Abstract
7-Ethyl-10-hydroxycamptothecin (SN-38) is 1000 times more cytotoxic than its prodrug Irinotecan hydrochloride (CPT-11). It is not used therapeutically because of its insolubility in acceptable solvents. The objective of the present study was to prepare chitosan nanoparticles (CsENP) of SN-38 by polyelectrolyte complexation method using the Box-Behnken design. CsENPs were evaluated for mean particle size, drug loading, entrapment efficiency and characterized by TEM, XRD, DSC and FTIR. The actual values represented good agreement with predicted values. Drug release behavior in simulated colonic fluid followed Higuchi kinetics. CsENPs were stable and can be used further for treatment of colon cancer by oral route.
Introduction
Oral chemotherapy is the need of today’s cancer sufferers. This route is attractive because of its convenience and ease of administration, particularly in conscious conditions. 7-ethyl-10-hydroxycamptothecin (SN-38) is a highly cytotoxic, nearly 100-1000 times more cytotoxic than its prodrug Irinotecan hydrochloride (CPT-11) (Atyabi et al. Citation2009). CPT-11 is FDA approved chemotherapeutic agent available in the market as Camptosar® (Pharmacia Corp., Peapack, NJ) and SN-38 is at present in clinical trials by some of the pharmaceutical companies, e.g. Enzon Pharma, Neopharm, Insys. It is cytotoxic against cancer-like colorectal cancer (Carie et al. Citation2011). CPT-11 is converted to SN-38 in liver and tumor cells by the enzyme carboxylesterases (Jansen et al. Citation1997). It has an advantage over its camptothecin precursors; it does not require activation by the liver, thereby eliminating the interpatient variability (Zhang et al. Citation2013). The metabolic conversion rate of SN-38 is about 2-8% of the original volume of CPT-11(Zhang et al. Citation2004).Thus, further efficient use of SN-38 might be of great advantage and may be attractive for cancer treatment. It is a challenge for the researchers to develop a formulation of SN-38 because of its insolubility in number of pharmaceutically acceptable solvents and its pH-dependent activity. It exists in two forms, e.g. lactone (at low pH, active form) and carboxylate (at high pH, inactive form) (Yang et al. Citation2005). Delivery systems like polymeric micelles, polymer drug conjugates, antibody conjugates, liposomes, nanoparticles (NPs) have been formulated and evaluated for its cytotoxicity against cancer cell lines (Bala et al. Citation2013). Oral formulations of SN-38 were investigated for permeability through Caco-2 monolayers but needs to be more investigated at the backdrop of other biological factors.
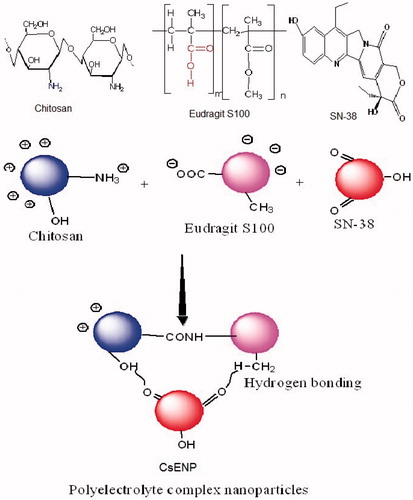
In this study, a novel approach is applied where polymeric nanoparticles are developed using chitosan (cationic polymer) and Eudragit®S100® (anionic copolymers based on methacrylic acid and methyl methacrylate) by polyelectrolyte complexation and the nanoparticles formed are having positive surface charge due to coating of chitosan. As mucus is negatively charged, these NPs will stay for longer period of time inside gut. SN-38 is entrapped in these nanoparticles (NPs) in lactone form. Polymeric NPs can incorporate both hydrophilic and lipophilic drug, release the drug at a specific site improves oral delivery of drugs with poor chemical, enzymatic or metabolic stability and permeability (Patel et al. Citation2012). Eudragit®S100 (ES-100) dissolves when the pH is higher than 7 (Wang and Zhang Citation2012). Chitosan is a biodegradable polymer, which is soluble in acidic media only (Chen et al. Citation2013, Prasad and Suresh Citation2011) and is used for colonic delivery (Shukla and Tiwari Citation2012). Polyelectrolyte complexation is a novel approach for the formation of NPs as compared to other methods and it has a potential for oral controlled release (Hamman Citation2010). SN-38 is entrapped in NPs in lactone form and drug release characteristics showed great potential for colon delivery. As this system is meant for colon cancer, it has minimal release of drug at other sites thus preventing systemic side effects. This system was found suitable for colonic delivery of SN-38 in lactone form and can be used for colon cancer treatment.
