Abstract
The study highlights the synthesis of gold nanoparticles and silver nanoparticles by fresh leaves of Panax ginseng, an herbal medicinal plant. The reduction of auric chloride and silver nitrate led to the formation of gold and silver nanoparticles within 3 and 45 min, at 80°C, respectively. The developed methodology was rapid, facile, ecofriendly and the utmost significant is quite economical, which did not require subsequent processing for reduction or stabilization of nanoparticles. The nanoparticles were further characterized by Ultraviolet-visible spectroscopy (UV-vis) which showed the relevant peak for gold and silver nanoparticles at 578 and 420 nm, correspondingly. Field-emission transmission electron microscopy (FE-TEM) displayed the spherical shape of monodispersed nanoparticles. FE-TEM revealed that the gold nanoparticles were 10-20 nm and silver nanoparticles were 5–15 nm. The energy dispersive X-ray (EDX) and elemental mapping results indicated the maximum distribution of gold and silver elements in the respective nanoproducts, which further corresponds the purity. Further, the X-ray diffraction (XRD) results confirm the crystalline nature of synthesized nanoparticles. The biosynthesized AgNPs served as an efficient antimicrobial agent at 3 μg concentration against many pathogenic strains for instance, Escherichia coli, Salmonella enterica, Vibrio parahaemolyticus, Staphylococcus aureus, Bacillus anthracis and Bacillus cereus. In addition, AgNPs showed complete inhibition of biofilm formation by S. aureus and Pseudomonas aeruginosa at 4 μg/ml concentration. Moreover, the AuNPs and AgNPs found as a potent anticoagulant agent. Thus, the study claims the rapid synthesis of gold and silver nanoparticles by fresh P. ginseng leaf extract and its biological applications.
Introduction
Innovative methods for the synthesis of metal nanoparticles and their application in biological platforms have gained interest due to the fact that nanoparticles exhibit unique and tunable physiochemical properties (Raveendran et al. Citation2003). Gold and silver nanoparticles have emerged as a promising candidate for numerous applications resembles to their facile synthesis, optical, electrical, surface properties, excellent biocompatibility and simplicity in bioconjugation (Kemp et al. Citation2009). In addition, applications in bio-labeling, biosensors, biomedical science, imaging, and pathogens identification make them a valuable choice for many therapeutic and pharmacological applications (Huang Citation2006, Shah et al. Citation2014, Wang et al. Citation2003). Furthermore, these nanoparticles can be applied in cancer diagnostics and therapy, biological probes, brain implants, drug delivery, gene delivery, sterilization system, vaccine preparation, artificial skin and improving electrical signaling in the heart (Shah et al. Citation2014, Wang et al. Citation2003). Gold and silver nanoparticles have photothermal properties that can be explored in drug release by localized heating (Kumar et al. Citation2008, Wang et al. Citation2003). Thus, the various applications in biological, electrical and chemical field made them an interesting option for the recent research.
Over the past few decades, there has been considerable interest in developing nanoparticles by various physiochemical and biological method. There are several chemical and physical techniques for the synthesis of gold and silver nanoparticles has been reported, but the methodologies have several disadvantages including high energy consumption, utilization of toxic and highly reactive chemicals, which pose potential environmental and biological risks (Dahl et al. Citation2007). Biological synthesis of metal nanoparticles using natural products is an alternative. Nature provides a rich source of various biological diversity including plants and microorganisms, which shows the ability to synthesize nanoparticles. Among several biological species; various plant extracts, bacteria, algae and fungi have been studied for this action (Elavazhagan and Arunachalam Citation2011, Govindaraju et al. Citation2009, Pereira et al. Citation2014, Singh et al. Citation2014, 2015a, 2015b, Wang et al. Citation2015). This green route of synthesis minimizes the side effects of chemical and physical methods by avoiding the utilization of toxic chemicals and creating hazardous by products. Additionally, the green synthesis provides stabilization to the synthesized nanoparticles without any additional capping agent (Raveendran et al. Citation2003). In continuation of the efforts for synthesizing nanoparticles by a green route, a facile, green and rapid methodology for the biosynthesis of gold and silver nanoparticles using Panax ginseng leaf extract has been reported here.
