Abstract
We aimed to explore the role of NLRP3 inflammasome in Bifidobacterium longum-regulated visceral hypersensitivity of postinfectious irritable bowel syndrome (PI-IBS). Fifty NIH mice were divided into five groups (n = 10). The visceral sensitivities of PI-2W and PI-8W groups significantly exceeded than those of normal control groups (P < 0.05), which was significantly decreased in PI-B group (P < 0.05). Significantly more IL-18 and IL-1β were expressed in PI-2W and PI-8W groups than those in normal control groups (P < 0.05), which was significantly decreased in PI-B group (P < 0.05). Bifidobacterium longum may down-regulate IL-18 and IL-1β expressions by inhibiting NLRP3 inflammasome and thus reduce the visceral hypersensitivity of PI-IBS.
Introduction
Irritable bowel syndrome (IBS), as a common functional bowel disorder, is manifested as abdominal pain and (or) discomfort and is related with number and characteristics of bowel movements, but without a clear pathogenesis up to now. IBS has recently been attributed to gastrointestinal infections, referred to as postinfectious IBS (PI-IBS) (Lee and Park Citation2014). About 3.7–36.0% of patients with acute gastrointestinal infections finally suffer from IBS (Marshall et al. Citation2007), who usually have disordered intestinal flora (e.g., significant decrease of Lactobacillus and Bifidobacterium) (Kassinen et al. Citation2007). Meanwhile, the patients are evidently more prone to excessive growth and proliferation of small intestinal bacteria than normal people (Ford et al. Citation2009). Probiotics have been employed to treat IBS. For instance, Lactobacillus and Bifidobacterium can remarkably mitigate the symptoms of IBS and improve the quality of life, with an elusive mechanism (Rodes et al. Citation2013). Inflammasomes include 14 members, i.e., NLRP1-NLRP14. NLRP3 inflammasome plays an important role in intestinal flora and intestinal innate immunity. Upon depletion of NLRP3 gene, mice undergo gastrointestinal bleeding, colon shortening, obvious changes of intestinal flora and increased susceptibility to inflammatory bowel diseases (Dupaul-Chicoine et al. Citation2010, Zaki et al. Citation2010). However, the effects of NLRP3 on PI-IBS remain unknown. We herein aimed to explore whether Bifidobacterium longum could regulate the visceral hypersensitivity of PI-IBS model mice, and to clarify the role of NLRP3 inflammasome.
Materials and methods
Establishment of PI-IBS model and experimental grouping
This study has been approved by the ethics committee for animal use of Second Affiliated Hospital, Medical School of Xi’an Jiaotong University, and all experiments were performed according to “Guidance Suggestions for the Care and Use of Laboratory Animals (2006)” issued by the Ministry of Science and Technology of the People’s Republic of China. Fifty NIH mice were divided into five groups (n = 10), i.e., 2-week normal control group (NC-2W), 2-week postinfectious group (PI-2W), 8-week normal control group (NC-8W), 8-week postinfectious group (PI-8W) and 8-week postinfectious group with B. longum interference (PI-B).
PI-2W, PI-8W and PI-B groups were established according to a previous literature (Keating et al. Citation2008), and they were intragastrically administered with 350–400 Trichinella spiralis larvae that had been diluted with 0.2 ml of PBS. NC-2W and NC-8W groups were intragastrically given 0.2 ml of PBS without T. spiralis. PI-B group was intragastrically administered with 2 × 109 CFU/day B. longum for 1 week, 8 weeks after the infection, while PI-8W group was used as the control for intervention and given 0.9% NaCl solution with the same volume for 1 week.
Assessment of abdominal withdraw reflex scores
Visceral sensory functions were evaluated by using abdominal withdraw reflex (AWR) scores. In brief, mice were fasted for 24 h with free access to water, inserted with a balloon catheter through the anus and detected after adaptation. Gas was injected randomly by an operator until the target pressure that was maintained for 20 s, and the mice were scored by an observer who then returned the pressure to zero. Starting from 20 mmHg (1 mmHg = 0.133 kPa), pressure inside the balloon was elevated to 40, 60 and 80 mmHg every 5 min. The mice were scored five times at each pressure, and the averages were reported. The scoring was a double-blind procedure completed by the operator and the observer. AWR scoring criteria: 0, without response upon colorectal distension; 1 point: the body remained in stationary state and the head movement decreased upon colorectal distension; 2 points: the abdominal muscle contracted but did not leave the ground upon colorectal distension; 3 points: the abdominal muscle contracted and left the ground upon colorectal distension; 4 points: the pelvis raised, the body arched and the perineum left the ground upon colorectal distension.
