Abstract
Inflammatory diseases are considered to be highly dreadful ones responsible for higher mortality in the developed countries. This includes cancer, psoriasis, rheumatoid arthritis, and inflammatory bowel disease. The tremendous strides in the area of drug development to find newer molecules like non-steroidal and steroidal agents and immunosuppressant agents delivered by conventional formulation. These therapy have enhances the life expectancy of patient, but it provide the therapeutic benefits only to a limited extent. Recent advancement in liposomes based nanomedicines has led to the possibility of improves the efficacy and safety of the pharmacotherapy of inflammatory disorders. Of late, liposomes have been highly explored as one of the promising systems for delivering numerous anti-inflammatory drugs for attaining enhanced therapeutic outcomes. Over the conventional carriers, liposomal systems have numerous drug delivery merits including advantages in both passive and active targeting of drug molecules to the inflammatory lesions. The current review article, therefore, endeavors to provide a bird’s eye view account on the success of liposome-based therapeutic systems in the management of dreadful inflammatory disorders along with updated knowledge to pharmaceutical scientists in the field.
Introduction
Inflammation is part of the complex biological response of the body which involves processes like redness, swelling, heat, and pain. Moreover, it is triggered and elicited by injury, microbes, toxins, abnormal T cell expression, necrotic cells, histamine, bradykinin, and leukotriens, etc (Cotran and Majno Citation1964). Inflammation resides in the extra vascular space, where it elicits a series of cellular reactions that activates the inflammatory response. A comprehensive list of inflammatory diseases includes cancer, psoriasis, rheumatoid arthritis, and inflammatory bowel disease (IBD) (Cotran and Majno Citation1964, Coussens and Werb Citation2002). Although anti-inflammatory drug-loaded conventional formulations are available for the treatment of these dreadful maladies, yet they have several hiccups including their low bioavailability, non-targeted action, higher clearance and drug resistance, it often limits their efficacy in accomplishing desired therapeutic efficacy in controlling disease symptoms (Cotran and Majno Citation1964, Coussens and Werb Citation2002). Thus, significant need of an effective medication is the current demand. First time, vascular leakage was observed in inflammation in 1964 by Cotran and Majno and this concept has been widely utilizes in passive targeting of the drugs for treatment of inflammatory disorders (Cotran and Majno Citation1964). The over-expression of biological markers or receptors on cell surfaces is a common feature of inflammatory maladies. This phenomenon has been studied in the last few decades on liposomes as targeted drug delivery system on desired site (Cotran and Majno Citation1964, Coussens and Werb Citation2002). The diverse application of liposomes in drug delivery arena is strongly implicated due to optimum encapsulation efficiency, biocompatibility, enhanced specificity of drug molecules to the inflammatory sites via passive and active targeting (Cleland et al. Citation1979). Moreover, these protect from premature drug metabolism during transport at the biological barrier (Cleland et al. Citation1979). Macrophages play a key role in producing inflammatory response; their specific targeting is a potential anti-inflammatory therapy (De Silva et al. Citation1979). Therefore, active targeting approach is gaining novelty in this regard using specific ligand on the liposomal surface which delivered the drug to the target cell by phagocytosis, receptor mediated endocytosis, etc (De Silva et al. Citation1979). In a nutshell, this review provides understanding of pathogenesis, diagnostics, biomarkers, and liposome based targeted drug delivery for better management of dreadful inflammatory disorders.