Panax ginseng is belongs to the Araliaceae family; it is a slow growing, perennial herb, mostly found in Korea, northeastern China, and far-eastern Siberia (Hu Citation1977, Wen and Zimmer Citation1996). For a very long time, the plant has been cultivated and used as a tonic, restorative and anti-aging agent in traditional medicine in Korea, China and Japan (Radad et al. Citation2006). The principal active components of the plant are ginsenosides, which are responsible for most of the pharmacological activity. More than 40 ginsenosides have been reported for biological and pharmacological actions. These ginsenosides have numerous pharmacological effects on humans including anti-cancer, anti-diabetic, antioxidant, anti-inflammatory, immunomodulatory, radioprotective, anti-amnestic, anti-apoptotic and anti-stress properties (Kim et al. Citation2014). Moreover, research proposed that some of the active components of P. ginseng also exert beneficial effects on aging, central nervous system disorders and neurodegenerative diseases (Radad et al. Citation2006). The present study focus on the leaves of plant as the ginseng leaves contain numerous active ingredients, such as flavonoids, triterpenoids, polyacetylenic, alcohols, amino acids, peptides, polysaccharides, volatile oils and fatty acids. These active ingredients produce many important pharmacological effects on the central nervous system, as well as on the cardiovascular, reproductive and metabolic systems. Ginseng leaf extract also has anti-fatigue, anti-hyperglycemic, anti-obesity, anti-cancer, antioxidant and anti-aging properties (Wang et al. Citation2006). Thus, various pharmacological applications of ginsenosides have been explored and in the present study, the leaves of the plant were highlighted for the gold and silver nanoparticles synthesis. Consequently, several studies has been done using root of different form of P. ginseng; however, there is no report for the utilization of fresh leaf of P. ginseng for nanoparticle synthesis. Therefore, here we report the rapid synthesis of gold and silver nanoparticles using fresh leaves of P. ginseng in a quite rapid and facile manner.
Antimicrobial activity, biofilm inhibition, anticoagulant and anti-inflammatory effects of metal nanoparticles have been well studied, which make them an ideal candidate for many medical and biological applications (Kalishwaralal et al. Citation2010). In the present study, the synthesized nanoparticles were utilized for the antimicrobial potential against many pathogenic strains including Escherichia coli, Salmonella enterica, Vibrio parahaemolyticus, Staphylococcus aureus, Bacillus anthracis and Bacillus cereus. In addition, the biofilm inhibition and anticoagulant effect of nanoparticles were studied.
Materials and methods
Materials
Gold(III) chloride trihydrate (HAuCl4·3H2O) and silver nitrate (AgNO3) was purchased from Sigma-Aldrich Chemicals, St Louis, MO. Fresh P. ginseng leaves were collected from the four year old P. ginseng plants from Gochang, Republic of Korea. Of fresh leaves, 15 g were washed thoroughly and shade-dried for 6–8 h. Further, the leaves were cut into small pieces, grounded and boiled for 20 min in 100 ml of sterile water. After boiling, the collected extract was filtered and centrifuged at 10,000 rpm for 10 min. The total volume of filtrate was maintained at 100 ml with sterile water at the end of the process and stored at 4°C for further use. This 100 ml of stock filtrate was used further for the nanoparticles synthesis. The standard antibiotics discs used were vancomycin (VA30) 30 μg/disc, rifampicin (RD5) 5 μg/disc, oleandomycin (OL15) 15 μg/disc, penicillin G (P10) 10 μg/disc, novobiocin (NV30) 30 μg/disc and lincomycin (MY15) 15 μg/disc, purchased from Oxoid Ltd., Basingstoke, Hampshire. The pathogenic strains Bacillus anthracis (NCTC 10340), Vibrio parahaemolyticus (ATCC 33844), Salmonella enterica (ATCC 13076), Staphylococcus aureus (ATCC 6538), Escherichia coli (ATCC 10798), Bacillus cereus (ATCC 14579) and Pseudomonas aeruginosa (ATCC 27853) were used. The bacterial strains were cultured on nutrient agar media at 37°C and preserved at −70°C in glycerol stock vials for further study. Human blood was received from healthy male and female volunteers in Vacutainer tubes (Greiner Bio-One, Kremsmünster, Austria).
