Abstract
Artesunate-loaded nanostructured lipid carriers (ART-NLCs) were prepared by hot homogenization followed by ultrasonication technique. The optimized ART-NLC demonstrated a particle size of 117.5 ± 6.1 nm, with good stability regarding zeta-potential of −19.47 ± 0.9 mV and drug entrapment efficiency of 92.93 ± 1.47%. ART-NLC showed good cellular uptake in breast cancer cells, which was confirmed by confocal laser scanning microscopy (CLSM) and flow cytometry analysis. The significantly higher in vitro cytotoxicity of ART-NLCs against human breast cancer MCF-7, MDA-MB-231 cells as compared with the free ART was recorded. Hoechst 33342 staining indicated that ART-NLC induced higher apoptosis rates in MCF-7 as well as MDA-MB-231cells than free ART.
Introduction
Artesunate (ART), a semi-synthetic derivative of artemisinin, is the active component of the Chinese herb Artemisin annual. Along with anti-malarial activity, ART possesses a promising role in inhibiting the proliferation of cancer cells not only in in vitro and in vivo animal models, but also in humans (Meng et al. Citation2014). In some reported studies, ART potentially works against breast cancer by induction of tumor cell apoptosis or oncosis, inhibition of angiogenesis and down regulation of vascular endothelial growth factor expression, induction of DNA damage, suppression of the hyperactive Wnt/b-catenin pathway and inhibition of tumor invasion, and metastasis (Nguyen et al. Citation2015, Xu et al. Citation2011). Apoptosis is a normal physiological process that occurs during embryonic development and participates in the maintenance of tissue homeostasis. Controlling regulation of apoptosis is a potential strategy to inhibit cancer for ART. However, the application of ART is limited by its short circulation time, extreme hydrophobicity, rapid metabolism, and severe side effects (Chadha et al. Citation2012). Due to this, till date, there are few systems developed to carry ART as an anti-cancer agent, such as polymer lipid hybrid nanoparticles, PLGA nanoparticles, cyclodextrin inclusion (Chadha et al. Citation2012, Gabriels and Plaizier-Vercammen Citation2003, Nguyen et al. Citation2015, Xie et al. Citation2015).
Solid lipid nanoparticles (SLNs) – is a colloidal carrier well-known in chemotherapy due to controlled release, high bioavailability, and better tolerability. In addition, SLNs could effectively deliver loaded drugs into the cell and bypassed the P-gp dependent efflux, so it can improve intracellular drug concentration and drug cytotoxicity as well as reverse multidrug resistance (MDR) activity in MDR cancer cells (Miao et al. Citation2013). However, low drug-loading capacity and expulsion during storage could happen due to the high crystallization of the solid lipids or blends of solid lipids. To overcome the limitation of SLNs, nanostructured lipid carriers (NLCs) were developed, which consist of solid lipids and liquid lipids (Jia et al. Citation2012).
In this study, we aim to enhance characteristics and activity of ART by incorporating lipid carriers. The optimization process was performed to select the proper formulation. Afterward, the physicochemical characterization of the optimized batch was systematically investigated by dynamic light scattering (DLS), differential scanning calorimetry (DSC), powder X-ray diffraction (PXRD), and transmission electron microscopy (TEM). Further, dissolution study was conducted for drug release pattern. Finally, intracellular uptake, in vitro cytotoxicity and apoptosis study were characterized in different breast cancer cell-lines.
Materials and methods
Materials
Artesunate (ART) was provided by Sao Kim Pharma (Hanoi, Vietnam). Lecithin (Phospholipids GmBh; Ludwigshafen, Germany) is a combination of vegetable phospholipids. Polysorbate 80 (Tween-80), a non-ionic surfactant, was purchased from Duksan Chemical Co. (Ansan, Korea). Thiazolyl Blue Tetrazolium Blue (MTT) was purchased from Sigma–Aldrich Chemical Co. Ltd. (St Louis, MO). Compritol 888 ATO, Labrafil (Labrafil® M1944CS) were obtained from Gattefosse (St-Priest, France). Palmitoyl-2-{6-[(7-nitro-2–1,3-benzoxadiazol-4-yl)amino]hexanoyl}-sn-glycero-3-phosphocholine (NBD-PC) was provided by Avanti polar lipids, Inc. (Alabaster, AL), whereas Lysotracker Red was supplied by Thermo Fisher Scientific Inc. (Waltham, MA). All other chemicals were of analytical grade and were used without further purification.