Introduction to liposomes and its targeted drug delivery in inflammatory maladies
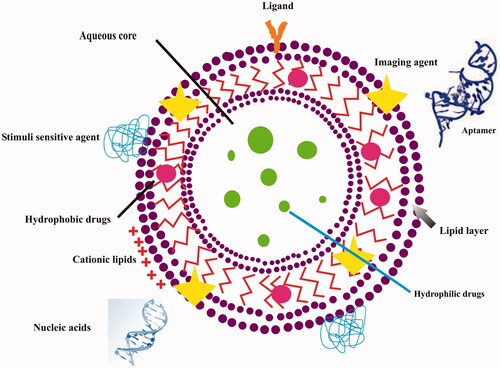
Liposomes first discovered by A. D. Bangham in 1961, which primarily composed of phospholipids (Bangham and Horne Citation1964). Its size range is 50–1000 nm and it resembles with natural membranes (Bangham and Horne Citation1964). Vesicles have ability to swell osmotically and they may vary in lipid composition and capability of surface functionalization makes preferred vesicular carriers (Bangham et al. Citation1965). It has moved a long way from biophysical research to becoming a novel pharmaceutical carrier (Lian and Ho Citation2001). Vesicles have a hydrophilic core and hydrophobic bilayers, which may encapsulate both hydrophilic and hydrophobic drugs, respectively (Lian and Ho Citation2001). Further it can be single layer (unilamellars or single layer vesicle or SUV) and many concentric bilayer (multilamellar or multilamellar vesicles or MLV) vesicles (Lian and Ho Citation2001). Liposomes have certain limitations like as they interact with high density lipoproteins and low density lipoproteins; it cause instability in plasma and premature release and rapid clearance (Lian and Ho Citation2001). Another liposome is stealth liposome, it emerge as an option to overcome hiccups of conventional liposomes. The surface of liposomes are modified with inert, biocompatible, and hydrophilic polymers, such as polyethylene glycol (PEG), polyvinyl pyrrolidone (PVP), polyacryl amide (PAA), poly[N-(2-hydroxypropyl) methacrylamide], amphiphilic poly-N-vinylpyrrolidones (PVP), L-amino-acid-based biodegradable polymer–lipid conjugate, polyvinyl alcohol, poly-2-methyl-2-oxazoline (PMOZ), and poly(2-ethyl-2-oxazoline) (PEOZ) has been widely utilized for developing of long circulating liposomes or stealth liposomes (Immordino et al. Citation2006). Liposome surface may conjugate with monoclonal antibodies (mAb) or antifragment segments called as immunoliposomes (Alving et al. Citation1969). It has been utilizes as carrier in treatment of various disease and its preparation based upon the utilization of functional groups like amino, and carboxyl onto antibody molecule and uses of hydroxyl and amine groups present in phospholipids (Alving et al. Citation1969). They have greater binding avidity and higher stability and further its application is discussed in the treatment section (Alving et al. Citation1969). Another development is cationic lipids based liposome, it binds to anionic charge containing molecules such as oligonucleotides or genes, and this complex is called cationic lipoplexes or cationic liposome (Funk et al. Citation2007). Its application is widely reported in the treatment of inflammatory disorders including RA and psoriasis, etc. Another is stimuli based liposomes, which delivers the encapsulated molecules in response to pathological triggers, which are present in the disease tissues. The stimulus modifies the confirmation of liposomes, and further promoting the release of therapeutic molecules in specific biological environment (Ta and Porter Citation2013). Multifunctional liposomes are pictorially depicted in . The drug targeting approach in treatment of various inflammatory maladies is best supported by cancer targeted drug delivery such as passive, active, and combinational drug targeting. Passive targeting, the biodistribution of liposomes is mediated by physiological conditions of tumor cells environment such as pH, temperature, and specific enzymes to allow more accumulation of drug carrier at the target site (Mornet et al. Citation2006). There are several enzymes such as alkaline phosphatase and plasmins are founds in rich amount at the tumor site. Angiogenesis is prominent feature of tumor cell, their unmatured development lead to incomplete vasculatures of tumor which result to form leaky blood vessels (Maeda et al. Citation2000). This hyperpermeability of tumor vasculature causes accumulation of liposomes and release the loaded therapeutic molecules. This mechanism is called as Enhanced Permeation and Retention effect (EPR effect) (Maeda Citation2001). This mechanism is common in almost all inflammatory maladies. Their example based description is discussed in the various disease sections.
