuscript. All authors read and approved the final manuscript.
Acknowledgments
The authors thank the Department of Medical Nanotechnology, Faculty of Advanced Medical Science of Tabriz University, for all support provided. This work is funded by the 2015 Drug Applied Research Center Tabriz University of Medical Sciences Grant.
Declaration of interest The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abbasi E, Abolfazl A, Kouhi M, Milani M. 2014a. Graphene: Synthesis, Bio-Applications, and Properties. Artif Cells Nanomed Biotechnol. 1–7.
- Abbasi E, Aval SF, Akbarzadeh A, Milani M, Nasrabadi HT, Joo SW, et al. 2014b. Dendrimers: synthesis, applications, and properties. Nanoscale Res Lett. 9:247.
- Abbasi E, Milani M, Fekri Aval S, Kouhi M, Akbarzadeh A, Tayefi Nasrabadi H. 2014c. Silver nanoparticles: synthesis, properties, bio-applications and limitations. Crit Rev Microbiol. 1–8.
- Abbasi E, Milani M, Fekri Aval S, Kouhi M, Akbarzadeh A, Tayefi Nasrabadi H, et al. 2014d. Silver nanoparticles: Synthesis, properties, bio-applications and limitations. Crit Rev Microbiol. 1–8.
- Afsaneh S, Reza HH, Mohammad AB, Shayan Z, Nosratolah Z, Abolfazl A. 2014. The association between serum KALRN levels with polymorphism gene KALRN (rs9289231) with risk of early-onset coronary artery disease (CAD). Molecular Biology Reports.
- Ahmadi A, Shirazi H, Pourbagher N, Akbarzadeh A, Omidfar K. 2014. An electrochemical immunosensor for digoxin using core-shell gold coated magnetic nanoparticles as labels. Mol Biol Rep. 41: 1659–1668.
- Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, et al. 2013. Liposome: Classification, Preparation, and Applications. Nanoscale Res Lett, 8:102.
- Akbarzadeh A, Mikaeili H, Asgari D, Zarghami N, Mohammad R, Davaran S. 2012a.Preparation and in-vitro evaluation of doxorubicin-loaded Fe3O4 magnetic nanoparticles modified with biocompatible copolymers. Int J Nanomedicine. 7:511–526.
- Akbarzadeh A, Samiei M, Davaran S. 2012b. Magnetic nanoparticles: preparation, physical properties, and applications in biomedicine. Nanoscale Res Lett. 7:144.
- Akbarzadeh A, Samiei M, Davaran S. 2012c.Magnetic Nanoparticles: Preparation, Physical Properties and Applications in biomedicine. Nanoscale Res lett. 7:144–157.
- Akbarzadeh A, Samiei M, Joo SW, Anzaby M, Hanifehpour Y, Nasrabadi HT, Davaran S. 2012d. Synthesis, characterization and in vitro studies of doxorubicin-loaded magnetic nanoparticles grafted to smart copolymers on A549 lung cancer cell line. J Nanobiotechnology. 10:46–58.
- Akbarzadeh A, Zarghami N, Mikaeili H, Asgari D, Goganian AM, Khiabani HK, Davaran S. 2012e. Synthesis, characterization and in vitro evaluation of novel polymer-coated magnetic nanoparticles for controlled delivery of doxorubicin. Int J Nanotechnol Sci Environ. 5:13–25.
- Alimirzalu S, Akbarzadeh A, Abbasian M, Alimohammadi S, Davaran S, Younes H, et al. 2014. Synthesis and study of physicochemical characteristics of Fe3O4 magnetic nanocomposites based on poly(Nisopropylacrylamide)for anti-cancer drugs delivery. Asian Pac J Cancer Prev 15:49–54.
- Alizadeh E, Akbarzadeh A, Zarghami N, Eslaminejad BM, Hashemzadeh S, Nejati-Koshki K. 2014. Up-regulation of Liver enriched Transcription Factors (HNF4a and HNF6) and Liver Specific MicroRNA (miR-122) by Inhibition of Let-7b in Mesenchymal Stem Cells. Chem Biol Drug Des. 85:600–608.