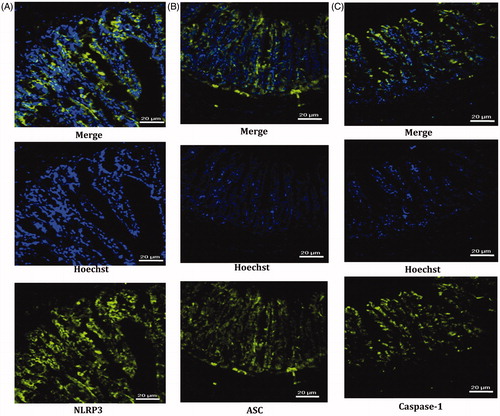
Location of NLRP3 and its ligands
The expressions of NLRP3 and its ligands in terminal ileal tissues of PI-8W group were located by immunofluorescent labeling. Terminal ileal tissues were fixed in 4% paraformaldehyde for 24 h, dehydrated, embedded, sliced into 4-μm-thick sections, deparaffinized, antigen-repaired at high pressure in pH 6.0 sodium citrate buffer, blocked in sheep immune sera at room temperature for 1 h, incubated with primary antibodies respectively at 4 °C overnight (NLRP3, Abcam, Cambridge, UK, 1:500 dilution; ASC, Santa Cruz, CA, 1:400 dilution; caspase-1, Abcam, 1:300 dilution; PBS was added to the negative control), washed with PBS at room temperature, and incubated with fluorescence-labeled secondary antibodies at 37 °C for 1.5 h (donkey anti-rabbit, Abcam, 1:1000 dilution; donkey anti-mouse IgG, Santa Cruz, CA, 1:1200 dilution). Afterwards, the nuclei were stained with Hoechst33258 (1:2000 dilution), and the sections were incubated at room temperature for 10 min, washed with PBS, sealed with antifade reagent (Life Technologies, Frederick, MD), and observed and photographed under a confocal microscope.
Detection of NLRP3 and related proteins
The expression levels of NLRP3 and related proteins were detected by Western blot. Fresh terminal ileal tissues (100 mg) were homogenized, added in protein lysis buffer, ground on ice for 30 min, and centrifuged at 4 °C and 12,000 g for 10 min, from which the supernatant was collected, added proportionally into loading buffer, denatured and stored in a −80 °C refrigerator. The supernatant (10 μl each) was used to detect the concentration of total protein by the BCA method. Total protein sample of each group (80 μl) was resolved on 12% SDS–PAGE, transferred to a membrane, and blocked in 5% skimmed milk–TBST buffer for 1 h. Then, the membrane was incubated with primary antibodies overnight at 4 °C (CD172a, Abcam, 1:1000 dilution; NLRP3, Abcam, 1:500 dilution; ASC, Santa Cruz, 1:400 dilution; caspase-1, Abcam, 1:300 dilution; IL-18, Abcam, 1:1000 dilution; IL-1β, Abcam, 1:1000 dilution), washed with TBST buffer, incubated with horseradish peroxidase-labeled secondary antibodies, washed again with TBST buffer, exposed, developed and fixed to record the hybrid signals. With GAPDH as the internal reference, relative concentration was expressed as Atarget protein/AGAPDH (A means absorbance).
Statistical analysis
The categorical data were expressed as x ± s. All data were analyzed by SPSS 17.0 (IBM Corp., Armonk, NY). Inter-group comparisons were performed by one-way ANOVA. The equality of variances was assessed by Levene's test. Fisher's least significant difference test was used for variance homogeneity, and Dunnett's test was used for variance heterogeneity to compare inter-group semi-quantitative measurements of proteins. Means between groups were compared with t test. P < 0.05 was considered statistically significant.
Results
Changes of visceral sensitivity
The AWR scores of PI-2W and PI-8W groups distended at 40 and 60 mmHg were significantly higher than those of NC-2W and NC-8W groups (P < 0.05), but the differences were insignificant at 20 and 80 mmHg (P > 0.05). PI-B group had significantly lower AWR scores than those of PI-8W group at 40 and 60 mmHg (P < 0.05), whereas the two groups had similar scores at 20 and 80 mmHg (P > 0.05) ().
Table 1. AWR scores at different pressures (point, x ± s).
Location of NLRP3 inflammasome and its ligands
NLRP3 inflammasome and its ligands ASC and caspase-1 were expressed in the mucous layer of ileal tissues and most abundantly in the cytoplasm of the intestinal epithelium, with the same locations ().
CD172a expressions
The degrees of intestinal inflammation were evaluated by measuring CD172a expression levels. PI-2W group has significantly higher CD172a level than that of NC-2W group (P < 0.05, ), but PI-8W and NC-8W groups or PI-B and PI-8W groups had similar levels (P > 0.05, ).