Passive targeting does not acquires sufficient internalization of conventional liposome by the targeted cell, therefore liposomes are modified with targeting ligands for active targeting. Active targeting of liposomes involves the conjugation of targeting moiety and interacted with corresponding receptor to facilitate the targeting of a carrier to a specific tumor cell (Ahmad et al. Citation2015, Zhang et al. Citation2002). The tumor cells over express certain specific receptors include aptamers, proteins, and antibodies, which can be efficiently targeted by drug loaded liposome to facilitate the active targeting. The conjugation of said ligands to the liposomes has been exploited in many areas for enhancement of therapeutic action to the specific tumor cell (Yoo and Park Citation2004). These ligands bind to specific receptor on the cellular surface and promote the internalization of therapeutic molecules which acts on cellular organelles, i.e., mitochondria, microtubules, nucleus, etc., via receptor-mediated endocytosis (RME) or also known as active drug targeting. Many over expressed growth factor receptors for selectively targeting the tumor cell or other disease cell and their description has been reviewed below (Carter et al. Citation2004).
Cancer
It is a second leading threatful cell death disorder across the globe. It involves numerous steps including cells signaling and apoptosis (Rahman et al. Citation2012a, Citation2012b). Tumor development involves activation of proto-oncogene to oncogene, lead to produce unmatured group of cells, it called as tumor. After achieving the size of 2 mm², they initiate to move to other parts of the body, this phenomenon is called as metastasis and it makes cancer incurable (Rahman et al. Citation2012a, Citation2012b). Moreover, they disturb the normal physiological functions of cells and ultimately cause apoptosis. Molecular heterogeneity and adaptive resistance in tumor cells make it challengeable for its treatment (Rahman et al. Citation2012a, Citation2012b). Conventional approaches available includes surgery, radiation, immunotherapy, and chemotherapy, they give suboptimal response with dose related toxicity and high risk damage of vital organs (Rahman et al. Citation2012a, Citation2012b). Hence, liposomal loaded anticancer drug delivery has gained great privilege in cancer therapeutics and has numerous advantages as pharmaceutical carriers including, biocompatibility, prevents from premature degradation of encapsulated drugs, entrapments of hydrophilic and hydrophobic drugs, targeted delivery, site avoidance, shorter treatment duration, and their size monitoring regulating biodistribution of liposomes (Sen and Mahitosh Citation2013). Besides, they have certain limitations like high production cost of raw material, oxidation of some phospholipids, fast elimination from systemic circulation and rapid removal by RES, non-specific uptake and instability due to aggregation, sedimentation, and hydrolysis (Akhter et al. Citation2012).
Conventional liposomes have significant merits but they have many limitations include shorter duration of action and non-selective action (Madni et al. Citation2014). Several anticancer drug loaded conventional liposome in clinical use, it include Myocet® (Sopherion Therapeutics or Cephalon in USA and Europe, respectively) containing doxorubicin (DOX), DaunoXome® (Galen) have daunorubicin and Marqibo® (Talon Therapeutics) carried vincristine sulfate (Madni et al. Citation2014). DOX is an anthracycline class of anticancer drug, which act through topoisomerase inhibition and have reported irreversible cardio toxicity (Madni et al. Citation2014). Oxaliplatin analogue loaded multilamellar liposome named as Aroplatin® (Antigenics Inc., Lexington, MA) has been developed by utilizing phospholipids dimyristoyl phosphatidylcholine (DMPC) and dimyristoyl phosphatidylglycerol (DMPG), which has been under clinical trials and found to reduced nephrotoxicity (Rahman et al. Citation2012a). Anamycin (3-deamino-4-epi-3-hydroxy-2-iodo-4-demethoxy doxorubicin) is encapsulated in liposome to provide higher encapsulation and enhanced therapeutic potential against cancer (Kaasgaard and Andresen Citation2010). Epirubicin (EPI), has been loaded in propylene glycol containing liposome with higher EE and study carried on MDA-MB 435 (lung cancer) and their mutant resistant (MDA-MB 435/ADR) cells, it received higher EPI absorption and greater tumor growth inhibition as compared to EPI solution (Zhao et al. Citation2013).