- Alizadeh E, Zarghami N, Eslaminejad BM, Akbarzadeh A, Barzegar A, Mohammadi SA. 2014. The Effect of Dimethyl Sulfoxide (DMSO) on Hepatic Differentiation of Mesenchymal Stem Cells. Artif Cells Nanomed Biotechnol. 1–8.
- Anganeh MT, Mirakabad FST, Izadi M, Zeighamian V, Badrzadeh F, Salehi R, et al. 2014a. The comparison between effects of free curcumin and curcumin loaded PLGA-PEG on telomerase and TRF1 expressions in calu-6 lung cancer cell line. Int J Biosci. 4:134–145.
- Anganeh TM, Mirakabad FST, Izadi M, Zeighamian V, Badrzadeh F, Salehi R, et al. 2014b. The comparison between effects of free curcumin and curcumin loaded PLGA-PEG on telomerase and TRF1 expressions in calu-6 lung cancer cell line. Int J Biosciences. 4: 134–145.
- Badrzadeh F, Akbarzadeh A, Zarghami N, Yamchi MR, Zeighamian V, Tabatabae FS, et al. 2014a. Comparison between effects of free curcumin and curcumin loaded NIPAAm-MAA nanoparticles on telomerase and PinX1 gene expression in lung cancer cells. Asian Pac J Cancer Prev. 15:8931–8936.
- Badrzadeh F, Rahmati-Yamchi M, Badrzadeh K, Valizadeh A, Zarghami N, Farkhani SM, Akbarzadeh A. 2014b. Drug delivery and nanodetection in lung cancer. Artif Cells Nanomed Biotechnol. Early Online:1–17.
- Barkhordari A, Rahmati YM, Fekri S, Pourhassan-Moghaddam M, Nejati KK, Davaran S, et al. 2014. Study of inhibitory effect of Helenalin on hTERT gene expression in breast cancer cell line by Real-time PCR. Bio infopublications. In Press 2014.
- Bharali DJ, Klejbor I, Stachowiak EK, Dutta P, Roy I, Kaur N, et al. 2005. Organically modified silica nanoparticles: a nonviral vector for in vivo gene delivery and expression in the brain. Pro Natl Acad Sci USA. 102:11539–11544.
- Bigger BW, Tolmachov O, Collombet JM, Fragkos M, Palaszewski I, Coutelle C. 2001. An araC-controlled bacterialcre expression system to produce DNA minicircle vectors for nuclear and mitochondrial gene therapy. J Biol Chem. 276:23018–23027.
- Chouly C, Pouliquen D, Lucet I, Jeune JJ, Jallet P. 1996Development of superparamagnetic nanoparticles for MRI: effect of particle size, charge and surface nature on biodistribution. J Microencapsul. 13:245–255.
- Chung JH, Kim YK, Kim KH, Kwon TY, Vaezmomeni SZ, Samiei M, et al. 2014. Synthesis, characterization, biocompatibility of hydroxyapatite-natural polymers nanocomposites for dentistry applications. Artif Cells Nanomed Biotechnol. 1–8.
- Conwell CC, Huang L. 2005. Recent advances in non-viral gene delivery. Adv Genet. 53:1–18.
- Dadashzadeh K, Milani M, Rahmati M, Akbarzadeh A. 2014a. Real-Time PCR detection of 16S rRNA novel mutations associated with Helicobacter pylori tetracycline resistance in Iran. Asian Pac J Cancer Prev. 15:8883–8886.
- Dadashzadeh K, Milani M, Rahmati M, Akbarzadeh A. 2014b.Real-Time PCR detection of 16S rRNA novel mutations associated with Helicobacter pylori tetracycline resistance in Iran. APJCP. 15: 8883–8886.
