Abstract
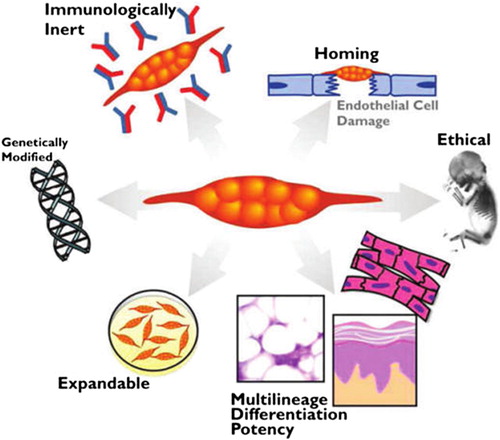
Mesenchymal stem cells (MSCs) are multipotent stromal cells that exist in bone marrow, fat, and so many other tissues, and can differentiate into a variety of cell types including osteoblasts, chondrocytes, and adipocytes, as well as myocytes and neurons. Moreover, they have great capacity for self-renewal while maintaining their multipotency. Their capacity for proliferation and differentiation, in addition to their immunomodulatory activity, makes them very promising candidates for cell-based regenerative medicine. Moreover, MSCs have the ability of mobilization to the site of damage; therefore, they can automatically migrate to the site of injury via their chemokine receptors following intravenous transplantation. In this respect, they can be applied for MSC-based gene therapy. In this new therapeutic method, genes of interest are introduced into MSCs via viral and non-viral-based methods that lead to transgene expression in them. Although stem cell-based gene therapy is a relatively new strategy, it lights a new hope for the treatment of a variety of genetic disorders. In the near future, MSCs can be of use in a vast number of clinical applications, because of their uncomplicated isolation, culture, and genetic manipulation. However, full consideration is still crucial before they are utilized for clinical trials, because the number of studies that signify the advantageous effects of MSC-based gene therapy are still limited.
Introduction
Mesenchymal stem cells (MSCs) are a population of multipotent progenitors which reside in bone marrow, fat, and some other tissues. The potential for self-renewal and multipotency are hallmarks of stem cells in common, and MSCs in particular (CitationMinayi et al. 2014). They can be easily isolated from bone marrow and fat for therapeutic goals (CitationMohammadian et al. 2013).
MSCs are considered to be the perfect candidates for applications in regenerative medicine, because of their significant potential in proliferation and differentiation. Increasing knowledge on the biology of MSCs has provided new insights into their possible clinical uses. The capacity of MSCs to differentiate into various cell lineages like adipocytes, chondrocytes, and osteocytes has led to a variety of investigational strategies to study whether they can be used for tissue engineering approaches. MSCs may also influence the course of chronic degenerative disorders through their trophic and regenerative potential (CitationMaumus et al. 2011). MSCs are also able to be recruited into the location of tumor. The ability of MSCs to migrate to and engraft into the site of solid tumors brought about the idea of MSC-based gene therapy for cancers (CitationJi et al. 2004, CitationTögel et al. 2005, CitationHerrera et al. 2007, CitationKlopp et al. 2011).
As MSCs have received significant attention in the field of regenerative medicine, recent studies focus on the genetic modifying feature of MSCs with the aim of reinforcement of their regenerative capacity (CitationWill et al. 2008, CitationGarcía-Castro et al. 2008). MSCs are used as the sources for delivering transgenes into patients (CitationUccelli et al. 2007, CitationJones and McTaggart 2008). They have newly emerged as a possible therapeutic vehicle for gene therapy and drug delivery, and their therapeutic usefulness is currently being assessed in a variety of disease models including inflammatory and autoimmune diseases, and cancer (CitationGalderisi et al. 2010). This is probably due to their intrinsic ability to migrate toward tumors (CitationWang et al. 2009). The unique properties of MSCs in migrating to the site of damage or repair make them one of the most versatile stem cell populations for use in gene therapy studies and trials (CitationNauta and Fibbe 2007).