Material and methods
7-Ethyl-10-hydroxycamptothecin was generously gifted by Avra Labs (Hyderabad, India). Eudragit® S100 was obtained as a gift sample from Evonik Industries (Mumbai, India). Chitosan (deacetylation degree ≥ 75%), Pluronic F 68, Dialysis bag (cut-off mol. wt. 8000 Dalton), HPLC grade acetonitrile and acetone were purchased from Hi Media Labs (Mumbai, India). All other chemicals and solvents were of analytical grade. Double distilled water was used throughout the study.
Methods
Preparation of chitosan and Eudragit® nanoparticles
Chitosan and Eudragit® nanoparticles (CsENP) were prepared by modified ionic gelation/polyelectrolyte complexation (Hamman Citation2010, Nimesh et al. Citation2014) method. Chitosan (in 1% glacial acetic acid) was stirred at room temperature and Pluronic F68 (Poloxamer 188) solution was added to it as a surfactant at different concentrations. The pH measured and was found to be between 4 and 5. To this emulsion SN-38 was added. This mixture was stirred for 15 min. To this Eudragit® S100 solution, acetone was added drop wise and at a very slow rate. The mixture was kept under magnetic stirring for 6 h. The mixture was centrifuged at 2500 rpm, 4 °C for 10 min to remove the polymer aggregates (Prasad and Dangi Citation2014). Supernatant taken and bath sonicated (Loba Life, 3.5L100, 230 V, 50 Hz, Mumbai, India) and again centrifuged (Remi cooling centrifuge) at 18,000 rpm for 10 min. Supernatant discarded and sediment washed thrice with double distilled water to remove the organic solvents till no drug is found in the washings as analyzed by HPLC (Young Lin, Korea), added 1% mannitol as cryprotectant and lyophilized for 48 h and kept in dessicator at 2-4 °C. The schematic diagram depicting the formation of polyelectrolyte complex nanoparticles and the structures of chitosan, Eudragit® S100 and SN-38 is shown in .
Preparation of CsENP as per experimental design
The CsENP formulations were prepared as per the experimental runs obtained from Design Expert software 9® (Stat-Ease Inc., Minneapolis, MN, USA). A 3-level experimental response surface methodology using the Box-Behnken design (Gaur et al. Citation2014) was used to study the effect of different variables on formulation properties like mean particle size (PS), Y1 and entrapment efficiency (% EE) (Y2) of the prepared CsENPs. Independent variables used in the study include polymer (Eudragit® S100) concentration (X1), surfactant concentration (X2), and sonication time (X3). These were chosen on the basis of the tested lower and upper values for each variable as per preformulation studies and literature research. A total of 17 runs were carried with 0 blocks and no categoric factors. In order to further optimize the CsENP formulations, response surface methodology was employed to identify the exact amount of surfactant and polymer, which produces CsENP with low particle size and more entrapment efficiency. The best fitted models for statistical analysis were considered significant, with probability limits of the P value < 0.05. Predicted R2 value and one-way ANOVA were estimated to confirm the best fit of the model. For optimization, the desirability function of particle size was at the minimum level, and that of entrapment efficiency and drug loading was at the maximum level. In this study, all the data are reported as mean ± standard deviation (n =3). Design-Expert software (version 9.0.1, trial version, Stat-Ease,Inc., Minneapolis, MN, USA) was utilized for statistical analysis and graph plotting. The quadratic model generated by the design was:
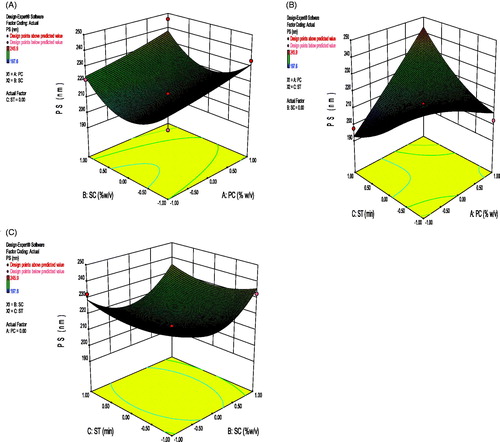
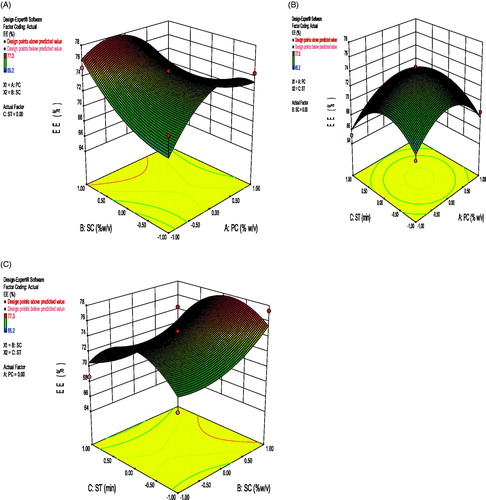
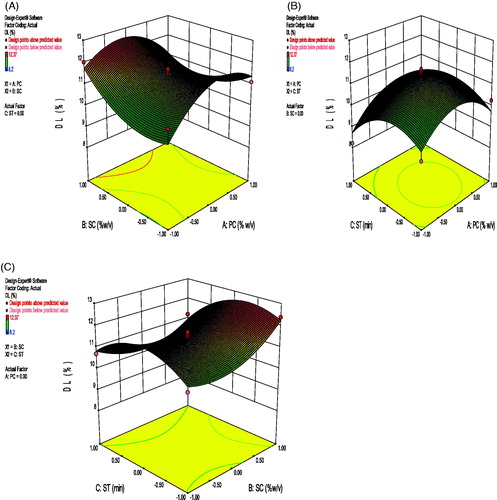
where, A0 – A9 are the coefficients of the respective variables and their interaction terms; Y is the measured response of the dependent variables allied with each factor-level combination; X1–X3 are the codes for independent variables. Independent variables were concentration of polymer (X1), concentration of surfactant (X2), and the sonication time (X3). These were represented by levels -1, 0 and +1 corresponding to the low, middle, and high values, respectively (given in ). The responses Y1 = particle size (PS), Y2 = entrapment efficiency (%EE), and Y3= drug loading (%DL) and the Box–Behnken design employed for the study is shown in . 3D response surface graphs, which were plotted to depict the effects of the preset factors on the measured responses are helpful in elucidating the relationship between independent variables and dependent variables. These plots can depict the qualitative effect of each variable on each response parameter.