- Daraee H, Eatemadi A, Abbasi E, Fekri Aval S, Kouhi M, Akbarzadeh A. 2014a. Application of gold nanoparticles in biomedical and drug delivery. Artif Cells Nanomed Biotechnol. 1–13.
- Daraee H, Etemadi A, Kouhi M, Alimirzalu S, Akbarzadeh A. 2014b. Application of liposomes in medicine and drug delivery. Artif Cells Nanomed Biotechnol. 1–11.
- Davaran S, Akbarzadeh A, Nejati-Koshki K, Alimohammadi S, Farajpour GM, Soghrati MM, et al. 2013. In vitro studies of NIPAAM-MAA-VP copolymer-coated magnetic nanoparticles for controlled anticancer drug release. JEAS. 3:108–115.
- Davaran S, Rezaei A, Alimohammadi S, Khandaghi AA, Nejati-Koshki K, Nasrabadi HT, Akbarzadeh A. 2014. Synthesis and physicochemical characterization of biodegradable star-shaped poly lactide-co-glycolide– β -cyclodextrin copolymer nanoparticles containing albumin. ANP. 3:14–22.
- Davoudi Z, Akbarzadeh A, Rahmatiyamchi M, Movassaghpour AA, Alipour M, Nejati-Koshki K, et al. 2014. Molecular Target Therapy of AKT and NF-kB Signaling Pathways and Multidrug Resistance by Specific Cell Penetrating Inhibitor Peptides in HL-60 Cells. APJCP. 15:4353.
- Deelman L, Sharma K. 2009. Mechanisms of kidney fibrosis and the role of antifibrotic therapies. Curr Opin Nephrol Hypertens. 18: 85–90.
- Dizaj SM, Jafari S, Khosroushahi AY. 2014. A sight on the current nanoparticle-based gene delivery vectors. Nanoscale Res Lett. 9:252.
- Dobson J. 2006Gene therapy progress and prospects: magnetic nanoparticle-based gene delivery. Gene Ther 13:283–287.
- Eatemadi A, Daraee H, Karimkhanloo H, Kouhi M, Zarghami N, Akbarzadeh A, et al. 2014a. Carbon nanotubes: properties, synthesis, purification, and medical applications. Nanoscale Res Lett. 9:1–13.
- Eatemadi A, Daraee H, Zarghami N, Melat YH, Akbarzadeh A, Hanifehpour Y. 2014b. Nanofiber; synthesis and biomedical applications Artif Cells Nanomed Biotechnol. 1–11.
- Ebrahimi E, Abbasi E, Akbarzadeh A, Khandaghi AA, Davaran S. 2014a. Novel drug delivery system based on doxorubicin-encapsulated magnetic nanoparticles modified with PLGA-PEG1000 copolymer. Artif Cells Nanomed Biotechnol. 1–8.
- Ebrahimi E, Khandaghi AA, Valipour F, Babaie S, Asghari F, Motaali S, et al. 2014b. In vitro study and characterization of doxorubicin-loaded magnetic nanoparticles modified with biodegradable copolymers. Artifi Cells Nanomed Biotechnol. Early Online:1–9.
- Ebrahimnezhad Z, Zarghami N, Keyhani M, Amirsaadat S, Akbarzadeh A, Rahmati M, et al. 2013. Inhibition of hTERT gene expression by silibinin-loaded PLGA-PEG-Fe3O4 in T47D breast cancer cell line. Bioimpacts. 3:67–74.
- Fallahzadeh S, Bahrami H, Akbarzadeh A, Tayarani M. 2010. High- isolation dual-frequency operation patch antenna using spiral defected microstrip structure. Antenn Wirel Pr Letters, IEEE. 9:122–124.
- Fekri Aval S, Akbarzadeh A, Yamchi MR, Zarghami F, Nejati-Koshki K, Zarghami N. 2014. Gene silencing effect of SiRNA-magnetic modified with biodegradable copolymer nanoparticles on hTERT gene expression in lung cancer cell line. Artif Cells Nanomed Biotechnol. Early Online:1–6.