Recently, paclitaxel (PTX) loaded liposomes has been developed and d-α-tocopherol PEG 1000 succinate–triphenylphosphine conjugate (TPGS-TPP) has been utilizes to targeting the mitochondrial sub-organelles in human lung cancer A549 cells and drug resistant lung cancer A549/cDDP cells of xenograft nude mice (Zhou et al. Citation2013). As compared to paclitaxel alone, PTX loaded liposomes show strongest anticancer efficacy through enhanced cellular uptake and cause apoptosis by higher accumulation in mitochondria against drug resistant A549/cDDP xenograft tumor model (Zhou et al. Citation2013). Another is stealth liposomes, clinically; Doxil is the first USFDA approved sterically stabilized liposomes in Kaposi sarcoma and ovarian cancer treatment. It consists of 2 mg/ml of DOX, and their 80–100 nm size produces systemic circulation up to longer duration and promotes passive targeting (Barenholz Citation2012). After 6 months, Daunosome have taken FDA approval. They are composed of dipalmitoylphosphatidylcholine (DPPC), cholesterol, total lipid concentration, PEG, and drug concentration was 1 mg ml−1. The drug was encapsulated by applied pH gradient technique (O'Byrne et al. Citation2002). Moreover they remain stable in the circulation, due to membrane neutrality and strong mechanical adhesion nature (Barenholz Citation2012, O'Byrne et al. Citation2002). Thus, both Doxil and Daunosome show superior therapeutic effects, lesser toxicity, and higher biocompatibility in Kaposi sarcoma patient over conventional liposomes (Barenholz Citation2012, O'Byrne et al. Citation2002).
In another study oxaliplatin has been loaded into transferrin (TF) conjugated PEG liposomes, and their study on solid tumor which over-expressed TF receptor has shown as targeted potential carriers (Suzuki et al. Citation2008). DOX has been loaded in RGD modified PEGylated liposomes and show enhanced cytotoxicity against melanoma (Noble et al. Citation2014). Cationic liposome has been effective in treatment of tumor cells resistance (Nakamura et al. Citation2011). Paclitaxel (MBT-0206) has been loaded into cationic liposomes and it showed higher selective uptake by angiogenic tumor endothelial cells in solid tumors and prevents metastases (Immordino et al. Citation2006). Endo TAG™-1 (Medi Gene A.G., Martinsried, Germany) is currently at II phase of clinical trial against pancreatic cancer (Eichhorn et al. Citation2006). However, they strongly interact with blood components and are unable to reach the therapeutic target. To overcome this, they are functionalized with PEG to become more efficacious in cancer therapy (Eichhorn et al. Citation2006). PEG coated cationic liposomes provides higher loading efficiency over similar size of neutral liposomes (Rahman et al. Citation2012a). Oxaliplatin PEG cationic liposome has been developed, which are effective in tumor targeting. This may due to readily binding to endothelial cells in the tumor vasculature by virtue of electrostatic interactive forces between cationic charge of liposome and negative charge of plasma membrane of endothelial cells (Abu Lila et al. Citation2009).
Moreover, PEG also utilized as linker between antibodies and liposomes. An interesting example of immunoliposomes is loading of monoclonal nucleosome (NS) specific antibody 2C5 into liposome with PEG-PE (Lukyanov et al. Citation2004). Other example is loading of monoclonal antibodies (MAbN-12A5) in stealth liposome and it shows superior action against breast cancer via targeting of erbB-2 oncoprotein (Goren et al. Citation1996). Another example is DOX monoclonal (Doxil®) nucleosome (NS) specific 2C5 antibody (mAb2C5) has been loaded into stealth liposomes, shown higher antitumor efficacy as compared to conventional DOX-loaded liposomes (Lukyanov et al. Citation2004). pH sensitive liposomes, which are destabilized at particular pH, and produce pronounced effects in cancer targeted drug delivery. They easily bind to cell surface receptor by endocytosis and release therapeutic molecule by the action of peptidases and hydrolase (Cho et al. Citation2008). As, for example, DOX has been loaded into pH sensitive liposome, it results to get 98% EE and higher drug release at pH of 6–4 (Fritze et al. Citation2006). Recently, with the help of dextran, 3-methyl glutaric anhydride and egg yolk phosphatidylcholine as a lipid and loaded with oval-albumin (OVA) to develops pH sensitive liposome, which is highly unstable at weak acidic region (Yuba et al. Citation2014). These when administered to mice bearing E.G7-OVA tumor exhibit significant inhibition of tumor growth and prolonged mice survival (Yuba et al. Citation2014).