Preparation of artesunate-loaded NLCs
The lipid phase, containing compritol, labrafil 1944, lecithin, and ART were melted at 80 °C to form a uniform liquid. The molten lipid was then dispersed in aqueous phase including Tween-80 at the same temperature by high-speed stirring in an Ultra-Turrax T25 (IKA®-Werke, Staufen, Germany) at 13,500 rpm for 3 min. Subsequently, the coarse emulsion was ultra-sonicated using a probe sonicator (Vibracell VCX130; Sonics, Newtown, CT) for 5 min of 80% intensity sonication. The resulting suspensions were cooled down in ice bath allowing the crystallization of the lipid and following formation of the lipid nanoparticles. Lyophilization of NLCs dispersion was carried out with mannitol as cryoprotectant at concentration of 5% (m/v) using freeze dry machine (FDA5518, IlShin, South Korea). The dispersion was pre-frozen (−80 °C) for 12 h and subsequently lyophilized at a temperature of −25 °C for 24 h followed by a secondary drying phase of 12 h at 20 °C.
Physicochemical characterization
Particle size and zeta potential
The average NLCs size, polydispersity index (PDI), and zeta potential were determined by dynamic laser light scattering using a Zetasizer Nano ZS (Malvern Instruments, UK). All assays were performed in triplicate with suitable dilution before measurement.
Encapsulation efficiency and drug loading
The drug entrapment efficiency (EE) and drug-loading capacity (LC) of ART-NLC were determined indirectly by evaluating free drug in the dispersion medium. Ultrafiltration at 5000 rpm for 15 min using centrifugal 10-kDa molecular weight cut-off devices (Amicon Ultra, Millipore, Billerica, MA) was carried out to discard free drug. Unbound ART moved to the bottom compartment across the filter membrane then estimated by employing HPLC and drug entrapment efficiency (EE); drug-loading capacity (LC) were calculated by using the following equations.
where W is the weight (in mg).
The quantity of ART was measured by Hitachi HPLC system (Hitachi, Tokyo, Japan) consisting of a pump (Model L2100), an auto sampler (Model L2200), and an ultraviolet detector (Model L2420). A C18 analytic column (Inertsil® ODS3: 15 cm × 0.46 cm, 5 μm, GL Sciences Inc., Japan) was used. The mobile phase was the mixture of acetonitrile and phosphate buffer solution pH 3.0 (55:45, volume ratio). The effluent was monitored at a UV absorption wavelength of 216 nm, at a flow rate of 1.0 mL/min with the 50 μl of injection volume.
Transmission electron microscopy analysis
TEM analysis was conducted to observe the morphology of the obtained NLCs in the dispersion. Samples for TEM analysis were prepared by drying a dispersion of the particles on a copper grid coated with amorphous carbon film and then negative staining with 2% (w/v) phosphotungstic acid. Then, the sample was observed by a Hitachi H-7600 transmission electron microscope (Hitachi, Tokyo, Japan).
Differential scanning calorimetry
A differential scanning calorimeter (Q-2000, TA Instruments, New Castle, DE) was used to analyze the thermal properties of the compritol, ART, blank NLCs, and ART-NLCs. The samples were weighed in a standard open aluminum pan with an identical empty pan used as a reference. The samples were heated from 40 °C to 150 °C at 10 °C/min for each sample, while using nitrogen as a purge gas.
Powder X-ray diffraction
The PXRD analysis was performed on compritol, ART, lyophilized blank NLCs, and ART-NLC using a Panalytical diffractometer (PANanalyical, Almelo, The Netherlands) with Cu Kα radiation (λ = 1.54060) at 40 kV and 30 mA between 10° and 50° (2θ) at room temperature with a scan step size of 0.02.
In vitro drug release study
In vitro release study was conducted with the dialysis tube (molecular weight cut off 3500 Da). Approximately 2 mL of the NLCs dispersion was poured into the dialysis tube, the end sealed and immersed into the 40 mL of PBS (pH 7.4) and put into the shaker bath (temperature = 37 °C, 100 rpm). At predetermined time intervals, 0.5 mL of samples were withdrawn and then evaluated with HPLC method as mentioned above. After each sampling, 0.5 mL of fresh release medium was added to maintain sink condition.