Table I. Formulation variables in coded and actual values and their respective responses in different batches of CsENP.
Physicochemical characterization of CsENPs
Fourier transform infrared spectroscopy analysis
SN-38, Chitosan, ES100, CsENP and physical mixture of drug and polymers thus obtained were analyzed with Fourier transform infrared spectroscopy (FTIR, IR Solutions, Sunrise, FL, USA) by KBr pellet method. 1 mg of powdered samples is mixed in a small mortar and pestle with purified and desiccated 200 mg of KBr. It was placed in KBr pellet and force is applied for several minutes to form uniform pellet and scanned from 400 to 4000 cm−1. A background measurement was taken on a pellet holder with a pellet of KBr only.
Differential scanning calorimeter studies
DSC of drug, polymers, CsENP was taken by DSC 60 (FC 60-A, Shimadzu, Japan). Samples were sealed in hermetic aluminum pans, run from 25°C and heated at a rate of 10 °C/min up to 300 °C under a nitrogen atmosphere at a flow rate of 40 cm3/min. A similar empty pan was used as a reference. The results were analyzed using Universal Analysis software (TA instruments Inc, New Castle, DE, USA.
Particle size, zeta potential and polydispersity index
The mean particle size (z-average) of the CsENPs, and the polydispersity index (PDI), as measures of the width of particle size distribution, were determined by dynamic light scattering (DLS), using particle size analyzer (SALD 2201, Shimadzu, Kyoto, Japan) and Zetasizer (Nano ZS 90, Malvern Instruments Ltd., Malvern, United Kingdom) at 25°C with a scattering angle of 90°. 2 mg of CsENPs were suspended in 2 ml deionized water. Particle size was measured with G6 cuvette after dilution. The surface charge was estimated by measuring the zeta potential of CsENPs based on the Helmholtz-Smoluchowski equation, using the same equipment at 25°C, with an electric field strength of 23 V/cm. Zeta potential (ZP) was measured using (zeta cuvette clear) without dilution.
where, ξ is the zeta potential, EM is the elecrophoretic mobility, η is the viscosity of the dispersion medium, and ɛ is the dielectric constant.
Transmission electron microscopy
The morphological examination of CsENP suspension was performed with transmission electron microscopy (TEM; FEI Philips Morgagni 268D, 100 kV, and Magnification: up to 2, 80,000×). A drop of diluted sample was placed on the surface of carbon-coated copper grid and stained with a drop of 2% (w/w) aqueous solution of phosphotungstic acid (negative stain) for 30 s. Excessive staining solution was wiped off by filter paper and a thin aqueous film was left on the surface. After staining, samples were dried at room temperature for 10 min to carry out the investigation.
X-ray diffraction study
X-ray diffraction (XRD) study was performed to analyze crystalline or amorphous nature of the SN-38 loaded CsENP. X-ray powder diffraction studies of pure SN-38, ES-100, Chitosan, and SN-38 loaded CsENP were taken by Bench top XRD instrument (Miniflex, Rigaku, Woodlands, TX, USA). Temperature was set between 18 and 22 °C. 2θ angle was set between 20° and 80°. Step width was 0.01, count time 0.1 s, voltage was 30 kV and 15 mA current.
Entrapment efficiency and drug loading
A total of 5 mg of CsENP was taken and dispersed in 2 ml of dichloromethane. 5 ml of acetonitrile was added to the above solution and centrifuged (Remi Instruments Ltd., Mumbai, India) at 20,000 rpm for 20 min. The supernatant was analyzed after dilutions for SN-38 at 265 nm with HPLC (Young Lin, Korea) (Prasad and Dangi Citation2015).