Synthesis of nanoparticles
For the synthesis of gold nanoparticles and silver nanoparticles, 5 ml from 100 ml of ginseng leaf stock filtrate was mixed with 25 ml of sterile water. The HAuCl4·3H2O and AgNO3 solution was added with a final concentration of 1 mM in the corresponding reaction mixtures, separately. The reaction mixture was then kept at 80°C for reduction of Au3+ to Au° and Ag+ ions to Ag atoms in the corresponding reaction mixtures. A color change was observed continuously, which indicated the formation of nanoparticles in the relevant reaction mixtures. After the synthesis, the nanoparticles were collected by centrifugation at 16,000 rpm for 15 min, washed thoroughly with sterile water, kept overnight for air dry and used further for characterization.
Characterization of nanoparticles
To verify reduction of gold and silver ions, each solution was scanned in the range of 300–800 nm in a UV–VIS spectrophotometer (Ultrospec 2100 Pro, Amersham Biosciences, Biochrom Ltd, Cambridge, United Kingdom). The size, shape, morphology and distribution of the nanoparticles were analyzed using field-emission transmission electron microscopy (FE-TEM) with a JEM-2100F (JEOL) instrument operated at 200 kV. The fringe spacing and selected area diffraction (SEAD) pattern were studied using FE-TEM. The size distribution profile of nanoparticles was studied by dynamic light scattering (DLS), Photal Otsuka Electronics, Hirakata, Japan. The X-ray diffraction (XRD) analyses were performed on X-ray diffractometer, D8 Advance, Bruker, Germany, operated at 40 kV, 40 mA, with CuKα radiation, at a scanning rate of 6°/min, step size 0.02, over the 2θ range of 20–80°.
The stability of nanoparticles was observed before and after the addition of sodium hydroxide to the nanoparticles. The effect of the change in pH on the stability of the nanoparticles in the range of 3–12 pH was studied. The nanoparticles were also scanned at different time interval range from one day to one month.
Antimicrobial activity
The antimicrobial activity of nanoparticles were studied against pathogenic microorganisms such as E. coli, S. enterica, V. parahaemolyticus, S. aureus, B. anthracis and B. cereus on Mueller-Hinton agar (MHA) plates using the disc diffusion method. In this assay, the MHA plates were spread evenly with 100 μL of overnight log culture of test organisms and each standard antibiotic disc of vancomycin, rifampicin, oleandomycin, penicillin G, novobiocin and lincomycin was kept on it, maintained as control. Next, the sterile paper disc was further impregnated into 30 μL (100 mg/L) of freshly prepared and partially purified silver and gold nanoparticles solution, and then, placed onto the MHA plates. The plates were incubated at 37°C for 24 h. After the incubation period, the zones of inhibition around each disc were compared (Singh et al. Citation2015c). The study was done in duplicates and the results were interpreted in terms of its mean ± standard deviation.
Determination of biofilm inhibition activity
The biofilm inhibition activity of AgNPs was determined by colorimetric method against S. aureus and P. aeruginosa. Briefly, the wells of 96-well micro-titer plates were filled with 100 μL of overnight grown log phase of S. aureus and P. aeruginosa. After culturing for 24 h, different concentrations of AgNPs ranging from (1–4 μg) were added. The cell culture plates were then incubated for 4 h at 37°C. After incubation, the media were removed and the wells were washed three times with 200 μL sterile water. Then, the microtiter plate was kept for air dry, for 45 min. Then, 200 μL of a 0.1% (v/v) crystal violet solution in water were added in each well and kept for 45 min. The wells were then washed three times with 300 μL sterile water to remove excess stain. The dye incorporated by the adherent cells was solubilized with 200 μL of 95% (v/v) ethanol. The absorbance of each well was measured at 595 nm using a microtiter ELISA reader. The experiments were performed in duplicates and results were interpreted in terms of mean ± SD (Singh et al. Citation2015a).
Anti-coagulant assay
Human blood from healthy male and female volunteers (age ranges from 25–45 years old) was collected in three Vacutainer tubes, labeled, A, B and C. Tube A and B received the blood with gold nanoparticles and silver nanoparticles, respectively, synthesized by using 5 ml of ginseng leaf extract, at 1:0.005 ml (v/v), respectively, and Tube C received blood without any anti-coagulant. After some time, the anticoagulant effect of the nanoparticles was examined visually (Kalishwaralal et al. Citation2010).