Stem cells
Stem cells are undifferentiated pluripotent cells which are found in multicellular organisms. They can differentiate into specialized cells, and are capable of self-renewal to produce more stem cells (CitationTuch 2006). Stem cells can be classified as embryonic or adult, depending on their tissue of origin. The role of adult stem cells is to sustain an established repertoire of mature cell types in essentially steady-state numbers over the lifetime of the organism. Adult tissues with a high turnover rate, such as blood, skin, and intestinal epithelium are maintained by tissue-specific stem cells (CitationDrosse et al. 2008). Adult stem cells from the bone marrow are the most broadly used and characterized stem cells. Adult bone marrow includes a heterogeneous population of cells, including hematopoietic stem cells and their progeny, MSCs, adipocytes, fibroblasts, endothelial cells, etc. (CitationSalem and Thiemermann 2010).
Stem cells are directed to differentiate into particular cell types indefinitely, which suggests the presence of a renewable source of alternative cells to treat various disorders (CitationAbdi et al. 2008).
These cells have a great therapeutic tendency to repopulate damaged tissues, as well as the potential of being genetically manipulated and used in cell-based gene therapy (CitationHodgkinson et al. 2010, CitationHuang et al. 2010).
Mesenchymal stem cells
More than 30 years ago, Friedenstein pioneered the concept that the marrow microenvironment resided within the so-called stromal cells of the marrow that were capable of abandoning the hematopoietic microenvironment to ectopic sites (CitationFriedenstein 1991, CitationFriedenstein et al. 1974). Since then, scientists began to explore the full potential of these cells, leading to the realization that this population of cells has the properties of true stem cells. These cells were officially termed MSCs (CitationCaplan 1991). MSCs are non-hematopoietic progenitor cells that can proliferate and actively differentiate into various cell lineages such as osteoblasts, adipocytes, and chondrocytes. MSCs can be simply isolated from bone marrow aspirates, adipose tissue, and many other sources by their special feature of plastic adherence. They can be identified by expression of some surface molecules like CD73, CD90, and CD105, and the lack of specific hematopoietic markers including CD34, CD45, and HLA-DR. Subsequently, they can be expanded and genetically altered in vitro and then used for cancer therapeutic strategies in vivo (CitationMohammadian et al. 2013). The wide distribution of MSCs throughout the body leads to the assumption that these cells may play a critical role in organ homeostasis (CitationGotherstrom et al. 2005). They are promising tools for regenerative medicine, in the repair of injured tissues. Many studies have focused on two significant features of MSC therapy: (I) systemically administered MSCs home to sites of ischemia or injury, and (II) MSCs can modulate immunological responses (CitationKhakoo et al. 2006, CitationQiao et al. 2008, CitationSecchiero et al. 2010, CitationLi et al. 2010, CitationLi et al. 2011, CitationOtsu et al. 2009, CitationTian et al. 2011, CitationDvorak 1986, CitationKucerova et al. 2007, CitationKidd et al. 2008) ().
MSC application in regenerative medicine
MSCs have attracted a lot of attention in clinical situations, mostly due to their potential in regenerative medicine and tissue engineering. They can be applied for tissue reconstruction, and repair occurs both at the bench and by the bedside. MSCs isolated from bone marrow are presently being examined for their therapeutic features in a diversity of disorders including kidney failure, cardiovascular injury, hematological and non-hematological malignancies, and neurological and bone disorders (CitationHodgkinson et al. 2010, CitationHuang et al. 2010).
MSCs have exclusive therapeutic capacity because of their immunosuppressive and anti-inflammatory properties, via both innate and adaptive immune systems (CitationEnglish et al. 2009). The immunomodulatory features of MSCs allow the sustained release of therapeutic molecules, which elicits no undesirable immune responses even in the allogeneic or xenogeneic transplantations (CitationSeo et al. 2011). Furthermore, the possible role of MSCs in angiogenesis is under consideration. Improved neovascular genesis has been related with regeneration of infracted myocardium by bone marrow-derived MSCs (CitationFuchs et al. 2001).
Homing of MSCs
Homing is the process by which cells migrate to, and engraft within the sites of ischemia or damage. It is a multistep process that begins with interactions between flowing MSCs and the vascular endothelium at the target tissue, mediated via some homing receptors expressed on MSCs that connect to the related endothelial co-receptors (CitationYagi et al. 2010).