The release rate constants
Drug release data from ART-NLCs were fitted to various mathematical models. Values for the AIC (Akaike criteria) were used as basis for comparison (EquationEq. (1)(1) ). The models with the smallest AIC were considered to fit the release data best.
(1)
where, n is the sample size, σ is the residual standard error which was calculated using S-plus 8.0 statistical software (TIBCO Software Inc., Palo Alto, CA), and p is the number of parameters in the model.
In vitro cell study
In vitro cytotoxicity of ART loaded NLCs
The in vitro cytotoxicity studies against MCF-7 and MDA-MB-231 cells were assessed by MTT assay. Cells were seeded in 96-well plates at a density of 1 × 104 cells per well and incubated for 24 h. Then, cells were treated with blank NLCs, free ART (dissolved in cell-grade DMSO at safe concentration), and ART-NLCs at various concentrations. After incubation for 24 and 48 h, the medium was removed, and a MTT solution (1.25 mg/mL) was added to the wells. After another 4 h, DMSO was added to the wells and the absorbance was read at 570 nm by a microplate reader (Multiskan EX, Thermo Scientific, Waltham, MA). Cell viability was calculated using the following formula (Tran et al. Citation2014c):
Flow cytometry study
For intracellular uptake assays, the cells were seeded in 6-well plates at a density of 2 × 105 cells/well and incubated for 24 h. The culture medium was replaced with 1 mL of serum-free medium containing NBD-PC labeled NLCs and incubated at 37 °C for 30 min. Subsequently, the cells were rinsed twice with PBS, detached by scraping and centrifuged at 1000 rpm for 5 min to obtain a cell pellet. The cells were resuspended in 0.5 mL of PBS then analyzed using a flow cytometer (BD FACSVerse™, BD Biosciences, San Jose, CA).
Confocal laser scanning microscopy
MCF-7 and MDA-MB-231 cells were seeded on the coverslip in 12-well plates at a density of 2 × 105 cells per well in 2 mL of RPMI 1640 medium and incubated in a humidified 5% CO2 atmosphere for 24 h. The NBD-PC-loaded NLCs were diluted with media and incubated in the cell for 0.5 h at 1 μg/mL in the incubator. Afterwards, the incubation medium was replaced by normal cell culture medium with Lyso Tracker Red at concentration of 1 μg/mL for 10 min. At last, the cells were washed and fixed with 4% formaldehyde (Choi et al. Citation2014, Darvishi et al. Citation2013).
In the apoptosis assay, cells were seeded in 12-well plates at a density of 2 × 105 cells per well. Cells were treated with free drug and ART-NLC for 24 h at concentration 10 μg/mL, later the cells were further washed three times with 500 μL of PBS and stained with 200 μL of Hoechst 33342 (Sigma–Aldrich, St Louis, MO) (10 μg/mL) for 8 min. The coverslips were mounted on micro-slides and the cellular localization of NBD-PC-NLCs and nuclei morphology were observed using a laser scanning confocal microscope Leica TCS SP2 (Leica Co., Wetzlar, Germany) (Shen et al. Citation2014).
Statistical analysis
The data are presented as the mean ± standard deviation. Depending on the number of the samples for comparison, the statistical significance was determined using Student’s t-test or one-way analysis of variance (ANOVA). The P values <0.05 were considered as significant difference.
Results and discussion
Preparation of ART-NLCs
The high lipophilicity of drug limits the bioavailability due to low solubility, resulting in poor absorption and rapid metabolism, elimination from the body (Mehnert and Mader Citation2001). In this study, nanostructured lipid carriers were developed which include solid and liquid lipids to enhance the pay-load of drug, avoid drug expulsion and degradation during storage and then potentially treat the tumor. In this study, compritol has been used to form the solid outer shell of the nanoparticles, labrafil 1944 as a liquid lipid, known to enhance the encapsulation of lipophilic drugs. Several formulations were prepared and then evaluated to find out the proper one for further studies (). The average particle size and zeta potential of ART-NLC formulations are shown in . In all formulations, particle sizes are in range of 120–180 nm with particle size distribution being narrow (PDI ∼0.2) and zeta potential measurements showed that the surface charges are consistently negative (−11.3 to −28.3 mV). There was a significant decrease in the particle size with an increase of liquid/solid lipid ratio (; P < 0.05); meanwhile they are almost maintained when drug was loaded into formulations. The particle size along with PDI were increased when drug amount was >12.5 mg per formulation, which could be due to the presence of excess drug. In the primary studies, it was found that labrafil 1944 showed highest solubility (data not shown). In the presence of compritol and labrafil, NLCs showed high encapsulation efficiency related to the ART lipophilic nature and high affinity toward the inner oil phase (). In detail, the entrapment efficiency reached to more than 75% (78–97%), whereas loading capacity was from 0.65% to 1.55%.