- Ghalhar MG, Akbarzadeh A, Rahmati M, Mellatyar H, Dariushnejad H, Zarghami N, Barkhordari A. 2014. Comparison of inhibitory effects of 17-AAG nanoparticles and free 17-AAG on HSP90 gene expression in breast cancer. Asian Pac J Cancer Prev. 15:7113–7118.
- Ghasemali S, Nejati-Koshki K, Akbarzadeh A, Tafsiri E, Zarghami N, Rahmati-Yamchi M, et al. 2013. Study of Inhibitory Effect of β-Cyclodextrin-HelenalinComplex on HTERT Gene Expression in T47D Breast Cancer Cell Line by Real TimeQuantitative PCR (q-PCR). Asian Pac J Cancer Prev. 14:6949–6953.
- Harris LA, Goff JD, Carmichael AY, Riffle JS, Harburn JJ, St. Pierre TG, Saunders M. 2003. Magnetite nanoparticle dispersions stabilized with triblock copolymers. Chem Mater, 15:1367–1377.
- Harris TJ, Green JJ, Fung PW, Langer R, Anderson DG, Bhatia SN. 2010. Tissue-specific gene delivery via nanoparticle coating. Biomaterials. 31:998–1006.
- Hendriks WT, Ruitenberg MJ, Blits B, Boer GJ, Verhaagen J. 2004. Viral vector-mediated gene transfer of neurotrophins to promote regeneration of the injured spinal cord. Prog Brain Res. 146: 451–476.
- Herizchi R, Abbasi E, Milani M, Akbarzadeh A. 2014. Current methods for synthesis of gold nanoparticles. Artif Cells Nanomed Biotechnol. Early Online:1–7.
- Higashi T, Khalil IA, Maiti KK, Lee WS, Akita H, Harashima H, Chung SK. 2009. Novel lipidated sorbitol-based molecular transporters for non-viral gene delivery. J Cont Rel. 136:140–147.
- Hosseininasab S, Pashaei-Asl R, Ahmad KA, Nasrabadi HT, Nejati-Koshki K, Akbarzadeh A, et al. 2014. Synthesis, characterization, and In vitro studies of PLGA-PEG nanoparticles for oral Insulin delivery. Chem Biol Drug Des. 84:307–315.
- Hughes C, Galea-Lauri J, Farzaneh F, Darling D. 2001. Streptavidin paramagnetic particles provide a choice of three affinity-based capture and magnetic concentration strategies for retroviral vectors. Mol Ther. 3:623–630.
- Huth S, Lausier J, Gersting SW, Rudolph C, Plank C, Welsch U, Rosenecker J. 2004. Insights into the mechanism of magnetofection using PEI-based magnetofectins for gene transfer. J Gene Med. 6:923–936.
- Indira TK, Lakshmi PK. 2010. Magnetic nanoparticles - A review. Int J Pharm Sci Nanotechnol. 3:1035–1042.
- Jalil K-H, Mobasseri M, Akbarzadeh A, Soodabeh D, Ostad-Rahimi A, Hamishehkar H, et al. 2014. Preparation of pH sensitive insulin-loaded Nano hydrogels and evaluation of insulin releasing in different pH conditions. Molecular Biology Reports. Mol Biology Rep. 41:6705–6712.
- Jin L, Zeng X, Liu M, Deng Y, He N. 2014. Current progress in gene delivery technology based on chemical methods and nano-carriers. Theranostics. 4:240–55.
- Kafshdooz T, Kafshdooz L, Akbarzadeh A, Hanifehpour Y, Joo SW. 2014. Applications of nanoparticle systems in gene delivery and gene therapy. Artif Cells Nanomed Biotechnol. Early Online:1–7.
- Kami D, Takeda S, Itakura Y, Gojo S, Watanabe M, Toyoda M. 2011. Application of magnetic nanoparticles to gene delivery. Int J Mol Sci. 12:3705–3722.