An astonishing attribute of MSCs is their noticeable ability to migrate to the site of injury and engraft there, in vivo. They express chemokine receptors and ligands involved in the processes of cell migration and homing (CitationMarlow et al. 2007, CitationKim et al. 2013). In this regard, MSCs are very promising candidates for clinical applications in cell-based replacement therapies (CitationMohammadian et al. 2013). For example, numerous investigations have shown that transplanted MSCs can home to injured tissues in animal models, including myocardial injury (CitationPenna et al. 2008) and acute renal failure (CitationNauta and Fibbe 2007). For purposes of cell-based regenerative therapy, MSCs can be injected in close proximity to injured tissue, which may not be clinically possible in many cases due to potential invasiveness, for example, into the heart or brain. Moreover, locally injected cells often die because of diffusion limitations of nutrients and oxygen, before considerably exerting their therapeutic effects (CitationKarp and Teo 2009, CitationMuschler et al. 2004). The key to the therapeutic possibility and success of systemically injected MSCs is that MSCs have been shown to selectively migrate to the damaged or inflamed tissues (CitationKarp and Teo 2009, CitationSpaeth et al. 2008).
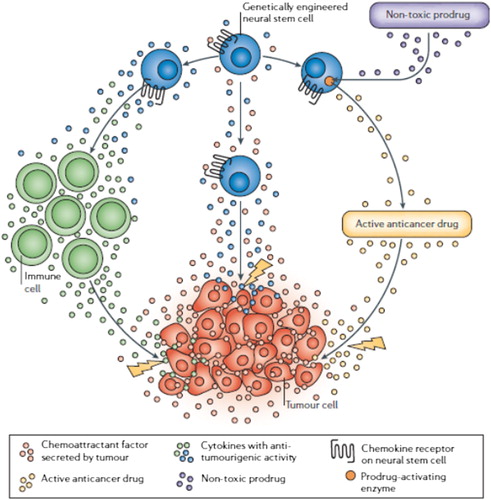
In addition, MSCs can recruit to the site of inflammation and tissue damage/repair, as well as toward the tumor environment. The tumor-trophic capacity of MSCs allows them to search and wipe out undetectable tumor cells (CitationJi et al. 2004, CitationTögel et al. 2005, CitationHerrera et al. 2007, CitationKlopp et al. 2011). The differences in the effects of MSCs on tumor growth depend on the tumor model, and on the dose and time of cell-based therapy (CitationAiuti et al. 2002). In vitro studies have expressed that cell contact between MSCs and tumor cells is not necessary for MSC antitumorigenic activity, as the antiproliferative effect was also observed with the MSC-conditioned medium (CitationHacein-Bey-Abina et al. 2002, CitationAboody et al. 2000, CitationBenedetti et al. 2000, CitationHerrlinger et al. 2000). In addition to the timing of delivery and dose, the delivery route is recognized to be a key factor determining the success of MSC therapy (CitationKarp and Teo 2009).
Detailed research regarding MSC migration and the factors influencing this tropism, has made MSC-based gene therapies probable (CitationDao et al. 1997, CitationNolta et al. 1994, CitationPereira et al. 1998, CitationRingden et al. 2006, CitationBrooke et al. 2007).
Stem cell-based gene therapy
Gene therapy is a new therapeutic branch of modern medicine which is still exceedingly experimental, but is progressively going forward toward becoming an important treatment strategy. Modifying the properties of stem cells may be required to fully utilize their potential. Genetic engineering, with plenty of methodologies to induce gene expression in a precise and well- controlled manner, is especially attractive for this goal (CitationNowakowski et al. 2013). Therapeutic genes should be introduced into the cell used for therapy. There are viral and non-viral-based methods of genetic manipulation (CitationGracey Maniar et al. 2013). Genes may be introduced into cells by transfection or transduction. Transfection utilizes chemical or physical methods for entry of DNA encoding the gene of interest into the target cells (CitationMa et al. 2003). Transduction is the stable integration of the gene of interest into the cell using viruses, which leads to stable transgene expression at high efficiencies and preserves the transgene over several passages during cell division and differentiation (CitationZare et al. 2014).