Figure 2. Expressions of NLRP3 inflammasome and related proteins. (A) Comparisons between PI-2W and NC-2W groups; (B) comparisons between PI-8W and NC-8W groups; (C) comparisons between PI-2W and PI-8W groups; (D) comparisons between PI-8W and PI-B groups. *P < 0.05. n = 10, each experiment was performed in triplicate, and the mean was used.
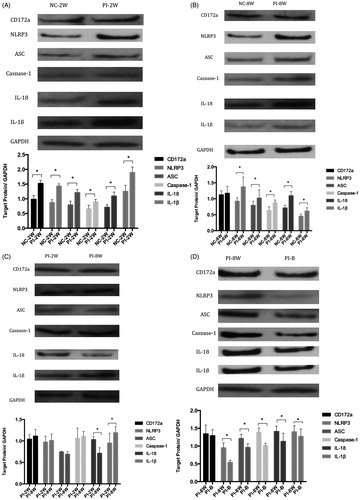
Expressions of NLRP3 inflammasome and ligand proteins
Significantly more NLRP3 was expressed in PI-2W and PI-8W groups than that in NC-2W and NC-8W groups (P < 0.05, ), but PI-2W and PI-8W groups had similar levels (P > 0.05, ), and the level of PI-B group was significantly lower than that of PI-8W group (P < 0.05, ).
Significantly more ASC and caspase-1 were expressed in PI-2W and PI-8W groups than those in NC-2W and NC-8W groups (P < 0.05, ), whereas PI-8W and PI-2W groups had similar levels (P > 0.05, ), and the levels of PI-B group were significantly lower than those of PI-8W group (P < 0.05, ).
Expressions of downstream cytokine proteins
Significantly more IL-18 and IL-1β were expressed in PI-2W and PI-8W groups than those in NC-2W and NC-8W groups (P < 0.05, ), but the levels in PI-8W group were significantly lower and higher than those of PI-2W group (P < 0.05, ). Moreover, the levels of PI-B group were significantly lower than those of PI-8W group (P < 0.05, ).
Discussion
In this study, the PI-IBS mouse model established by infection of T. spiralis for 8 weeks had visceral hypersensitivity that was alleviated by administration of B. longum. In model mice, the expression levels of NLRP3 inflammasome and downstream cytokines IL-18, IL-1β were significantly elevated, which were reduced by B. longum intervention.
Being one of the characteristics of IBS, visceral hypersensitivity has been ascribed to local intestinal immune disorder and increased intestinal permeability. PI-IBS is accompanied by intestinal mucosal immune activation that leads to long-term mild inflammation, in which immune cells such as mononuclear macrophages, mast cells and dendritic cells play crucial roles (Ohman and Simren Citation2013). In addition, IBS patients and model animals are all vulnerable to intestinal flora disturbance. By analyzing the fecal flora of IBS patients and normal subjects, Malinen et al. (Citation2005) found that distinct differences between microbial types. Meanwhile, diarrheal and constipated IBS patients also had different flora. Krogius-Kurikka et al. (Citation2009) reported that Proteobacteria and Firmicutes increased in the intestinal tract of constipated IBS patients but Actinomycetes and Bacteroides decreased. By infecting the intestinal tract with Campylobacter jejuni, Pimentel et al. (Citation2008) found clinical symptoms of IBS such as changes in the number and characteristics of bowel movements as well as achroacytosis, suggesting that transplantation of microorganisms into the intestinal tract induced PI-IBS-like symptoms.
NLRP3, as one of the members of the NLR family, forms inflammasome after binding ASC and caspase-1, which predominantly controls immune response and disease onset as an essential component of innate immunity. By activating caspase-1, NLRP3 cleaves inflammatory cytokines such as IL-1β and IL-18 and induces immune response by maturing them and releasing them outside cells (Wang et al. Citation2004). IBS patients commonly have NLRP3 expression disorder, rising IL-1β and caspase-1 expressions and intestinal flora disturbance that further aggravate intestinal inflammation, indicating that NLRP3 dominantly participates in regulation of intestinal flora and maintenance of intestinal homeostasis (Pimentel et al. Citation2000; Posserud et al. Citation2007). In this study, the expressions of NLRP3, ASC and caspase-1 in PI-IBS model mice significantly exceeded that of control groups, but the levels were similar in chronic and acute inflammation phases. Hence, NLRP3 inflammasome participated in the intestinal immune activation of PI-IBS. Besides, the expression level of IL-18, a downstream cytokine of the NLRP3 inflammasome signaling pathway, was up-regulated 8 and 2 weeks after infection compared with those of control groups, and the level was lower in the postinfectious eighth week. Therefore, IL-18 negatively regulated this pathway. IL-1β may also be involved in the onset of PI-IBS based on similar outcomes.