In vitro release of SN-38
The in vitro release studies were conducted by a dialysis membrane method (Souza Citation2014, Dutta and Sahu Citation2012) with modification. The membrane was soaked in double-distilled water for about 12 h, prior using the membrane. CsENP containing SN-38 were enclosed in a dialysis bag MWCO 8 kDa (Hi Media, Mumbai, India) and immersed in 100 ml of simulated gastric fluid (SGF) (pH 1.2 ± 0.5), in simulated intestinal Fluid (SIF) (pH 6.8 ± 0.5), in simulated colonic fluid (SCF) (pH 7.4 ± 0.5) for 48 h. The tests were conducted in a constant temperature shower mixer at 100 rpm at 37°C ± 1°C. At specified time intervals, 1 ml of the release medium was taken out to determine drug concentration and 1 ml of the fresh medium was refilled. The released amount of SN-38 was measured by HPLC at 265 nm. All assays were performed in triplicate.
In vitro drug release kinetics
The release pattern of CsENP in simulated colonic fluid was put in different release models, i.e. zero order (graph plotted between cumulative % release (Q) and time (t)), first order (graph plotted between log of Q and t) and Higuchi kinetics (graph plotted between Q and √t) (Dash et al. Citation2010). The correlation coefficient (r2) value of each plot was found out.
Stability studies
Stability studies were done as per the International Conference on Harmonization (ICH) guidelines. Accelerated stability studies were done (Humidity and Temperature Control Cabinet, MAC, India) at a temperature of 40°C ± 2°C with humidity 75% ± 5% for 6 months (0, 3, 6 months). The samples were characterized with respect to particle size, ZP, PI, EE, and drug loading of CsENP dispersions initially and after 3 and 6 months.
Results and discussions
Preparation of CsENP
Polyelectrolyte complex formation is one of the recent, easiest, and most feasible methods for the formation of chitosan-based nanoparticles. It is based on complexation between anionic and cationic polymer. Eudragit-S100 is often used for coating of nanoparticles (Subudhi et al. Citation2015, Jain et al. Citation2010) for colon targeting because of its pH-dependent solubility above pH 7. Here its ionic property as well as its pH sensitive properties were exploited for the formation of pH sensitive nanoparticles. Ultrasonication improved the formation of smaller nanoparticles with pH sensitive properties. ES-100 is mostly used for coating of nanoparticles and formation of microparticles (Jain et al. Citation2012, Thakral et al. Citation2010). The magnetic stirring speed was optimized at 2500 rpm for 6 h. Amount of cationic polymer chitosan was kept fixed at 0.25% (w/v).
Optimization of polymer (anionic) concentration
The amount of anionic polymer (ES-100) was varied (1, 1.5, and 2%) and added at different pH between 4-5, 5-6, 7-8. A formulation without any clumps and aggregation was formed at pH 4-5. So, this pH was maintained while stirring by adding 0.1 N glacial acetic acid. At 1.5% of ES-100 entrapment efficiency, drug loading was maximum and particle size was optimum and, so 1.5% of ES-100 was found to be at optimum concentration of CsENP formation.
Optimization of surfactant concentration
Different surfactants were used for the preparation of CsENP, i.e. Tween 80, Polyvinyl alcohol (hot and cold water soluble), and Pluronic F68 (Poloxamer 188). Pluronic F68 is a triblock copolymer of poly(ethylene oxide) (PEO) and poly(propylene oxide) (PPO) with the structure PEO–PPO–PEO, are highly surface-active compounds. These are widely used as carriers for anticancer drugs (Zhao et al. Citation2012) and for stabilizing nanoparticles (Vauthier and Bouchemal Citation2009). In addition, it can easily manipulate the p-glycoprotein efflux system and cytochrome P-450, thus improving oral absorption of hydrophobic drugs (Desai et al. Citation2012). On the basis of preformulation studies and literature survey, the surfactant concentrations ranging from 0.1% to 0.3% (w/v) were selected for assessing the effect of change in surfactant concentration on mean particle size and % drug entrapment efficiency. At 0.3% surfactant concentration, the values of mean particle size is 232.5 ± 1.12 nm and that of entrapment efficiency (75.3 ± 0.85%) and drug loading (12.37 ± 1.2%) are maximum. So, from above results 0.3% surfactant concentration is found to be optimum.