Stem cell-based gene therapy is a relatively new and highly experimental approach in the treatment of human disease. However, it has recently lit new hope for the treatment of various disorders. Researchers have of late applied MSCs as delivery vehicles for gene therapy, in part due to their accessibility for genetic modification in vitro and mostly due to the simplicity of their culture and expansion in vitro (CitationAboody et al. 2000, CitationBao et al. 2010, CitationMaumus et al. 2011). These cells are appropriate for gene transfer, and as such, are excellent candidates for genetic manipulation to promote proliferation, enhance differentiation to the specific cell lineage, and avoid detrimental cellular dedifferentiation and senescence (CitationLi et al. 2004).
There is hope to bring the results of research to clinical use. For instance, genetic manipulation of MSCs to express angiogenic factors is a promising approach for further improving the efficacy of stem cells for therapeutic angiogenesis. Virally modified VEGF-overexpressing MSCs were reported to increase angiogenesis in vivo and improve myocardial function (CitationJabbarzadeh et al. 2008, CitationMatsumoto et al. 2005). Furthermore, genetically engineered MSCs have been used for expansion of hematopoietic stem cells in stem cell transplantation following myeloablative chemotherapy (CitationDominici et al. 2006, CitationDimmeler et al. 2005, CitationGussoni et al. 1999, CitationZhu et al. 2006, CitationYu et al. 2008, CitationDjouad et al. 2006, CitationRamasamy et al. 2007, CitationWong 2011) ().
Delivery routes
Currently, human stem cells are being used to test new drugs. New medications are subjected to experiments on safety of differentiated cells generated from human pluripotent cell lines. Other types of cell lines have a long history of being used in this method. Cancer cell lines, for instance, are used to screen potential antitumor drugs. The viability of pluripotent stem cells would allow drug testing in a broader range of cell types. Stem cells, directed to separate into particular cell types, suggest the possibility of a renewable source of alternate cells and tissues to treat diseases including macular degeneration, spinal cord injury, stroke, burns, heart disease, diabetes, osteoarthritis, and rheumatoid arthritis. For instance, it may become probable to make healthy heart muscle cells in the laboratory and then to transplant those cells into patients with chronic heart disease (CitationAbdi et al. 2008). In addition to the timing of delivery and dose, the delivery route is recognized to be a key factor determining the success of MSC therapy (CitationKarp and Teo 2009). MSCs can be injected close by to injured tissue, which, though, may not be clinically possible in many cases due to its potential invasiveness—for example, into the heart or brain, and locally injected cells often die before considerably exerting the therapeutic effects because of diffusion limitations of nutrients and oxygen (CitationKarp and Teo 2009, CitationMuschler et al. 2004). The key to the therapeutic possibility and success of systemically injected MSCs is that MSCs have been shown to selectively “home” to injured or inflamed tissues (CitationKarp and Teo 2009, CitationSpaeth et al. 2008). For example, numerous investigations have shown that transplanted MSCs can home to injured tissues in animal models, including myocardial injury (CitationPenna et al. 2008) and acute renal failure (CitationNauta and Fibbe 2007).
Rheumatologic disorders
Increasing knowledge on the biology of MSCs has provided new insights into their possible clinical uses, mainly for rheumatologic disorders. Their ability to differentiate into cells of the bone and cartilage lineages has led to a variety of investigational strategies to study whether MSCs can be used for tissue engineering approaches. Presently, an emerging field of study comes from the possibility that these cells, through their trophic/regenerative potential, may also influence the course of chronic degenerative disorders and put off cartilage degradation in osteoarthritis (CitationMaumus et al. 2011). Bone engineering strategies are warranted in cases of large bone defects or non-union fractures, which remain a serious problem as the associated loss of function considerably impairs the quality of life of affected patients. For successful tissue engineering approaches, implantation of these cells will need the use of growth and differentiation factors that will allow the induction of the particular differentiation pathways and the preservation of the bone or chondrocyte phenotype, together with a suitable scaffold to supply a three-dimensional environment (CitationDrosse et al. 2008, CitationWill et al. 2008).