Results and discussion
Synthesis of nanoparticles
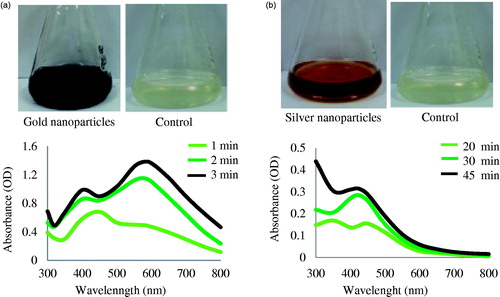
The synthesis of gold and silver nanoparticles was monitored by change in color of the reaction mixture with time. The reduction of Au3+ to Au° was completed within 3 min of reaction at 80°C, and the reaction mixture completely turned to a dark purple which resembles the collective oscillations of electrons in a nanoparticle upon interaction with light, and specific for gold nanoparticles (Geethalakshmi and Sarada Citation2012). Thus, the appearance of a dark purple color in the reaction mixture indicated the formation of gold nanoparticles (). For silver nanoparticles, the reaction mixture completely turned to brown after 45 min of incubation at 80°C (), whereas no change in control flask containing only leaf extract and sterile water in same proportion was observed. The brown color of reaction mixture corresponds to surface plasmonace of silver nanoparticles formed in the reaction mixture. The presence of phenolic acids, flavonoids, ginsenosides, and polysaccharides in P. ginseng leaves must have influenced the formation of nanoparticles. Previously, many reports showed the nanoparticles synthesis potential of P. ginseng plant. For instance, gold nanoparticles were synthesized using red ginseng root powder in which the gold nanoparticles were prepared by using 1 g of Korean red ginseng root powder and 1 ml of 0.1 M HAuCl4 solution under ultrasonic condition for 2 h. The study was suggested that various phytochemicals within the plant effectively reduce gold salts to produce gold nanoparticles, and the saponin glycosides provide additional stability by providing a coating on the gold nanoparticles (Leonard et al. Citation2011). Additionally, biosynthesis of gold and silver nanoparticles using red ginseng root has been studied by Singh et al. Citation2015a. Panax ginseng (Chinese ginseng) dried root powder has been reported for the silver nanoparticles synthesis in an ecofriendly manner (Vimalanathan et al. Citation2013). Moreover, the P. ginseng fresh root extract were reported for gold and silver nanoparticles synthesis in 5 min and 2 h (Singh et al. Citation2015b). However, this is the first report for the facile synthesis of gold and silver nanoparticles by utilization of fresh leaves of four year old P. ginseng plant. Comparatively, by using fresh leaf extract, the gold and silver nanoparticles can be synthesized within 3 and 45 min, respectively, which is quite rapid and facile. Moreover, the synthesized nanoparticles were quite stable and monodisperse in nature.
Characterization of gold and silver nanoparticles
Visual and UV–VIS analysis
The reduction of gold and silver ions was followed by UV–VIS spectroscopy which showed a highest peak at 578 nm and 420 nm for gold and silver reaction mixtures, which is a characteristic peak for gold nanoparticles and silver nanoparticles (), corresponds to surface resonance plasmon of respective nanoparticles (Ahmad et al. Citation2013, Geethalakshmi and Sarada Citation2012). The peak originated at 400 nm in the UV spectrum of gold nanoparticles, due to some organic moieties present in the reaction mixture. The absorption spectrum graph was found to be similar to previous reports, which demonstrated the biological synthesis of gold nanoparticles by leaf extract of Cassia auriculata and fungus, Fusarium semitectum (Kumar et al. Citation2011). Panax ginseng leaves are known to have phenolic acids and flavonoids, which exhibit very potent antioxidant activities (Wang et al. Citation2006). In addition, the leaves also contain many ginsenosides including Rb1, Rb2, Rc, Rd, Re, Rg1, F1, F2 and F4 (Ligor et al. Citation2005). These components of P. ginseng leaf may play an important role in rapid synthesis and stabilization of nanoparticles (Leonard et al. Citation2011). In preceding studies for the biological synthesis of gold and silver nanoparticles by bacteria (Reddy et al. Citation2010) and fungi (Kumar et al. Citation2008), etc. the time required for synthesis ranged from one to several days. This is one of the limitations of the biosynthetic procedure that must be addressed if the biological methodology is compared with physical and chemical methods. The decrease in reaction time from some days to 3 min for gold nanoparticles and 45 min for silver nanoparticles was observed when using the fresh leaf extract of P. ginseng. This represents a major significant step toward achieving the goal of ecofriendly, cheap and rapid synthesis of gold and silver nanoparticles.