- Kordi S, Zarghami N, Rahmati YM, Ghasemali S, Tozihi M, Nejati-Koshki K, Akbarzadeh A. 2014. The Comparison of inhibitory effect of nanocapsolated Helenalin and free Helenalin on Telomerase gene expression in breast cancer cell line by Real-time PCR. Artif Cells Nanomed Biotechnol. Early Online:1–17.
- Kostarelos K, AD Miller. 2005. Synthetic, self-assembly ABCD nanoparticles; a structural paradigm for viable synthetic non-viral vectors. Chem Soc Rev. 34:970–994.
- Kouhi M, Vahedi A, Akbarzadeh A, Hanifehpour Y, Sang Woo J. 2014. Investigation of quadratic electro-optic effects and electro absorption process in GaN/AlGaN spherical quantum dot. Nanoscale Res Lett. 9:131–136.
- Lee J-M, Yoon T-J, Cho Y-S. 2013. Recent developments in nanoparticle-based siRNA delivery for cancer therapy. Biomed Res Int. 2013:782041
- Li C, L Li, AC Keates. 2012. Targeting cancer gene therapy with magnetic nanoparticles. Oncotarget. 3:365–70.
- Lu Y, 2009. Transcriptionally regulated, prostate-targeted gene therapy for prostate cancer. Adv Drug Deliv Rev. 61:572–588.
- Mah C, Fraites TJ Jr, Zolotukhin I, Song S, Flotte TR, Dobson J, et al. 2000. Microsphere-mediated delivery of recombinant AAV vectors in vitro and in vivo. Mol Ther. 1:S239.
- Mah C, Fraites TJ Jr, Zolotukhin I, Song S, Flotte TR, Dobson J, et al. 2002. Improved method of recombinant AAV2 delivery for systemic targeted gene therapy. Mol Ther. 6:106–112.
- Mastrobattista E, van der Aa MA, Hennink WE, Crommelin DJ. 2006. Artificial viruses: a nanotechnological approach to gene delivery. Nat Rev Drug Discov. 5:115–121.
- Mayrhofer P, Schleef M, Jechlinger W. 2009. Use of minicircle plasmids for gene therapy, in Gene Therapy of Cancer. Springer, pp. 87–104.
- McBain SC, Yiu HH, Dobson J. 2008. Magnetic nanoparticles for gene and drug delivery. Int J Nanomedicine. 3:169.
- Mellatyar H, Akbarzadeh A, Rahmati M, Ghalhar MG, Etemadi A, Nejati-Koshki K, Zarghami N, Barkhordari A. 2014. Comparison of inhibitory effect of 17-DMAG nanoparticles and free 17-DMAG in HSP90 gene expression in lung cancer. Asian Pac J Cancer Prev. 15:8693–8698.
- Mollazade M, Nejati-Koshki K, Abolfazl A, Younes H, Zarghami N, Sang Woo J. 2013. PAMAM dendrimers arugment inhibitory effect of curcumin on cancer cell proliferation: possible inhibition of telomerase. Asian Pac J Cancer Prev. 14:6925–6928.
- Morachis JM, Mahmoud EA, Sankaranarayanan J, Almutairi A, 2012. Triggered rapid degradation of nanoparticles for gene delivery. J Drug Deliv. 2012:291219
- Morishita N, Nakagami H, Morishita R, Takeda S, Mishima F, Terazono B, et al. 2005. Magnetic nanoparticles with surface modification enhanced gene delivery of HVJ-E vector. Biochem Biophys Res Commun. 2005. 334:1121–1126.
- Namiki Y, Namiki T, Yoshida H, Ishii Y, Tsubota A, Koido S, et al. 2009.A novel magnetic crystal “lipid nanostructure for magnetically guided in vivo gene delivery. Nat Nanotechnol. 4:598–606.
- Nasrabadi HT, Abbasi E, Davaran S, Kouhi M, Akbarzadeh A. 2014. Bimetallic nanoparticles: Preparation, properties, and biomedical applications. Artif Cells Nanomed Biotechnol. 1–5.