Immunologic characteristics
Considerably, MSCs, which, as discussed, are immunologically privileged, have extremely strong immunosuppressive effects on a diversity of immune cells via cell–cell contact or soluble factors (summarized in ) (CitationJones and McTaggart 2008, CitationNauta and Fibbe 2007).
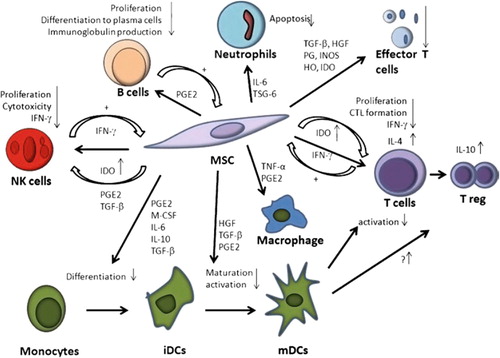
Researchers have found that the direct contact between MSCs and T cells, which modulates the expression of cytokine receptors and transduction molecules for cytokine signaling, also contributes remarkably to the inhibition of T cell proliferation (CitationAbdi et al. 2008, CitationNauta and Fibbe 2007).
Conclusion
MSCs have huge potential for regenerative medicine due to their intrinsic capacity of self-renewal and differentiation, as well as immunomodulatory activity. In addition, MSCs, because of their ability of mobilization to the site of damage, can be used to restore tissue function by providing some paracrine trophic effects to target tissue. The last feature, in addition to the simplicity of isolation and culture, make them an excellent tool for cell-based regenerative medicine to improve damaged heart tissue. Moreover, they can be utilized for gene therapy by manipulation their DNA by so many genetic engineering methods.
Although MSCs might suggest a great hope for regenerative medicine, full consideration is still essential before they are marketed for clinical trials. Yet, the number of studies that signify advantageous effects of MSC-based gene therapy is hard to dismiss.
Authors’ contributions
MM and EA conceived of the study and participated in its design and coordination. AA participated in the sequence alignment and drafted the manuscript. All authors read and approved the final manuscript.
Acknowledgments
The authors thank the Department of Medical Nanotechnology, Faculty of Advanced Medical Science of Tabriz University, for all support provided. This work is funded by a 2014 grant by the Drug Applied Research Center, Tabriz University of Medical Sciences.
Declaration of interest
The authors have no declaration of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abdi R, Fiorina P, Adra CN, Atkinson M, Sayegh MH. 2008. Immunomodulation by mesenchymal stem cells: a potential therapeutic strategy for type 1 diabetes. Diabetes. 57:1759–1767.
- Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, et al. 2000. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci USA. 97:12846–12851.
- Aiuti A, Slavin S, Aker M, Ficara F, Deola S, Mortellaro A, et al. 2002. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science. 296:2410–2413.
- Bao Q, Zhao Y, Renner A, Niess H, Seeliger H, Jauch KW, Bruns CJ. 2010. Cancer stem cells in pancreatic cancer. Cancers. 2:1629–1641.
- Benedetti S, Pirola B, Pollo B, Magrassi L, Bruzzone MG, Rigamonti D, et al. 2000. Gene therapy of experimental brain tumors using neural progenitor cells. Nat Med. 6:447–450.
- Brooke G, Cook M, Blair C, Han R, Heazlewood C, Jones B, et al. 2007. Therapeutic applications of mesenchymal stromal cells. Semin Cell Dev Biol. 18:846–858
- Caplan AI. 1991. Mesenchymal stem cells. J Orthop Res. 9:641–650
- Dao MA, Pepper KA, Nolta JA. 1997. Long-term cytokine production from engineered primary human stromal cells influences human hematopoiesis in an in vivo xenograft model. Stem Cells. 15:443–454.
- Dimmeler S, Zeiher AM, Schneider MD. 2005. Unchain my heart: the scientific foundations of cardiac repair. J Clin Invest. 115:572–583.
- Djouad F, Bony C, Apparailly F, Louis-Plence P, Jorgensen C, Noel D. 2006. Earlier onset of syngenic tumors in the presence of mesenchymal stem cells. Transplantation. 82:1060–1066.
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. 2006. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement Cytotherapy. 8:315–317.