FE-TEM, SEAD and elemental mapping analysis
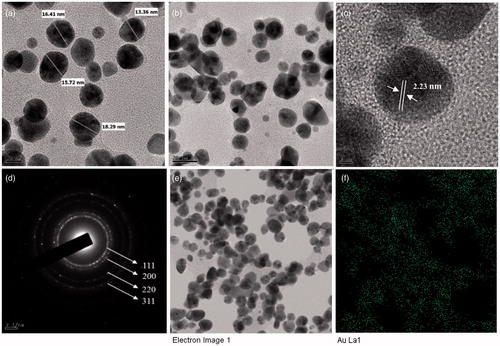
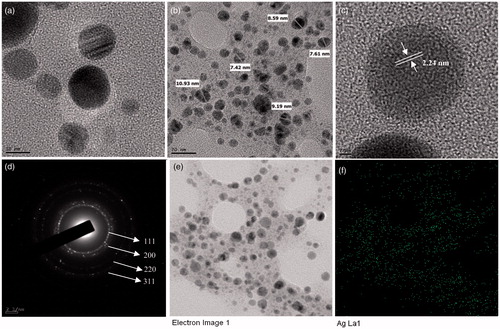
The results obtained from the FE-TEM study gives a clear indication regarding the shape and size of the nanoparticles. Previously, the gold nanoparticles synthesized by red ginseng root powder were reported of spherical shape and 2–40 nm (Leonard et al. Citation2011). The gold nanoparticles synthesized in the present study were 10–20 nm and spherical (). showed the fringe spacing of 2.23 nm of spherical gold nanoparticles. shows the selected electron diffraction (SEAD) pattern of purified spherical gold nanoparticles, with rings corresponding to the [111], [200], [220] and [311] reflections. Further, biosynthesized gold nanoparticles were also characterized by elemental mapping. The elemental mapping results revealed that, in the electron micrograph region of synthesized product, the distribution of gold element were the predominant (). The silver nanoparticles synthesized in the present study were spherical and range from 5 to 15 nm in size (). Previously, the silver nanoparticles formed by dried Chinese ginseng-P. ginseng root extract were in the range of 100 nm (Vimalanathan et al. Citation2013). Comparatively, using leaves resources, the size of obtained silver nanoparticles was quite small. The fringe spacing of spherical silver nanoparticle was of 2.24 nm (). shows the SEAD image of silver nanoparticle, with rings corresponding to the (111), (200), (220) and (311) reflections. The elemental mapping results also indicate maximum distribution of silver element in the purified nanoproducts (). The obtained results were similar to the diffraction pattern of gold and silver nanoparticles synthesized by using heparin and hyaluronan, as both reducing and stabilizing agents (Kemp et al. Citation2009).
EDX and XRD analysis
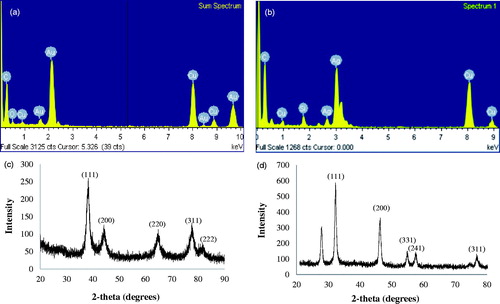
In EDX analysis, strong signals were observed from the gold and silver atoms in the nanoparticles at 2.15 and 3 keV, which is a characteristic peak of metallic gold and silver nanocrystallites (). The results were in line with previous reports of EDX spectra of gold and silver nanoparticles (Geethalakshmi and Sarada Citation2012). Other signals originated from carbon and copper, which is because of the TEM grid. Weak signals for oxygen and silicon were also found, which perhaps originate from biomolecules and other components of P. ginseng. The XRD analyses were performed to confirm the monocrystalline nature of the gold and silver nanoparticles. showed the XRD pattern of gold nanoparticles. The gold nanoparticles exhibited intense peaks in the whole spectrum of 2θ value ranging from 20 to 80 corresponding to (111), (200), (220), (311) and (222). This pattern was similar to the Bragg’s reflection of gold nanocrystals indicating that the purified product is composed of pure crystalline gold. Earlier study showed the similar XRD pattern of gold nanoparticles which were biologically synthesized by endophytic Aspergillus clavatus (Verma et al. Citation2011). The XRD spectrum of silver nanoparticles confirmed that the silver nanoparticles formed were in the form of nanocrystals, as evidenced by the peaks at 2θ values corresponding to (111), (200), (331), (241) and (311) Bragg reflections of silver, respectively () (Vivek et al. Citation2011).