Probiotics work for a part of IBS patients by effectively reliving related symptoms and by improving the quality of life (Horvath et al. Citation2011; O'Mahony et al. Citation2005). Although the mechanism is unknown, it is now believed that probiotics can regulate intestinal flora and motility, recover the balance between inflammatory cytokines, enhance the mucosal barrier, and mitigate visceral hypersensitivity (Korpela and Niittynen Citation2012). After being intragastrically administered with B. longum herein, the visceral sensitivities of PI-IBS model mice were significantly alleviated compared with those without such treatment. In the meantime, the expressions of NLRP3 inflammasome, its ligands ASC and caspase-1, as well as downstream cytokines IL-18 and IL-1β were significantly down-regulated by B. longum administration, by which B. longum exerted regulatory effects on the visceral hypersensitivity of PI-IBS.
In summary, NLRP3 inflammasome was involved in the onset of PI-IBS, the pathway of which was affected by B. longum to regulate visceral hypersensitivity.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Dupaul-Chicoine J, Yeretssian G, Doiron K, Bergstrom KS, McIntire CR, LeBlanc PM, et al. 2010. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity. 32, 367–378.
- Ford AC, Spiegel BM, Talley NJ, Moayyedi P. 2009. Small intestinal bacterial overgrowth in irritable bowel syndrome: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 7:1279–1286.
- Horvath A, Dziechciarz P, Szajewska H. 2011. Meta-analysis: Lactobacillus rhamnosus GG for abdominal pain-related functional gastrointestinal disorders in childhood. Aliment Pharmacol Ther. 33:1302–1310.
- Kassinen A, Krogius-Kurikka L, Makivuokko H, Rinttila T, Paulin L, Corander J, et al. 2007. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 133:24–33.
- Keating C, Beyak M, Foley S, Singh G, Marsden C, Spiller R, Grundy D. 2008. Afferent hypersensitivity in a mouse model of post-inflammatory gut dysfunction: role of altered serotonin metabolism. J Physiol. 586:4517–4530.
- Korpela R, Niittynen L. 2012. Probiotics and irritable bowel syndrome. Microb Ecol Health Dis. 23:19–25.
- Krogius-Kurikka L, Lyra A, Malinen E, Aarnikunnas J, Tuimala J, Paulin L, et al. 2009. Microbial community analysis reveals high level phylogenetic alterations in the overall gastrointestinal microbiota of diarrhoea-predominant irritable bowel syndrome sufferers. BMC Gastroenterol. 9:95.
- Lee YJ, Park KS. 2014. Irritable bowel syndrome: emerging paradigm in pathophysiology. World J Gastroenterol. 20:2456–2469.
- Malinen E, Rinttila T, Kajander K, Matto J, Kassinen A, Krogius L, et al. 2005. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. Am J Gastroenterol. 100:373–382.
- Marshall JK, Thabane M, Borgaonkar MR, James C. 2007. Postinfectious irritable bowel syndrome after a food-borne outbreak of acute gastroenteritis attributed to a viral pathogen. Clin Gastroenterol Hepatol. 5:457–460.
- Ohman L, Simren M. 2013. Intestinal microbiota and its role in irritable bowel syndrome (IBS). Curr Gastroenterol Rep. 15:323.
- O'Mahony L, McCarthy J, Kelly P, Hurley G, Luo F, Chen K, et al. 2005. Lactobacillus and Bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 128:541–551.
- Pimentel M, Chatterjee S, Chang C, Low K, Song Y, Liu C, et al. 2008. A new rat model links two contemporary theories in irritable bowel syndrome. Digest Dis Sci. 53:982–989.
- Pimentel M, Chow EJ, Lin HC. 2000. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol. 95:3503–3506.
- Posserud I, Stotzer PO, Bjornsson ES, Abrahamsson H, Simren M. 2007. Small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Gut. 56:802–808.
- Rodes L, Coussa-Charley M, Marinescu D, Paul A, Fakhoury M, Abbasi S, et al. (2013). Design of a novel gut bacterial adhesion model for probiotic applications. Artif Cells Nanomed Biotechnol. 41:116–124.
- Wang LH, Fang XC, Pan GZ. 2004. Bacillary dysentery as a causative factor of irritable bowel syndrome and its pathogenesis. Gut. 53:1096–1101.
- Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, Kanneganti TD. 2010. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 32:379–391.