- Drosse I, Volkmer E, Capanna R, De Biase P, Mutschler W, Schieker M. 2008. Review tissue engineering for bone defect healing: an update on a multi-component approach. Injury. 39:9–20.
- Dvorak HF. 1986. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing N Engl J Med. 315:1650–1659.
- English K, Ryan JM, Tobin L, Murphy MJ, Barry FP, Mahon BP. 2009. Cell contact, rostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4 + CD25(High) forkhead box P3 + regulatory T cells. Clin Exp Immunol. 156:149–160.
- Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. 1974. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 17:331–340.
- Friedenstein AJ. 1991. Osteogenic stem cells in the bone marrow. Bone Miner. 7:243–272.
- Fuchs D, Fuchs LS, Thompson A, Al Otaiba S, Yen L, Braun M, O’Connor RE. 2001. Is reading important in reading-readiness programs? A randomized field trial with teachers as program implementers. J Educ Psychol. 93:251–267.
- Galderisi U, Giordano A, Paggi MG. 2010. The bad and the good of mesenchymal stem cells in cancer: boosters of tumor growth and vehicles for targeted delivery of anticancer agents. World J Stem Cells. 2:5–12.
- García-Castro J, Trigueros C, Madrenas J, Pérez-Simón JA, Rodriguez R, Menendez P. 2008. Mesenchymal stem cells and their use as cell replacement therapy and disease modelling tool. J Cell Mol Med. 12:2552–2565.
- Gotherstrom C, West A, Liden J, Uzunel M, Lahesmaa R, Le Blanc K. 2005. Difference in gene expression between human fetal liver and adult Bone marrow mesenchymal stem cells. Haematologica. 90:1017–1026.
- Gracey Maniar LE, Maniar JM, Chen ZY, Lu J, Fire AZ, Kay MA. 2013. Minicircle DNA vectors achieve sustained expression reflected by active chromatin and transcriptional level. Mol Ther..21:131–138.
- Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, et al. 1999. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 401:390–394.
- Hacein-Bey-Abina S, Le Diest F, Carlier F, Bouneaud C, Hue C, De Villartay JP, et al. 2002. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N Engl J Med. 346:1185–1193.
- Herrera MB, Bussolati B, Bruno S, Morando L, Mauriello-Romanazzi G, Sanavio F, et al. 2007. Exogenous mesenchymal stem cells localize to the kidney by means of CD44 following acute tubular injury. Kidney Int. 72:430–441.
- Herrlinger U, Woiciechowski C, Sena-Esteves M, Aboody KS, Jacobs AH, Rainov NG, et al. 2000. Neural precursor cells for delivery of replication-conditional HSV-1 vectors to intracerebral gliomas. Mol Ther. 1:347–357.
- Hodgkinson CP, Gomez JA, Mirotsou M, Dzau VJ. 2010. Genetic engineering of mesenchymal stem cells and its application in human disease therapy. Hum Gene Ther. 21:1513–1526
- Huang H, Chen L, Sanberg P. 2010. Cell therapy from bench to bedside translation in CNS neurorestoratology era. Cell Med. 1:15–46.
- Jabbarzadeh E, Starnes T, Khan YM, Jiang T, Wirtel AJ, Deng M, et al. 2008. Induction of angiogenesis in tissue-engineered scaffolds designed for bone repair: a combined gene therapy-cell transplantation approach. Proc Natl Acad Sci USA. 105:11099–11104.
- Ji JF, He BP, Dheen ST, Tay SS. 2004. Interactions of chemokines and chemokine receptors mediate the migration of mesenchymal stem cells to the impaired site in the brain after hypoglossal nerve injury. Stem Cells..22:415–427.
- Jones BJ, McTaggart SJ. 2008. Immunosuppression by mesenchymal stromal cells: from culture to clinic. Exp Hematol. 36:733–741.
- Karp JM, Teo G. 2009. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 4:206–216.
- Khakoo AY, Pati S, Anderson SA, Reid W, Elshal MF, Rovira II, et al. 2006. Human mesenchymal stem cells exert potent anti-tumorigenic effects in a model of Kaposi's sarcoma. J Exp Med. 203:1235–1247.