DLS and pH stability analysis
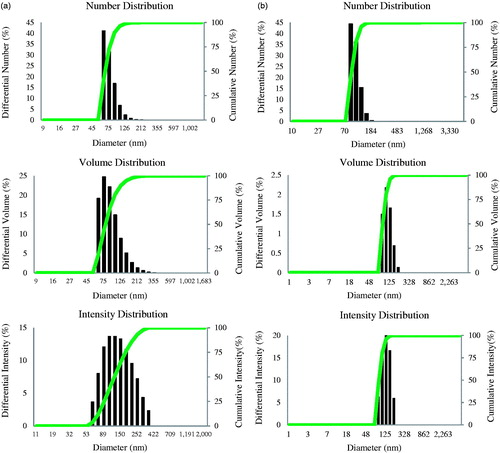
The DLS analysis results showed the number, volume and intensity distribution profile of nanoparticles. The size of gold nanoparticles was range from 50 to 150 nm with 0.191 polydispersity index (PDI) (). The particles size for silver nanoparticles was in the range of 70–140 with PDI 0.13 . The results interpreted that the average particles size (hydrodynamic diameter) was 97 and 80 nm for gold and silver nanoparticles, individually. The pH of gold and silver nanoparticles synthesized using P. ginseng was 5.6 and 6 for the respective reaction mixtures, and these nanoparticles were stable with no major shift when 0.1 M sodium hydroxide was added. On studying the effect of pH, a range from 3 to 12 resulted in no major change in the absorbance, which confirmed the stability of the nanoparticles. In addition, even after three weeks to one month of synthesis, there was no observable variation in the UV–VIS spectrum of the respective reaction mixture, which directed the stabile nature. Thus, the leaf extract, itself act as a reducing and stabilizing agent. The stability of the gold and silver nanoparticles over a wide range of pH implies the possibility of using P. ginseng mediated gold and silver nanoparticles in drug delivery applications.
Antimicrobial and biofilm inhibition effect of silver nanoparticles
The antimicrobial activity results showed that the E. coli, S. enterica and V. parahaemolyticus were completely resistant to standard antibiotics and gold nanoparticles; however, exhibited sensitivity to silver nanoparticles at 30 μL (100 mg/L) concentrations. The strains S. aureus, B. anthracis and B. cereus were sensitive to the standard antibiotics as well as silver nanoparticles also. The gold nanoparticles not showed any antimicrobial effect. Thus, the antimicrobial activity results clearly showed that all the tested strains were sensitive to silver nanoparticles at 30 μL (100 mg/L) concentration. The results were interpreted in .
Table 1. Antimicrobial activity of silver and gold nanoparticles against E. coli, S. enterica, V. Parahaemolyticus, S. aureus. B. anthracis and B. cereus.
Microorganisms in biofilm-associated infections on indwelling medical devices, such as catheters are the major concern. Even after antibiotic therapy, it may persist, sometime results in the removal of the device. In order to reduce the costs and patient's sufferings, several efforts have been made. Reports suggested that, silver nanoparticles showed remarkable action in this respect. The results in the present study for biofilm inhibition activity showed that the complete biofilm inhibition was observed for S. aureus and P. aeruginosa, at 4 μg/ml concentration of silver nanoparticles () (Gurunathan et al. Citation2014).