Optimization and statistical analysis of experimental data using Design expert software®
The Box-Beckhen design with response surface models is an accurate approach for getting optimized formulation with limited number of experimental runs, thus preventing wastage of time. A total of 17 experimental runs were performed using the Box-Beckhen design for three levels and three factors with three different responses. The variables used in the study were selected on the basis of preformulation studies and literature survey. The values of responses or dependent variables ranged from 65.2 to 75.3% for EE, 197.6 to 245.9 nm for PS, and from 8.2 to 12.37% for DL. The quadratic model obtained was best fitted for all the responses studied. The statistical validation of the polynomial equations was established by Analysis of variance (ANOVA). Therefore, the optimum values of the variables were determined from the obtained experimental data, based on the criterion of desirability presented in . The average experimental values of the three responses of optimized CsENP prepared were very close to the predicted values as shown in . There is low percentage bias, which suggests that the optimized formulation was consistent. The values of various statistical parameters like F-value, P value, multiple correlation coefficients (R2), coefficient of variation, predicted residual sum of squares, standard deviation, and mean square values are shown in . From the design graphical optimization results, optimized CsENPs with high %EE, high % of DL, and small mean particle size, were obtained at polymer concentration of -0.23, surfactant concentration of 0.17, and sonication time 0.02 (coded values). The lack of fit was not significant in case of drug loading at 95% confidence level. All the remaining parameters were significant, at P < 0.05 or lower.
Table II. Comparison of the observed and predicted values of CsENP prepared under predicted optimum conditions.
Table III. Summary of the results of regression analysis for responses, and analysis of variance for particle size, EE, and DL.
Effects on particle size
Particle size varied from 197.6 nm (CsENP 1) to 245.9nm (CsENP 12) for various factor level combination. The effect of independent variables on particle size can be explained by the following quadratic equation:
Correlation coefficient (r2) of the above equation was found to be 0.9132 indicating a good fit. The variable X1 had a significant and positive effect on PS as revealed by the positive value in the quadratic equation. This means PS rapidly increased as the amount of polymer increased. Positive value before X2 revealed increasing the surfactant concentration resulted into an increased PS. It may be due to aggregation of nanoparticles at higher surfactant concentration and the molecular weight of the surfactant. Negative value before X3 shows decrease in PS with increase in sonication time. As increase in sonication time results in smaller particles. The interaction term X1X2, X2X3 and X1X3 shows positive effect on PS. 3D response surface graph depicting the effect of PC-SC, SC-ST, PC-ST is shown in . Particle size and surface charge play important roles in the drug release kinetics and intracellular uptake of polymeric NPs. An average size of 200–500 nm is appropriate for oral delivery of polymeric NPs (Guo et al. Citation2013). Moreover, cationic PNPs could more easily interact through electrostatic forces with the negative surface of the cell membrane and subsequently enhance cellular uptake.
Effects on entrapment efficiency
The entrapment efficiency varied from 65.2% (CsENP 1) to 75.3% (CsENP 5) for various combinations of factors at different level. The independent factors affecting the EE are Eudragit-S100 (%w/v), Poloxamer 188 (%w/v), and sonication time (min). The effect of independent variables on entrapment efficiency can be explained by the following quadratic equation:
where, X1, X2, and X3 represent the coded values of the polymer concentration, surfactant concentration, and sonication time, respectively. The positive values of a factor in the above quadratic equation depict a synergistic effect or an augmentation of that particular response, and vice versa. The variable X1 had a significant and positive effect on EE as revealed by the positive value in the quadratic equation. This means EE rapidly increased as the amount of polymer increased.
Positive value before X2 revealed increasing the surfactant concentration resulted into an increased EE. It may be due to better stabilization of nanoparticles at higher surfactant concentration (Rao and Geckeler Citation2011). Negative value before X3 shows decrease in EE with increase in sonication time. As increase in sonication time may cause particle aggregation and causing lesser entrapment. The interaction terms X1X2 and X2X3 show negative effect on EE and X1X3 shows positive effect on EE. Correlation coefficient (r2) of the above equation was found to be 0.8584 indicating a good fit. 3D response surface graph depicting the effect of PC-SC, SC-ST ans PC-ST is shown in .
Effects on drug loading
Drug loading varied from 8.2 (CsENP 1) to 12.37 (CsENP 5) for various factor level combinations. The effect of independent variables on drug loading can be explained by the following quadratic equation:
Correlation coefficient (r2) of above equation was found to be 0.8601, which indicated a good fit. The variables X1 and X2 had significantly positive effect on drug loading, as polymer concentration and surfactant concentration increases drug loading increased. This is due to the fact that increased polymer concentration provides extra space for adjusting more drug; and similarly increase in surfactant concentration increases the capability of more drug loading by reducing the interfacial tension and increasing the core flexibility (Picone and Cunha Citation2013). X3 had negative effect on DL. Ultrasonication generates heat and energy, which reduces the particle size and while reducing the particle size the drug loading capacity may decrease. X1X2 and X2X3 terms had negative effect on DL. X1X3 had positive effect on DL. 3D response surface graph depicting the effect of PC-SC, SC-ST, and PC-ST is shown in .