- Kidd S, Spaeth E, Klopp A, Andreeff M, Hall B, Marini FC. 2008. The (in) auspicious role of mesenchymal stromal cells in cancer: be it friend or foe. Cytotherapy. 10:657–667.
- Kim SW, Kim SJ, Park SH, Yang HG, Kang MC, Choi YW, et al. 2013. Complete regression of metastatic renal cell carcinoma by multiple injections of engineered mesenchymal stem cells expressing dodecameric TRAIL and HSV-TK. Clin Cancer Res. 19:415–427.
- Klopp AH, Gupta A, Spaeth E, Andreeff M, Marini F III. 2011. Concise review: dissecting a discrepancy in the literature: do mesenchymal stem cells support or suppress tumor growth? Stem Cells. 29: 11–19.
- Kucerova L, Altanerova V, Matuskova M, Tyciakova S, Altaner C. 2007. Adipose tissuederived human mesenchymal stem cells mediated prodrug cancer gene therapy. Cancer Res. 67:6304–6313.
- Li GC, Ye QH, Xue YH, Sun HJ, Zhou HJ, Ren N, et al. 2010. Human mesenchymal stem cells inhibit metastasis of a hepatocellular carcinoma model using the MHCC97-H cell line. Cancer Sci. 101: 2546–2553.
- Li L, Tian H, Chen Z, Yue W, Li S, Li W. 2011. Inhibition of lung cancer cell proliferation mediated by human mesenchymal stem cells. Acta Biochim Biophys Sin. 43:143–148.
- Li Y, Tew SR, Russell AM, Gonzalez KR, Hardingham TE, Hawkins RE. 2004. Transduction of passaged human articular chondrocytes with adenoviral, retroviral, and lentiviral vectors and the effects of enhanced expression of SOX9. Tissue Eng. 10:575–584.
- Ma Y, Ramezani A, Lewis R, Hawley RG, Thomson JA. 2003. High-level sustained transgene expression in human embryonic stem cells using lentiviral vectors. Stem Cells. 21:111–117.
- Marlow LAV, Waller J, Wardle J. 2007. Public awareness that HPV is a risk factor for cervical cancer. Br J Cancer. 97:691–694.
- Matsumoto R, Omura T, Yoshiyama M, Hayashi T, Inamoto S, Koh KR, et al. 2005. Vascular endothelial growth factor-expressing mesenchy-mal stem cell transplantation for the treatment of acute myocardial infarction. Arterioscler Thromb Vasc Biol. 25:1168–1173.
- Maumus M, Guérit D, Toupet K, Jorgensen C, Noël D. 2011. Mesenchymal stem cell-based therapies in regenerative medicine: applications in rheumatology. Stem Cell Res Ther. 2:14.
- Minayi N, Alizadeh S, Dargahi H, Soleimani M, Khatib ZK, Tayebi B, et al. 2014. The effect of miR-210 up-regulation on proliferation and survival of mouse bone marrow derived mesenchymal stem cell. Int J Hematol Oncol Stem Cell Res. 8:15–23.
- Mohammadian M, Shamsasenjan K, Lotfi Nezhad P, Talebi M, Jahedi M, Nickhah H, et al. 2013. Mesenchymal stem cells: new aspect in cell-based regenerative therapy. Adv Pharm Bull. 3:433–437.
- Muschler GF, Nakamoto C, Griffith LG. 2004. Engineering principles of clinical cell-based tissue engineering. J Bone Joint Surg Am. 86A:1541–1558.
- Nauta AJ, Fibbe WE. 2007. Immunomodulatory properties of mesenchymal stromal cells. Blood. 110:3499–3506.
- Nolta JA, Hanley MB, Kohn DB. 1994. Sustained human hematopoiesis in immunodeficient mice by cotransplantation of marrow stroma expressing human interleukin-3: analysis of gene transduction of long-lived progenitors. Blood. 83:3041–3051.
- Nowakowski A, Andrzejewska A, Janowski M, Walczak P, Lukomska B. 2013. Genetic engineering of stem cells for enhanced therapy. Acta Neurobiol Exp. 73:1–18.