Anticoagulant activity of nanoparticle
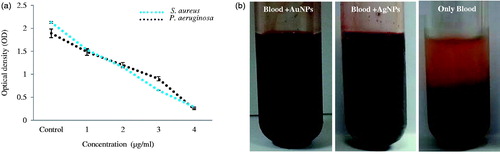
Metal nanoparticles have been known to have anti-platelet properties, specifically gold nanoparticles are well known for showing an anti-coagulant effect. In the present study, the human blood coagulation was tested in the presence of gold and silver nanoparticles. The results demonstrate that the formation of blood clots was inhibited effectively in the presence of gold and silver nanoparticles. In comparison, control showed completely coagulation of blood. In addition, after 24 h, no significant change was observed, which confirmed the stability of nanoparticles activity (). Similarly, the previous studies demonstrate the anticoagulant effect of nanoparticles (Kalishwaralal et al. Citation2010). Thus, biosynthesized nanoparticles by leaf extract of P. ginseng can be applicable in drug and gene delivery or as biosensors, where direct contact with blood occurs. The obtained nanoparticles can be further modified and used as nanocarriers in vivo with reduced risk of coagulation.
Conclusion
A green and rapid method of gold and silver nanoparticles biosynthesis was developed by using P. ginseng fresh leaf extract. This facile methodology overcomes the limitation of physiochemical methods including safety, cost, chemicals toxicity, hazardous byproducts and energy consumption. The synthesized nanoparticles were monodisperse and stable. The results obtained from physicochemical, biostability, antimicrobial, biofilm inhibition activity and blood compatibility of nanoparticles supports its application in medical field. In the future, selection of such medicinal plants with high therapeutic importance will create a new platform for the nanoparticles synthesis.
Declaration of interest
This research was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (KIPET NO. 313038-03-2-SB020).
References
- Ahmad T, Wani IA, Manzoor, N, Ahmed J, Asiri AM. 2013. Biosynthesis, structural characterization and antimicrobial activity of gold and silver nanoparticles. Colloids Surf B Biointerfaces. 107:227–234.
- Dahl JA, Maddux BL, Hutchison JE. 2007. Toward greener nanosynthesis. Chem Rev. 107:2228–2269.
- Elavazhagan T, Arunachalam KD. 2011. Memecylon edule leaf extract mediated green synthesis of silver and gold nanoparticles. Int J Nanomedicine. 6:1265–1278.
- Geethalakshmi R, Sarada DV. 2012. Gold and silver nanoparticles from Trianthema decandra: synthesis, characterization, and antimicrobial properties. Int J Nanomedicine. 7:5375–5384.
- Govindaraju K, Kiruthiga V, Kumar VG, Singaravelu G. 2009. Extracellular synthesis of silver nanoparticles by a marine alga, Sargassum wightii Grevilli and their antibacterial effects. J Nanosci Nanotechnol. 9:5497–5501.
- Gurunathan, Han SJW, Kwon DN, Kim JH. 2014. Enhanced antibacterial and anti-biofilm activities of silver nanoparticles against Gram-negative and Gram-positive bacteria. Nanoscale Res Lett. 9:373.
- Hu SY. 1977. A contribution to our knowledge of ginseng. Am J Chin Med. 5:1–23.
- Huang SH. 2006. Gold nanoparticle-based immunochromatographic test for identification of Staphylococcus aureus from clinical specimens. Clin Chim Acta. 373:139–143.
- Kalishwaralal K, Deepak V, Pandian SRK, Muniasamy Kottaisamy M, BarathManiKanth S, Kartikeyan B, et al. 2010. Biosynthesis of silver and gold nanoparticles using Brevibacterium casei. Colloids Surf B Biointerfaces. 77:257–262.
- Kemp MM, Kumar A, Mousa S, Park TJ, Ajayan P, Kubotera N, et al. 2009. Synthesis of gold and silver nanoparticles stabilized with glycosaminoglycans having distinctive biological activities. Biomacromolecules. 10:589–595.
- Kim YJ, Jeon JN, Jang MG, Oh JY, Kwon WS, Jung SK, et al. 2014. Ginsenoside profiles and related gene expression during foliation in Panax ginseng Meyer. J Ginseng Res. 38:66–72.
- Kumar SA, Peter YA, Nadeau JL. 2008. Facile biosynthesis, separation and conjugation of gold nanoparticles to doxorubicin. Nanotechnology. 19:495101.
- Kumar VG, Gokavarapu SD, Rajeswari A, Dhas TS, Karthick V, Kapadia Z, et al. 2011. Facile green synthesis of gold nanoparticles using leaf extract of antidiabetic potent Cassia auriculata. Colloids Surf B Biointerfaces. 87:159–163.
- Leonard K, Ahmmad B, Okamura H, Kurawaki J. 2011. In situ green synthesis of biocompatible ginseng capped gold nanoparticles with remarkable stability. Colloids Surf B Biointerfaces. 82:391–396.