Validation of experimental design
The responses were set as per the desirability with maximum entrapment efficiency and drug loading with minimum particle size. The factors were set in goal. The desirability value obtained from Design-Expert software is 0.743. Therefore, a new batch of CsENP with the predicted levels of formulation factors was prepared to confirm the validity of the optimization procedure. The optimized formulation was achieved with the formulation composition having 1.41% (w/v) PC, 0.22% (w/v) SC, and 7.50 min ST. Particle size, entrapment efficiency, and drug loading of the optimized formulation were 212.5 ± 1.61 nm, 75.3 ± 1.01% and 11.1 ± 0.6% drug loading, respectively, which represented good agreement with the predicted values. The predicted values of the responses and % bias are given in . Poydispersity index (PdI) and zeta potential (ZP) of the optimized formulation is 0.318 ± 0.062 and 25.6 ± 0.47 mV, respectively. High zeta potential (positive or negative) indicates stability of nanoparticles dispersion as particles having high charge cause less aggregation and thus lesser PdI. Positive zeta potential is due to the chitosan concentration being more than ES-100.
FTIR
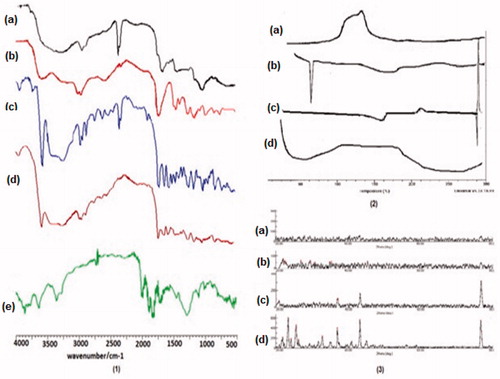
The Fourier transform infrared spectroscopy analysis of pure SN-38, chitosan, ES100, CsENP, and physical mixture were done to investigate the interaction of drug and polymer as shown in (1). In spectra of SN-38 hydrogen bonded OH (3579 cm−1), C-H stretching alkanes (3248, 2978, and 2885 cm−1), C-O stretching and O-H bending (2630 cm−1) and saturated C = O (1735 cm−1) functional groups are present. In physical mixture, peaks for hydrogen bonded OH (3579 cm−1), C-H stretching alkanes (2947 cm−1) and other peaks of polymers and drug are present showing no incompatible reaction between the components. CsENP shows peaks between 3600 and 4000 cm−1 depicting extensive H-bonding, C-H stretching alkanes (2924 cm−1), lactone group (1809 cm−1). In CsENP peaks between 1600 and 1690 shows amide bond formation between COOH group of ES-100 and NH2 group of chitosan. Thus, extensive H-bonding occurred between SN-38, Chitosan, and ES-100. Moreover, lactone form is predominant in CsENP, which is required for its cytotoxic activity and there is no incompatible reaction between SN-38, ES-100, and Chitosan in CsENP formation.
DSC
Differential scanning calorimetry studies are done to investigate the thermal properties, nature of solid powder, and compatibility of drug, polymer and excepients. The thermographs are shown in (2). It shows the endothermic and exothermic peaks of SN-38, Chitosan, ES100, and CsENP. ES100 has endothermic peak starts at 56.67 °C and peak is obtained at 58.67 °C. Similarly for SN-38 peaks are at 160.49 °C, 206.96 °C, and 287.11 °C, chitosan has an exothermic peak at 140.32 oC. Sharp peaks of ES-100, SN-38 shows crystalline nature but CsENP has no such peaks showing that SN-38 is fully dispersed in the CsENPs and it is converted into amorphous form and all other excepients are compatible with SN-38 in molecular form.
Transmission electron microscopy
Size and shape of the optimized batch of nanoparticles were evaluated by transmission electron microscopy (TEM). TEM images of the CsENPs confirmed spherical shape of nanoparticles with narrow size distribution and non-aggregation and clumping of nanoparticles (). The size of nanoparticles was in the range of 135-205 nm as observed in the electron microscopic images.
X-ray diffraction study
X-ray diffractograms ((3)) showed a sharp diffraction peak in spectra of pure SN-38 with a maximum d-value of 4.050 at 21.930 (2θ) and flex value of 0.235 at an intensity of 219 depicting its crystalline structure. Eudragit S100 is crystalline and chitosan is amorphous. In CsENP, there is no evident peak of SN-38, as the peak is deformed indicating the relative reduction in the diffraction intensities. The change in diffraction pattern shows the conversion of crystalline drug to amorphous form contributing enhanced solubility of the drug.