Folate receptor is over expressed on tumor cells and quantitatively minimal on normal cells and thus it targeting is interesting for drug delivery in cancer treatment (Akhter et al. Citation2013). Recently, irinotecan [camptothecin (CPT)-11]-loaded folate targeted liposome has been developed and they apply by i.v. administration in tumor-bearing mice. The distribution of CPT-11 in tumor was significantly higher as compared to CPT-11 injection (Akhter et al. Citation2011). It may attribute due to folate mediated targeting by the folate receptor on tumor cells. Moreover, they also exhibit dose dependent tumor growth inhibition and superior therapeutic efficacy. Most recent dual-targeting approach (passive and active) has gained synergistic anticancer effects over alone targeting approach (Akhter et al. Citation2011).
The dual targeting approach displayed a great potential for optimal combination cancer therapy. Recently, in dual targeting approach, pH sensitive response with mitochondrial targeting action has developed (Jiang et al. Citation2015). Recently, PTX has been loaded into liposomes, which utilize peptide D[KLAKL AK](KLA)2 modified with 2,3-dimethylmaleic anhydride (DMA) and combined with 1,2-distearoyl-sn-glycero-3-phosphoethanolamine (DSPE) to form DSPE-KLA-DMA (DKD) lipid and further mixed with other commercial lipid (Jiang et al. Citation2015). Tumor extracellular exhibit pH of ∼6.8 promotes cellular internalization and further PTX reach to mitochondria and shows targeting action. This dual functional liposome founds greatest efficacy (tumor growth inhibition 86.7%) against A549 cells, A549/Taxol cells and drug-resistant lung cancer A549/Taxol cells xenografted onto nude mice (Jiang et al. Citation2015). Recently, paclitaxel (PTX) and DNA were loaded into hyaluronic acid (HA) and folate (FA)-conjugated liposomes. As compared to FA-conjugated PTX/DNA co-loaded liposome, they have superior in protecting the liposome from aggregation and degradation by DNase I (Liu et al. Citation2014). Furthermore, they have to apply on murine malignant melanoma cell line (B16) and human hepatocellular carcinoma cell line (HepG2) and found significantly improved transfection efficiency and effective cellular internalization into the tumor cell take place (Liu et al. Citation2014). Moreover, it showed no toxicity on B16 cell line. The human epidermal growth factor receptor 2 (HER2) is a tyrosine kinase receptor and is over expressed in 25–35% breast carcinoma cells (Slamon et al. Citation1989). Moreover, dimerization is required for activation of this signal pathway, which responsible for the transactivation of tyrosine kinase (Olayioye et al. Citation2000). Monoclonal antibodies have been administered to target the HER2 receptor and suppress the dimerization and further blocking the activation of HER2 signal pathway. Herceptin is a FDA approved monoclonal antibody used in treatment of breast and ovarian cancer by HER2 receptor targeting (Kirpotin et al. Citation1977). Researcher has developed anti-HER2 immunoliposomes containing metallic nanoparticles (MNPs), which act as combinational targeted cancer therapy. They have to exert HER2 mediated antiproliferative effects on SKBr3 breast cancer by chemotherapy with hyperthermia, which resulted to strong cytoxicity effects (Akira et al. Citation2004). Other example is conjugation of stealth liposomes with Tf mediated delivery has been described for effective delivery of phosphorothioate antisense oligodeoxyribonucleotide (ODN) (G3139, oblimerson sodium, or Genasense TM) in a leukemia cells treatment via Bcl-2 regulation (Chiu et al. Citation2006). The combination of PEGylated siBcl-2 lipoplex and 5-FU show enhanced anticancer efficacy in DLD-1 xenograft model (Jiang et al. Citation2015). Overall, this dual approach is beneficial to overcome the MDR in cancer treatment.