Figure 1. Effect of (A) liquid/solid lipid ratio, (B) drug concentration on formulation parameters: particle size, polydispersity index (PDI), zeta potential (ZP). Data are expressed as mean ± SD (n = 3).
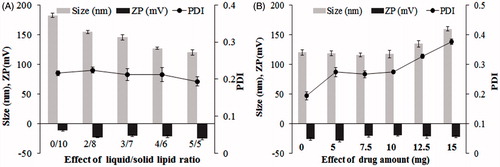
Table 1. Composition of Artesunate-loaded nanostructured lipid carriers.
Table 2. Drug entrapment efficiency and loading capacity of Artesunate-loaded nanostructured lipid carriers.
The particle size of the NLC is a critical factor that could impact on the rate and the extent of drug release as well as drug absorption. The smaller droplet size provides a larger interfacial surface area for drug absorption (Madane and Mahajan Citation2015). On another hand, the particles having average diameter <200 nm could be expected to improve intra-tumoral delivery via enhanced permeability and retention (EPR) effect (Ramasamy et al. Citation2014a, Torchilin Citation2011). In addition, the high loading capacity is essential to reduce the administrated formulation resulting in decrease in the side effects. Based on these criteria, Form.8 was chosen for further studies with favorable properties such as small size (117.5 ± 6.1 nm), high loading capacity (1.24 ± 0.02%), and good stability.
Characterization of ART-NLCs
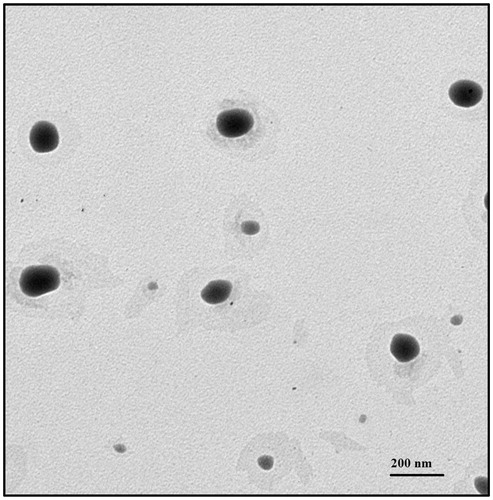
The morphology of ART-NLC observed by TEM was shown in . The TEM study indicated that the particles had almost spherical and uniform shapes and were well dispersed. The mean diameter was in the range of 100–150 nm which is consistent with DLS data.
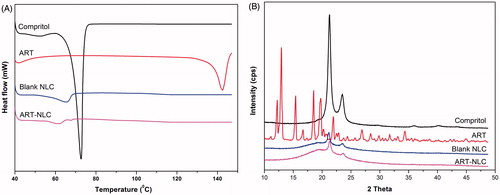
In order to identify the physical state of ART incorporated in NLC, DSC and XRD were performed. shows DSC pattern of Compritol 888 ATO, ART, blank NLC, and ART-NLC lyophilized powder. The thermogram of ART, Compritol demonstrated the melting peaks of ART, Compritol at around 142 °C and 73 °C, respectively. These melting peaks indicated natural properties of materials (Nguyen et al. Citation2015, Tran et al. Citation2014a). However, the melting peak for the ART around 142 °C disappeared in the thermograms of the lyophilized ART-NLC with blank NLC pattern as the control. It suggested that ART in lipid was not in crystalline state and it could be in amorphous state within the matrix of nanoparticles (Madane and Mahajan Citation2015, Ramasamy et al. Citation2014b). On the other hand, the X-ray diffraction patterns of the materials are revealed in . The diffractogram of ART exhibits many sharp peaks. On the contrary, these sharp peaks disappeared or the intensities of these peaks decrease drastically in the ART-NLC, confirming the amorphous or molecular disperse of ART in the nanoparticles. The diffraction curve of compritol presents sharp peaks at 2θ around 22° and 24°, which also appear with low intensity in ART-NLC due to liquid lipid which decrease the degree of crystallite. The imperfection of crystallite of compritol proposed more space for drug accumulation, resulting in enhanced drug-loading capacity as well as stability (Teeranachaideekul et al. Citation2007).