- Nejati-Koshki K, Akbarzadeh A, Pourhasan-Moghaddam M, Abhari A, Dariushnejad H. 2013. Inhibition of Leptin and Leptin Receptor Gene Expression by Silibinin-Curcumin Combination. Asian Pac J Cancer Prev. 14:6595–6599.
- Nejati-Koshki K, Mesgari M, Ebrahimi E, Abhari A, Aval FS, Khandaghi AA, Akbarzadeh A. 2014. Synthesis and In-vitro study of Cisplatin-loaded Fe3O4 Nanoparticles Modified with PLGA-PEG6000 Copolymers in Treatment of Lung Cancer. J Microencapsulation. 1–9.
- Neuberger T, Schöpf B, Hofmann H, Hofmann M, von Rechenberg B 2005. Superparamagnetic nanoparticles for biomedical applications: possibilities and limitations of a new drug delivery system. J Magn Magn Mater, 293:483–496.
- Niidome T, Huang L. 2002. Gene therapy progress and prospects: nonviral vectors. Gene Ther, 9:1647–1652.
- Nitta SK, Numata K. 2013. Biopolymer-based nanoparticles for drug/gene delivery and tissue engineering. Int J Mol Sci. 14:1629–1654.
- Pack DW, Hoffman AS, Pun S, Stayton PS. 2005. Design and development of polymers for gene delivery. Nat Rev Drug Discov. 4: 581–593.
- Pankhurst QA, Thanh NTK, Jones SK, Dobson J. 2003. Applications of magnetic nanoparticles in biomedicine. J Phys D: Appl Phys. 36:R167.
- Park TG, Jeong JH, Kim SW. 2006. Current status of polymeric gene delivery systems. Adv Drug Deliv Rev. 58:467–486.
- Philippi C, Loretz B, Schaefer UF, Lehr CM 2010. Telomerase as an emerging target to fight cancer-opportunities and challenges for nanomedicine. J Cont Rel. 146:228–240.
- Pourhassan-Moghaddam M, Rahmati-Yamchi M, Akbarzadeh A, Daraee H, Nejati-Koshki K, Hanifehpour Y, Sang Woo J. 2013. Protein detection through different platforms of immuno-loop-mediated isothermal amplification. Nanoscale Res Lett. 8:485.
- Pourhassan-Moghaddam M, Zarghami N, Mohsenifar A, Rahmati-Yamchi M, Gholizadeh D, Akbarzadeh A, de la Guardia M, Nejati-Koshki K. 2014. Watercress-based gold nanoparticles: biosynthesis, mechanism of formation and study of their biocompatibility in vitro. IET Digital Library. 9:345–350.
- Pouton CW, Seymour LW. 2001. Key issues in non-viral gene delivery. Adv Drug Deliv Rev. 46:187–203.
- Prijic S, G Sersa 2011. Magnetic nanoparticles as targeted delivery systems in oncology. Radiol Oncol. 45:1–16.
- Putnam D, 2006. Polymers for gene delivery across length scales. Nat Mater. 5:439–451.
- Rahimzadeh A, Tabatabaei Mirakabad FS, Movassaghpour A, Shamsasenjan K, Kariminekoo S, Talebi M, et al. 2014. Biotechnological and biomedical applications of mesenchymal stem cells as a therapeutic system. Artifi Cells Nanomed Biotechnol. Early Online:1–12.
- Rapti K, Chaanine AH, Hajjar RJ. 2011. Targeted gene therapy for the treatment of heart failure. Can J Cardiol. 27:265–283.
- Rezaei-Sadabady R, Zarghami N, Barzegar A, Eidi A, Akbarzadeh A, Rezaei-Tavirani M. 2013. Studies of the relationship between structure and antioxidant activity in interesting systems, including tyrosol, hydroxytyrosol derivatives indicated by quantum chemical calculations. Soft. 2:13–18.