Psoriasis
Psoriasis is immune-based skin disarray, followed by keratinocyte hyperproliferation. It has spread across the globe and effect nearly about 2–3% of the world population. It appears in different forms such as plaque psoriasis, guttate psoriasis, pustular, and erythrodermic psoriasis. The most common among all is plaque psoriasis (Rahman et al. Citation2012c). It forms red scaly plaques over the scalp, lower back and extended up to the limbs. There are umpteen stimulator including streptococcal infection, cutaneous trauma, drugs, alcohol, cigarette smoking, stress, and ultraviolet (UV) radiation exposure (Rahman et al. Citation2012c). Perhaps relapsing and spontaneous remission is a great challenge which persists in psoriasis (Rahman et al. Citation2012c). An abnormal T lymphocyte function is a principal pathway in pathogenesis of psoriasis. Attacking of antigen on the surface of antigen presenting cells in skin layers to forms major histocompatibility complex (MHC) and move into lymph node to bind to T lymphocyte receptors and its activation leads to synopsis which include cluster differentiations 2 (CD2), and lymphocyte functional antigen 3, etc (Rahman et al. Citation2012c). Furthermore, the activated cloned T cell enters into circulatory system to different skin layers and produces cytokines, chemokines, and growth factors inducing hyperkeratosis in psoriatic skin (Rahman et al. Citation2012a, Rahman et al. 2015a). Psoriatic lesions have to founds enriched amount of dendritic cells (DCs) and macrophages, and they are associated with CD4 and CD8 T-cells, their integration responsible for production of various cytokines incl. interleukins (IL-12 and IL-23), tumor necrosis factors (TNF-α and TNF-β) (Rahman et al. 2015a). Moreover, all these mediators regulates the production and expansion of T helper (T1) and TH17/TH 22 cells, which produces IL-4, IL-13, and IL-5 in psoriasis cascade. To date, literature reports that TH 17 cell itself and produce IL-17A and IL-23R, which are strongly involved in keratinocytes hyperproliferation in psoriasis, and to date, it is the strong evidence in pathogenesis of psoriasis (Rahman et al. 2015a). Other factors which are involved in pathogenesis of psoriasis are abnormal alteration in eicosanoid and polyunsaturated fatty acids (PUFA) metabolism, free radical generation, lymphokines secretion, and gene expression (Rahman et al. 2015a). Various treatments () that provide short-term suboptimal benefits and severe adverse effects such as body weight gain, hypertension, and osteoporosis, etc. (Rahman et al. 2015a) are available. Thus, these treatment options in psoriasis treatment calls for cost effective and safe nanobullet drug delivery system. An extensive research has been carried on this realm and among all nanobullets; liposomes have gained unique identity in drug delivery (Rahman et al. 2015a). They improves patient compliance by virtue of it biocompatible nature, minimization of dose and lesser adverse effects as antipsoriatic drug delivery (Rahman et al. 2015a). Dithranol is one of the oldest therapies for psoriasis, it get beneficial effect by action on IL-receptor of keratinocyte cell. However, clinically it is not widely accepted, due to having irritation and staining action (Rahman et al. 2015a). To overcome this problem, dithranol loaded liposomes are developed and it has higher topical permeation (Rahman et al. 2015a). In another different study, dithranol loaded liposomes has been applied as an open label randomized double blind trials and compared with 1.15% commercially available dithranol ointment. It gets clear psoriasis plaques by 0.5% lesser dose and provides higher drug permeation by liposomes and it prevents from staining (Agarwal et al. Citation2002). Agarwal et al. (Citation2001) prepared 0.5% dithranol loaded liposomes and niosomes and both received better skin permeation delivery as compared to conventional ointment. Whereas liposomal formulation get enhanced skin permeation over niosomes (Agarwal et al. Citation2001). Methotrexate (MTX) is a potent immunosuppressant and widely used in psoriasis treatment. It acts via blocking the conversion of di-hydrofolate to tetra-hydrofolate (THF). THF is a cofactor for the formation of De-Nova synthesis. Moreover they inhibit aminoimidazole carboxamide ribotide transformylase, which results to accumulation of adenosine and they also act