Figure 3. (A) Differential scanning calorimetry, (B) X-ray diffraction patterns of Compritol, free ART, blank NLC, and ART-NLC.
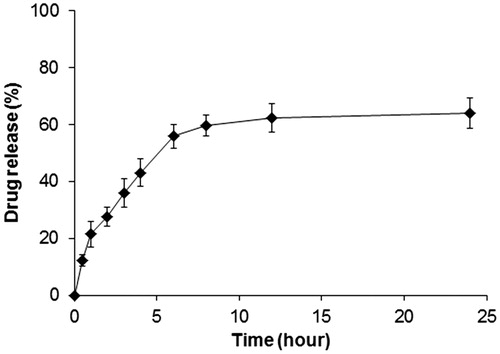
The cumulative drug release of ART from ART-NLC investigated in vitro over a period of 24 h. demonstrated that the drug release of ART follows bi-phasic manner with an initial steady drug release phase up to 6 h (∼60%) followed by a sustained release phase. Such release pattern could be explained that large amount of drug might bind to outer particle and hence they are easily released, whereas the encapsulated lipid core deep inside released slowly with erosion or degradation of lipid matrix (Tran et al. Citation2014b, Zhang et al. Citation2008).
Results of fitting of drug release data to mathematical models are presented in . The table shows that the Weibull model best describes the drug release kinetics from ART-NLC (AIC value is the smallest). Weibull model is an empirical model and can be successfully applied to almost all kinds of drug release curves. The second choice is the Korsmeyer–Peppas model, which is usually used to elucidate the drug release mechanism in a dosage form. The obtained diffusion exponent, n, of 0.334 shows that the mechanism of drug release follows square root versus time drug release kinetics.
Table 3. Summary of residual standard errors and AIC of drug release models.
In vitro cell studies
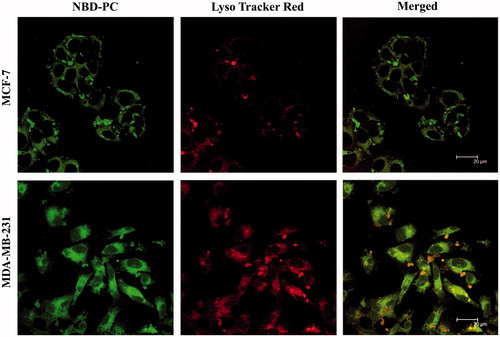
In order to investigate the cellular uptake of nanoparticles, NBD-PC was loaded into NLCs’ nanoparticles. The cellular uptake of dye incorporated nanoparticles was quantitatively assayed by fluorescence microscopy and fluorescence-activated cell sorting on breast cancer cells. As shown in and , after 30 min incubation, the labeled particles penetrated into MCF-7 and MDA-MB-231 cells. represents the flow cytometry results after treated with media (control), NBD-PC loaded NLCs showed that within 30 min, the intensity of formulation is higher than control indicating the uptake of particles. The internalized fraction and location of the nanoparticles taken up by the breast cancer cells were further evaluated by fluorescence microscopy. In detail, particles mainly stayed in the cytoplasm that were stained by Lysotracker Red, and it is expected to release drug for later action toward the nuclei or cell components. Despite the negatively charged particles, the high uptake of NLCs can be attributed by the lipophilicity of NLCs that improves the interaction with the membranes of cells. These findings are consistent with previously published data that also found an increased uptake of lipid carriers in resistant and sensitive human tumor cell lines (Doktorovova et al. Citation2014, Mussi et al. Citation2013). The clear improvement in the intracellular delivery efficiency was expected to lead to a more efficient anti-tumor drug action.
Figure 5. Quantitative uptake of analysis by flow cytometry of NLC on: (A) MCF-7 cells, and (B) MDA-MB-231 cells. Control (orange), treated NLC (blue).