- Ligor T, Ludwiczuk A, Wolski T, Buszewski B. 2005. Isolation and determination of ginsenosides in American ginseng leaves and root extracts by LC-MS. Anal Bioanal Chem. 383:1098–1105.
- Pereira L, Dias N, Carvalho J, Fernandes S, Santos C, Lima N. 2014. Synthesis, characterization and antifungal activity of chemically and fungal-produced silver nanoparticles against Trichophyton rubrum. J Appl Microbiol. 117:1601–1613.
- Radad K, Gille G, Liu L, Rausch WD. 2006. Use of ginseng in medicine with emphasis on neurodegenerative disorders. J Pharmacol Sci. 100:175–186.
- Raveendran P, Fu J, Wallen SL. 2003. Completely “green” synthesis and stabilization of metal nanoparticles. J Am Chem Soc. 125:13940–13941.
- Reddy AS, Chen CY, Chen CC, Jean JS, Chen HR, Tseng MJ, et al. 2010. Biological synthesis of gold and silver nanoparticles mediated by the bacteria Bacillus subtilis. J Nanosci Nanotechnol. 10:6567–6574.
- Shah M, Badwaik VD, Dakshinamurthy R. 2014. Biological applications of gold nanoparticles. J Nanosci Nanotechnol. 14:344–362.
- Singh P, Kim YJ, Wang C, Mathiyalagan R, El-Agamy Farh M, et al. 2015a. Biogenic silver and gold nanoparticles synthesized using red ginseng root extract, and their applications. Artif Cells Nanomed Biotechnol. [Epub ahead of print]. DOI:10.3109/21691401.2015.1008514.
- Singh P, Kim YJ, Wang C, Mathiyalagan R, Yang DC. 2015b. The development of a green approach for the biosynthesis of silver and gold nanoparticles by using Panax ginseng root extract, and their biological applications. Artif Cells Nanomed Biotechnol. [Epub ahead of print]. DOI:10.3109/21691401.2015.1011809.
- Singh P, Kim YJ, Singh H, Wang C, Hwang KH, Farh Mel A, Yang D C. 2015c. Biosynthesis, characterization, and antimicrobial applications of silver nanoparticles. Int J Nanomedicine. 10:2567–2577.
- Singh S, Vidyarthi AS, Nigam VK, Dev A. 2014. Extracellular facile biosynthesis, characterization and stability of gold nanoparticles by Bacillus licheniformis. Artif Cells Nanomed Biotechnol. 42:6–12.
- Verma VC, Singh SK, Solanki R, Prakash S. 2011. Biofabrication of anisotropic gold nanotriangles using extract of endophytic Aspergillus clavatus as a dual functional reductant and stabilizer. Nanoscale Res Lett. 6:16.
- Vimalanathan AB, Tyagi V, Rajesh A, Devan P, Tyagi MG. 2013. Biosynthesis of silver nanoparticles using Chinese white ginseng plant root Panax ginseng. Asian J Biomed Pharm Sci. 34:1–6.
- Vivek M, Kumar PS, Steffi S, Sudha S. 2011. Biogenic silver nanoparticles by Gelidiella acerosa extract and their antifungal effects. Avicenna J Med Biotechnol. 3:143–148.
- Wang CZ, Wu JA, McEntee E, Yuan CS. 2006. Saponins composition in American ginseng leaf and berry assayed by high-performance liquid chromatography. J Agric Food Chem. 54:2261–2266.
- Wang YF, Pang DW, Zhang ZL, Zheng HZ, Cao JP, Shen JT. 2003. Visual gene diagnosis of HBV and HCV based on nanoparticle probe amplification and silver staining enhancement. J Med Virol. 70:205–211.
- Wang C, Kim YJ, Singh P, Mathiyalagan R, Jin Y, Yang DC. 2015. Green synthesis of silver nanoparticles by Bacillus methylotrophicus, and their antimicrobial activity. Artif Cells Nanomed Biotechnol. [Epub ahead of print]. DOI:10.3109/21691401.2015.1011805.
- Wen J, Zimmer EA. 1996. Phylogeny and biogeography of Panax L. (the ginseng genus, araliaceae): inferences from ITS sequences of nuclear ribosomal DNA. Mol Phylogenet Evol. 6:167–177.