In vitro drug release
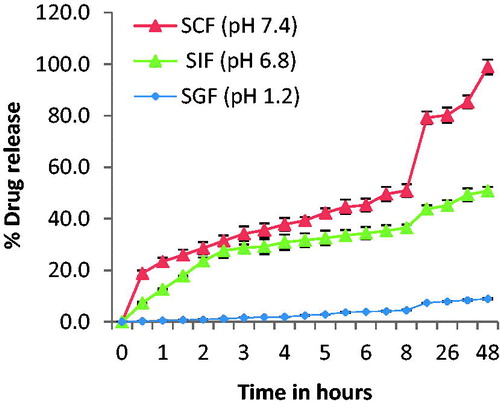
Drug release in simulated gastrointestinal fluids mimics the human body metabolic activity in gastric fluid, intestinal fluid, and colonic fluid. The release study of SN-38 from CsENP was conducted for 48 h in simulated gastrointestinal fluids at different pH. SN-38 release in SGF was negligible. In SIF, at pH 6.8, it released nearly 51% drug and in SCF the release was maximum (>90%) during 48 h. This pattern favored the objective to target the drug for colon cancer by oral route. It is shown graphically in .
In vitro drug release kinetics
Drug release kinetics in simulated colonic fluid followed Higuchi kinetics (r2 = 0.993, closer to 1). This release kinetics is followed by matrix system when the concentration of drug in the matrix is higher than its solubility. The release of drug is by diffusion process across the matrix.
Stability studies
The nanoparticles of SN-38 were found to be stable for 6 months with % deviations of less than ±5% from mean values of various parameters (i.e. particle size, zeta potential, entrapment efficiency, drug loading, and polydispersity index) during accelerated stability studies ().
Table IV. Stability studies of the optimized CsENP.
Conclusions
Polyelectrolyte complex nanoparticles of SN-38 (CsENP) was formulated using polymers, which are biodegradable (Chitosan), pH responsive (ES100) and are used for colon delivery. The CsENPs were optimized by the Box-Behnken factorial design with response surface methodology. Physicochemical characterization depicted no incompatible reactions between drug and excipients. CsENPs under optimized condition gave % EE of (75.3 ± 1.01), % of drug loading (11.1 ± 0.6) % and mean diameter of (212.5 ± 1.61) nm. Polydispersity index (<1) indicated a stable and evenly dispersed nanoparticles without aggregation. In vitro release studies confirmed its pH responsive behavior having maximum release in colonic fluid. FTIR peaks ascertain that lactone form of SN-38 is entrapped inside the nanoparticles. As compared to other delivery system (Bala et al. Citation2013) for SN-38, the present method adopted in the preparation of nanoparticles is less time consuming and is producing more effective results in terms of entrapment efficiency and loading of drugs. Particle size range (>200 nm) is suitable for oral delivery of CsENPs and entrapped lactone form of SN-38 will confirm its cytotoxic activity in colon area. Nanoparticles were found stable in accelerated conditions temperature and humidity and it is being further investigated for its cytotoxic effect on colon cancer by oral route.
Acknowledgements
The authors are also thankful to AIIMS, New Delhi for providing facilities for TEM, SAIL, RGPV, Bhopal for Zeta Sizer; Avra Labs, Hyderabad for gift sample of SN-38 and Evonik Industries, Mumbai for gift sample of Eudragit S100.
Declaration of interest
The authors report no declarations of interest. The corresponding author is thankful to the Department of Science and Technology, New Delhi, India for awarding Inspire fellowship to her.
References
- Atyabi F, Farkhondehfai A, Esmaeili F, Dinarvand R. 2009. Preparation of pegylated nano-liposomal formulation containing SN-38: in vitro characterization and in vivo biodistribution in mice. Acta Pharm. 59:133–144.
- Bala V, Rao S, Boyd BJ, Prestidge CA. 2013. Prodrug and nanomedicine approaches for the delivery of the camptothecin analogue SN38. J Control Release. 172:48–61.
- Carie A, Rios-doria J, Costich T, Burke B, Slama R, Skaff H, Sill K. 2011. IT-141, a polymer micelle encapsulating SN-38, induces tumor regression in multiple colorectal cancer models. J Drug Deliv. 9.
- Chen M, Mi FL, Liao ZX, Hsiao CW, Sonaje K, Chung M, Hsu L, Sung H. 2013. Recent advances in chitosan-based nanoparticles for oral delivery of macromolecules. Adv Drug Deliv. 65:865–879.
- Dash S, Murthy PN, Nath L, Chowdhury P. 2010. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol Pharm - Drug Res. 67:217–223.
- Desai PP, Date A, Patravale VB. 2012. Overcoming poor oral bioavailability using nanoparticle formulations opportunities and limitations. Drug Discov Today: Technol. 9:87–95.
- Dutta RK, Sahu S. 2012. Development of a novel probe sonication assisted enhanced loading of 5-FU in SPION encapsulated pectin nanocarriers for magnetic targeted drug delivery system. Eur J Pharm Biopharm. 82:58–65.
- Gaur PK, Mishra S, Kumar A, Panda BP. 2014. Development and optimization of gastroretentive mucoadhesive microspheres of gabapentin by Box–Behnken design. Artif Cells Nanomed Biotechnol. 42:167–177.