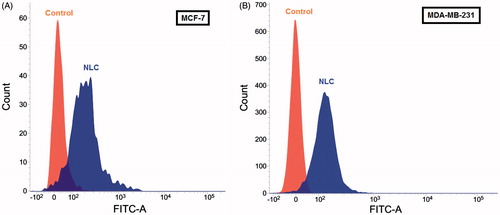
Figure 6. Intracellular uptake of NLC into MCF-7 cells and MDA-MB-231 cells by confocal laser scan microscope images. NLC containing NBD-PC (green) and Lysotracker Red (red) staining lysosome were used for this experiment.
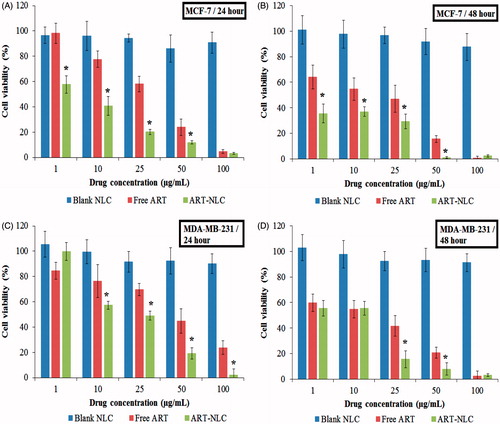
The anti-cancer potential of ART was evaluated by MTT assay. shows the cell viability of MCF-7 and MDA-MB-231 cells treated with blank NLCs, ART, and ART-NLCs at various concentrations, for 24 and 48 h, respectively. MCF-7 and MDA-MB-231cell growth were significantly inhibited by both free ART and ART-NLCs in a dose- and time-dependent manner. At the same concentration, ART-NLCs showed significantly higher inhibition rate compared with free ART. The viability of MCF-7 was about 60% after being treated with the ART at a concentration of 25 μg/mL for 24 h, while only 20% with ART-NLCs whereas after 48 h incubation the cell viability are 46% and 29% under exposure to ART and ART-NLCs, respectively. On the other hand, at 25 μg/mL ART-NLCs treating to MDA-MB-231, the survival rate is 49% after 24 h and 15% after 48 h. In contrast, the plain NLCs demonstrated no significant inhibition effect with more than 80% cell survival (Petersen et al. Citation2011, Tran et al. Citation2014c,). The better activities of ART-NLCs could be explained by improved solubility, stability of drug, as well as effective intracellular uptake (Martins et al. Citation2012).
Figure 7. In vitro cytotoxicity of blank NLCs, free ART, and ART-NLCs after 24 and 48 h exposure in MCF-7 and MDA-MB-231cells (P < 0.05). Data are expressed as mean ± SD (n = 8). *P <0.05, compared to the free drug.
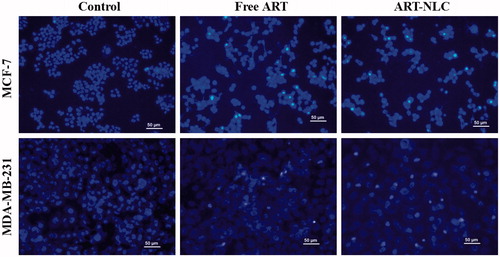
ART induced the apoptotic process in breast cancer via p53, Bcl2 family-mediated mitochondrial dysfunction, and enhanced reactive oxygen species (ROS) production (Hamacher-Brady et al. Citation2011). In order to see the effect of nanoparticles on cell apoptosis, free drug and ART-NLCs with equivalent ART dose of 10 μg/mL were treated to MCF-7 and MDA-MB-231 cells and then cells were stained with Hoechst 33342 using unexposed cells as controls. demonstrates that the control MCF-7 and MDA-MB-231 cells had a regular oval-shaped nucleus body and their nuclei were homogeneously stained with dim appearance. However, under exposure to free ART or ART-NLCs, some cells exhibited typical apoptotic features such as nucleus deformation, nucleus shrinkage, and the apoptotic cells are also brighter than the others. Furthermore, in both cell-lines, ART-NLCs had an increasing ability to induce the cell apoptosis as compared with free ART. It could be a strong evidence for improved cell growth inhibition as described above.
Stability studies
In order to evaluate the physical stability, ART-loaded NLC were stored at two different conditions, i.e., dispersed form or lyophilized form, and the particle size, zeta potential, entrapment efficiency, and loading capacity were evaluated over a period of one month. showed the particle size of ART-loaded NLC measured by DLS after one month of storage. The particle size and PDI increased in both conditions. The zeta potential was −19.47 ± 0.9 mV at the day of production. After one month of storage at dispersed and lyophilized form, a zeta potential of −22.5 ± 2.1 mV and –23.5 ± 2.8 mV was measured, respectively. On the other hand, the entrapment efficacy and loading capacity are reduced in both storage conditions (). It could be due to drug expulsion (Müller et al. Citation2002), but at limited level.