- Robbins PD, Ghivizzani SC. 1998. Viral vectors for gene therapy. Pharmacol Ther. 80:35–47.
- Sadat TMF, Nejati-Koshki K, Akbarzadeh A, Yamchi MR, Milani M, Zarghami N, et al. 2014. PLGA-Based Nanoparticles As Cancer Drug Delivery Systems. Asian Pac J Cancer Prev. 15:517–535.
- Scherer F, Anton M, Schillinger U, Henke J, Bergemann C, Krüger A, et al. 2002. Magnetofection: enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther. 9:102–109.
- Schlorf T, Meincke M, Kossel E, Glüer CC, Jansen O, Mentlein R. 2010. Biological properties of iron oxide nanoparticles for cellular and molecular magnetic resonance imaging. Int J Mol Sci. 12:12–23.
- Schwerdt JI., Goya GF, Calatayud MP, Hereñú CB, Reggiani PC, Goya RG, et al. 2012. Magnetic field-assisted gene delivery: achievements and therapeutic potential. Curr Gene Ther. 12:116–126.
- Sinha R, Kim GJ, Nie S, Shin DM. 2006Nanotechnology in cancer therapeutics: bioconjugated nanoparticles for drug delivery. Mol cancer Ther. 5:1909–1917.
- Sohrabi N, Valizadeh A, Farkhani SM, Akbarzadeh A. 2014. Basics of DNA biosensors and cancer diagnosis. Artif Cells Nanomed Biotechnol. Early Online:1–10.
- Tabatabaei Mirakabad FS, Akbarzadeh A, Milani M, Zarghami N, Taheri-Anganeh M, Zeighamian V, et al. 2014. A Comparison between the cytotoxic effects of pure curcumin and curcumin-loaded PLGA-PEG nanoparticles on the MCF-7 human breast cancer cell line. Artif Cells Nanomed Biotechnol. 1–8.
- Thomas CE, Ehrhardt A, Kay MA. 2003. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 4:346–358.
- Tozihi M, Zarghami N, Rahmati M, Nejati-Koshki K, Akbarzadeh A, Mohamadian J, et al. 2014. CDH1 and FGFR2 gene Polymorphisms in Breast Cancer Patients. Int J Biosci. InPress.
- Valizadeh A, Bakhtiary M, Akbarzadeh A, Salehi R, Frakhani SM, Ebrahimi O, et al. 2014. Preparation and characterization of novel electrospun poly(e-caprolactone)-based nanofibrous scaffolds. Artif Cells Nanomed Biotechnol. Early Online:1–6.
- Valizadeh A, Mikaeili H, Samiei M, Farkhani SM, Zarghami N, Kouhi M, et al. 2012. Quantum dots: synthesis, bioapplications, and toxicity. Nanoscale Res Lett, 7:276
- Widder KJ, Senyel AE, Scarpelli DG. 1978. Magnetic microspheres: a model system for site specific drug delivery in vivo. Proc Soc Exp Biol Med. 158:141–146.
- Wu W, He Q, Jiang C. 2008. Magnetic Iron Oxide Nanoparticles: Synthesis and Surface Functionalization Strategies. Nanoscale Res Lett, 3:397–415.
- Zheng X, Lu J, Deng L, Xiong Y, Chen J. 2009. Preparation and characterization of magnetic cationic liposome in gene delivery. Int Pharm. 366:211–217.
- Zhou J, Neff CP, Liu X, Zhang J, Li H, Smith DD, Swiderski P, et al. 2011. Systemic administration of combinatorial dsiRNAs via nanoparticles efficiently suppresses HIV-1 infection in humanized mice. Mol Ther. 19:2228–2238.
- Zohre S, Kazem NK, Abolfazl A, Mohammad RY, Aliakbar M, Effat A, et al. 2014. Trichostatin A-induced apoptosis is mediated by Kruppel-like factor 4 in ovarian and lung cancer. Asian Pac J Cancer Prev. 15:6581–6586.