- Guo M, Rong WT, Hou J, Wang DF, Lu Y, Wang Y, Yu SQ, Xu Q. 2013. Mechanisms of chitosan-coated poly(lactic-co-glycolic acid) nanoparticles for improving oral absorption of 7-ethyl-10-hydroxycamptothecin. Nanotechnology. 24:245101.
- Hamman JH. 2010. Chitosan based polyelectrolyte complexes as potential carrier materials in drug delivery systems. Mar Drugs. 8:1305–1322.
- Jain A, Jain SK, Ganesh N, Barve J, Beg AM. 2010. Design and development of ligand-appended polysaccharidic nanoparticles for the delivery of oxaliplatin in colorectal cancer. Nanomedicine. 6:179–190.
- Jain V, Gupta S, Pandey GK, Dubey BK, Jain PK, Saluja MS. 2012. Design and characterization of Eudragit coated chitosan microspheres of mesalazine for irritable bowel disease. Int J Drug Discov Herb Res. 2:301–307.
- Jansen WJ, Zwart B, Hulscher ST, Giaccone G, Pinedo HM, Boven E. 1997. CPT-11 in human colon-cancer cell lines and xenografts: characterization of cellular sensitivity determinants. Int J Cancer. 70:335–340.
- Nimesh S, Hembram KC, Prabha S, Chandra R, Ahmed B. 2014. Advances in preparation and characterization of chitosan nanoparticles for therapeutics. Artif Cells Nanomed Biotechnol. 42(4):1–10.
- Patel T, Zhou J, Piepmeier JM, Saltzman WM. 2012. Polymeric nanoparticles for drug delivery to the central nervous system. Adv Drug Deliv. 64:701–705.
- Picone CSF, Cunha RL. 2013. Chitosan–gellan electrostatic complexes: influence of preparation conditions and surfactant presence. Carbohydr Polym. 94:695–703.
- Prasad S, Dangi JS. 2014. Optimization and characterization of biodegradable polymeric nanocapsules of a camptothecin derivative. Int J Pharm Pharm Sci. 6:685–689.
- Prasad S, Dangi JS. 2015. Determination of SN-38 in nanocapsules by chromatographic and spectrophotometric methods. World J Pharm Res. 4(9):1227–1242.
- Prasad S, Suresh PK. 2011. Mucoadhesive submicron emulsion: a novel approach for periodontal diseases. Int J Curr Pharm Res. 3(2):80–90.
- Rao JP, Geckeler KE. 2011. Polymer nanoparticles: preparation techniques and size-control parameters. Prog Polym Sci. 36:887–913.
- Shukla RK, Tiwari A. 2012. Carbohydrate polymers: applications and recent advances in delivering drugs to the colon. Carbohydr Polym. 88:399–416.
- Souza SD. 2014. A review of in vitro drug release test methods for nano-sized dosage forms. Adv Pharm. 12.
- Subudhi MB, Jain A, Jain A, Hurkat P, Shilpi S, Gulbake A, Jain SK. 2015. Eudragit S100 coated citrus pectin nanoparticles for colon targeting of 5-fluorouracil. Materials (Basel). 8:832–849.
- Thakral NK, Ray AR, Majumdar DK. 2010. Eudragit S-100 entrapped chitosan microspheres of valdecoxib for colon cancer. J Mater Sci Mater Med. 21:2691–2699.
- Vauthier C, Bouchemal K. 2009. Methods for the preparation and manufacture of polymeric nanoparticles. Pharm Res. 26:1025–1058.
- Wang X, Zhang Q. 2012. pH-sensitive polymeric nanoparticles to improve oral bioavailability of peptide/protein drugs and poorly water-soluble drugs. Eur J Pharm Biopharm. 82:219–229.
- Yang X, Hu Z, Yung S, Cher B, Duan W, Chan E, Zhou S. 2005. Simultaneous determination of the lactone and carboxylate forms of irinotecan (CPT-11) and its active metabolite SN-38 by high-performance liquid chromatography: application to plasma pharmacokinetic studies in the rat. J Chromatogr B. 821:221–228.
- Zhang H, Wang J, Mao W, Huang J, Wu X, Shen Y, Sui M. 2013. Novel SN38 conjugate-forming nanoparticles as anticancer prodrug: in vitro and in vivo studies. J Control Release. 166:147–158.
- Zhang JA, Xuan T, Parmar M, Ma L, Ugwu S, Ali S, Ahmad I. 2004. Development and characterization of a novel liposome-based formulation of SN-38. Int J Pharm. 270:93–107.
- Zhao L, Du J, Duan Y, Zang Y, Zhang H, Yang C, Cao F. 2012. Curcumin loaded mixed micelles composed of Pluronic P123 and F68: preparation, optimization and in vitro characterization. Colloids Surf B Biointerfaces. 97:101–108.