Figure 9. Physical properties of ART-NLCs after 30 d storage in different conditions. Data are expressed as mean ± SD (n = 3).
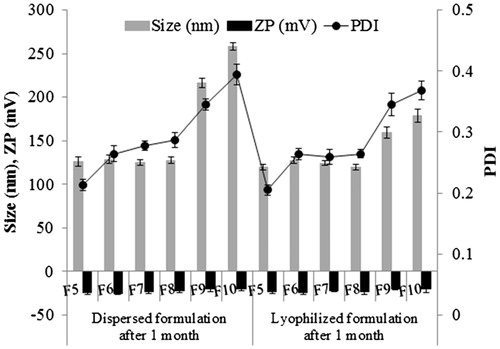
Table 4. Drug entrapment efficiency (EE) and loading capacity (LC) of Artesunate-loaded nanostructured lipid carriers after 30 d storage.
Conclusions
This study revealed that lipid nanoparticles are a promising carrier to deliver ART – the common anti-malarial drug for anti-tumor treatment. ART-NLCs with small particle size, good stability, and high entrapment efficiency were obtained. The in vitro drug release profile of ART-NLCs demonstrated that ART was sustainably released after burst release duration from NLCs. The ART-NLCs could well penetrate into cells and then express its activities. The in vitro cytotoxicity tests in breast cancer cell lines MCF-7 and MDA-MB-231 showed that ART-NLCs were more cytotoxic than free ART, which could be due to better cellular uptake and inducing apoptosis of tumor cells.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
This research is funded by Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 106-YS.05–2013.15.
References
- Chadha R, Gupta S, Pathak N. 2012. Artesunate-loaded chitosan/lecithin nanoparticles: preparation, characterization, and in vivo studies. Drug Dev Ind Pharm. 38:1538–1546.
- Choi JY, Ramasamy T, Tran TH, Ku SK, Shin BS, Choi H-G, Yong CS, Kim JO. 2014. Systemic delivery of axitinib with nanohybrid liposomal nanoparticles inhibits hypoxic tumor growth. J Mater Chem B. 3:408–416.
- Darvishi MH, Nomani A, Amini M, Shokrgozar MA, Dinarvand R. 2013. Novel biotinylated chitosan-graft-polyethyleneimine copolymer as a targeted non-viral vector for anti-EGF receptor siRNA delivery in cancer cells. Int J Pharm. 456:408–416.
- Doktorovova S, Souto EB, Silva AM. 2014. Nanotoxicology applied to solid lipid nanoparticles and nanostructured lipid carriers – a systematic review of in vitro data. . Eur J Pharm Biopharm. 87:1–18.
- Gabriels M, Plaizier-Vercammen J. 2003. Physical and chemical evaluation of liposomes, containing artesunate. J Pharm Biomed Anal. 31:655–667.
- Hamacher-Brady A, Stein HA, Turschner S, Toegel I, Mora R, Jennewein N, et al. 2011. Artesunate activates mitochondrial apoptosis in breast cancer cells via iron-catalyzed lysosomal reactive oxygen species production. J Biol Chem. 286:6587–6601.
- Jia L, Shen J, Zhang D, Duan C, Liu G, Zheng D, et al. 2012. In vitro and in vivo evaluation of oridonin-loaded long circulating nanostructured lipid carriers. Int J Biol Macromol. 50:523–529.
- Madane RG, Mahajan HS. 2015. Curcumin-loaded nanostructured lipid carriers (NLCs) for nasal administration: design, characterization, and in vivo study. Drug Deliv. 2015. [Epub ahead of print]. DOI: 10.3109/10717544.2014.975382.
- Martins S, Costa-Lima S, Carneiro T, Cordeiro-Da-Silva A, Souto EB, Ferreira DC. 2012. Solid lipid nanoparticles as intracellular drug transporters: an investigation of the uptake mechanism and pathway. Int J Pharm. 430:216–227.
- Mehnert W, Mader K. 2001. Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev. 47:165–196.