- Otsu K, Das S, Houser SD, Quadri SK, Bhattacharya S, Bhattacharya J. 2009. Concentration-dependent inhibition of angiogenesis by mesenchymal stem cells. Blood. 113:4197–4205.
- Penna C, Raimondo S, Ronchi G, Rastaldo R, Mancardi D, Cappello S, et al. 2008. Early homing of adult mesenchymal stem cells in normal and infarcted isolated beating hearts. J Cell Mol. 12:507–521.
- Pereira RF, O’Hara MD, Laptev AV, Halford KW, Pollard MD, Class R, et al. 1998. Marrow stromal cells as a source of progenitor cells for onhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfect. Proc Natl Acad Sci USA. 95:1142–1147.
- Qiao L, Xu Z, Zhao T, Zhao Z, Shi M, Zhao RC, et al. 2008. Suppression of tumorigenesis by human mesenchymal stem cells in a hepatoma model. Cell Res. 18:500–507.
- Ramasamy R, Lam EW, Soeiro I, Tisato V, Bonnet D, Dazzi F. 2007. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: impact on in vivo tumor growth. Leukemia. 21:304–310.
- Ringden O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lönnies H, et al. 2006. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 81:1390–1397.
- Salem HK, Thiemermann C. 2010. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. 28:585–596.
- Secchiero P, Zorzet S, Tripodo C, Corallini F, Melloni E, Caruso L, et al. 2010. Human bone marrow mesenchymal stem cells display anti-cancer activity in SCID mice bearing disseminated Non-Hodgkin's lymphoma xenografts. PLoS One. 6:e11140.
- Seo SH, Kim KS, Park SH, Suh YS, Kim SJ, Jeun SS, et al. 2011. The effects of mesenchymal stem cells injected via different routes on modified IL-12-mediated antitumor activity. Gene Ther. 18:488–495.
- Spaeth E, Klopp A, Dembinski J, Andreeff M, Marini F. 2008. Inflammation and tumor microenvironments: defining the migratory itinerary of mesenchymal stem cells. Gene Ther. 15:730–738.
- Tian LLH, Yue W, Zhu F, Li S, Li W. 2011. Human mesenchymal stem cells play a dual role on tumor cell growth in vitro and in vivo. J Cell Physiol. 226:1860–1867.
- Tögel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. 2005. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 289:F31–42.
- Tuch BE. 2006. Stem cells a clinical update. Aust Fam Physician. 35:719–721.
- Uccelli A, Pistoia V, Moretta L. 2007. Mesenchymal stem cells: a new strategy for Immunosuppression. Trends Immunol. 28:219–226.
- Wang H, Cao F, De A, Cao Y, Contag C, Gambhir SS, et al. 2009. Trafficking mesenchymal stem cell engraftment and differentiation in tumor-bearing mice by ioluminescence imaging. Stem Cells. 27:1548–1558.
- Will J, Melcher R, Treul C, Travitzky N, Kneser U, Polykandriotis E, et al. 2008. Porous ceramic bone scaffolds for vascularized bone tissue regeneration. J Mater Sci Mater Med. 19:2781–2790.
- Wong RS. 2011. Mesenchymal stem cells: angels or demons? J Biomed Biotechnol. 2011: 459510.
- Yagi H, Soto-Gutierrez A, Parekkadan B, Kitagawa Y, Tompkins RG, Kobayashi N, et al. 2010. Mesenchymal stem cells: mechanisms of immunomodulation and homing. Cell Transplant. 19:667.
- Yu JM, Jun ES, Bae YC, Jung JS. 2008. Mesenchymal stem cells derived from human adipose tissues favor tumor cell growth in vivo. Stem Cells Dev. 17:463–473.
- Zare M, Soleimani M, Mohammadian M, Akbarzadeh A, Havasi P, Zarghami N. 2014. Efficient biotechnological approach for lentiviral transduction of induced pluripotent stem cells. Artif Cells Nanomed Biotechnol.1–6.
- Zhu W, Xu W, Jiang R, Qian H, Chen M, Hu J, et al. 2006. Mesenchymal stem cells derived from bone marrow favour tumor cell growth in vivo. Exp Mol Pathol. 80:267–274.