- Meng H, Xu K, Xu Y, Luo P, Du F, Huang J, et al. 2014. Nanocapsules based on mPEGylated artesunate prodrug and its cytotoxicity. Colloids Surf B Biointerfaces. 115:164–169.
- Miao J, Du Y-Z, Yuan H, Zhang X-G, Hu F-Q. 2013. Drug resistance reversal activity of anticancer drug loaded solid lipid nanoparticles in multi-drug resistant cancer cells. Colloids Surf B Biointerfaces. 110:74–80.
- Müller R, Radtke M, Wissing S. 2002. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev. 54:S131–S155.
- Mussi SV, Silva RC, Oliveira MCD, Lucci CM, Azevedo RBD, Ferreira LM. 2013. New approach to improve encapsulation and antitumor activity of doxorubicin loaded in solid lipid nanoparticles. Eur J Pharm Sci. 48:282–290.
- Nguyen HT, Tran TH, Kim JO, Yong CS, Nguyen CN. 2015. Enhancing the in vitro anti-cancer efficacy of artesunate by loading into poly-d, l-lactide-co-glycolide (PLGA) nanoparticles. Arch Pharm Res. 38:716–724.
- Petersen S, Steiniger F, Fischer D, Fahr A, Bunjes H. 2011. The physical state of lipid nanoparticles influences their effect on in vitro cell viability. Eur J Pharm Biopharma. 79:150–161.
- Ramasamy T, Haidar ZS, Tran TH, Choi JY, Jeong J-H, Shin BS, et al. 2014a. Layer-by-layer assembly of liposomal nanoparticles with PEGylated polyelectrolytes enhances systemic delivery of multiple anticancer drugs. Acta Biomater. 10:5116–5127.
- Ramasamy T, Tran TH, Choi JY, Cho HJ, Kim JH, Yong CS, Choi H-G, Kim JO. 2014b. Layer-by-layer coated lipid–polymer hybrid nanoparticles designed for use in anticancer drug delivery. Carbohydr Polym. 102:653–661.
- Shen J, Sun H, Meng Q, Yin Q, Zhang Z, Yu H, Li Y. 2014. Simultaneous inhibition of tumor growth and angiogenesis for resistant hepatocellular carcinoma by co-delivery of sorafenib and survivin small hairpin RNA. Mol Pharm. 11:3342–3351.
- Teeranachaideekul V, Muller RH, Junyaprasert VB. 2007. Encapsulation of ascorbyl palmitate in nanostructured lipid carriers (NLC)-effects of formulation parameters on physicochemical stability. Int J Pharm. 340:198–206.
- Torchilin V. 2011. Tumor delivery of macromolecular drugs based on the EPR effect. Adv Drug Deliv Rev. 63:131–135.
- Tran TH, Choi JY, Ramasamy T, Truong DH, Nguyen CN, Choi H-G, Yong CS, Kim JO. 2014a. Hyaluronic acid-coated solid lipid nanoparticles for targeted delivery of vorinostat to CD44 overexpressing cancer cells. Carbohydr Polym. 114:407–415.
- Tran TH, Ramasamy T, Truong DH, Choi HG, Yong CS, Kim JO. 2014b. Preparation and characterization of fenofibrate-loaded nanostructured lipid carriers for oral bioavailability enhancement. AAPS Pharm Sci Tech. 15:1509–1515.
- Tran TH, Ramasamy T, Truong DH, Shin BS, Choi HG, Yong CS, Kim JO. 2014c. Development of vorinostat-loaded solid lipid nanoparticles to enhance pharmacokinetics and efficacy against multidrug-resistant cancer cells. Pharm Res. 31:1978–1988.
- Xie H, Yang B, Wang F, Zhao Y. 2015. Host–guest inclusion system of artesunate with β-cyclodextrin and its derivatives: characterization and antitumor activity. J Mol Struct. 1085:90–96.
- Xu Q, Li ZX, Peng HQ, Sun ZW, Cheng RL, Ye ZM, Li WX. 2011. Artesunate inhibits growth and induces apoptosis in human osteosarcoma HOS cell line in vitro and in vivo. J Zhejiang Univ Sci B. 12:247–255.
- Zhang XG, Miao J, Dai YQ, Du YZ, Yuan H, Hu FQ. 2008. Reversal activity of nanostructured lipid carriers loading cytotoxic drug in multi-drug resistant cancer cells. Int J Pharm. 361:239–244.