as immunosuppressant action (Ali et al. Citation2008). MTX widely given in psoriasis patient by oral and parenteral routes. However, its application is limited for long duration therapy due to adverse effects including mucosal ulceration, stomatatis, bone marrow suppression, loss of appetite, and cirrhosis (Ali et al. Citation2008). Presently, it is available in topical formulation, but they have limited drug skin permeation which results in producing suboptimal pharmacodynamic effects in psoriasis (Ali et al. Citation2008). To overcome these hiccups, liposomal loaded MTX has been developed and clinically applied on six patients, higher clearance of psoriasis were found and one patient had complete recovery (Ali et al. Citation2008). Recently, Trotta et al. (Citation2002) has been formulated MTX encapsulated liposomes by using dipotassium glycirrhizinate (KG) and when applied on porcine skin, it results to show 3–4 times higher skin permeation over conventional liposomes. Recent research had revealed that higher concentration of cyclic 3,5'-adenosine monophosphate (cAMP) and guanosine 3anosine 3siguanine monophosphate (cGMP) involved in keratinocyte hyperproliferation. Further they also induce TNF-α, IL-12, and IL-23, which actively involved in psoriasis (Iancu et al. Citation1979). Dyphylline is a derivative of theophylline; it acts as cAMP phosphodiastrease inhibitor which readily inactivates cyclic AMP or cGMP (Touitou et al. Citation1992). Limited permeation of dyphylline is a challenge for its effective application (Touitou et al. Citation1992). To overcome the challenge, it is loaded into stealth liposomes to provide enhance circulation time as well as passive targeting. Furthermore, it showed higher permeation comparative to non-PEGylated liposome loaded dyphylline (Touitou et al. Citation1992). Tacrolimus (FK506) is implicated as immunosuppressant in psoriasis. Biologically it is obtained from fermentation broth of Streptomyces tsukubaensis. It acts through by binding on to cytoplasmic receptor which is a FKBP immunophilin and showed reduced T lymphocyte activation (Remitz et al. Citation1999). The major adverse effect associated with tacrolimus is burning sensation, pruritus, and risk of cutaneous infections. Topical formulation is available, but they have limited poor penetration in psoriatic skin. Recently, tacrolimus has been encapsulated into liposomes and found to be higher entrapment efficiency with larger localization of said drug in dermis (Pople and Singh Citation2011). Tamoxifen (TAM) is a prodrug and metabolized into 4-hydroxy-tamoxifen, it bind to DNA and hindered their synthesis. Moreover, they also arrest the keratinocyte cell cycle which remains in the G0 and G1 phase. Their use is limited in psoriasis due to higher side effects and inappropriate permeation (Bhatia et al. Citation2004). To minimizes the problem, it is developed into TAM loaded multilamellar liposomes, further skin permeation studies revealed that 59.87 μg/cm2/h concentration of TAM, whereas solution shows only 21.65 μg/cm2/h (Bhatia et al. Citation2004). In another work, it is encapsulated into flexible vesicle and skin permeation studies found higher permeation and strong inhibition of epidermal keratinocytes cells in psoriasis (Bhatia et al. Citation2014). Temoporfin (mTHPC), is a second generation potent photosensitizer, it has lower skin penetration capability. To improve its pentration ability, recently it is loaded into cationic flexible liposomes (Dragicevic-Curic et al. Citation2009). The presence of cation on lipid allows higher skin permeation as compares to conventional liposomes (Dragicevic-Curic et al. Citation2009). In another studies, it is developed into liposomal gel to receive higher skin permeation (Dragicevic-Curic et al. Citation2010). Recently, the New Jersey Centre for Biomaterials has been develops a new potential topical antipsoriatic drug therapy which named as tyrosine based polymeric nano micelles or called as TyroSpheres (Kilfoyle et al. Citation2012). It delivers multiple drugs such as vitamin D3 analogues, betamethasone dipropionate, and paclitaxel at a time which meant for the purpose of combinational drug targeting in psoriatic drug therapy (Kilfoyle et al. Citation2012). It composed of copolymers of oligomer of desaminotyrosyl tyrosine esters, diacids, and hydrophilic blocks of PEG. It ex vivo study on human cadaver skin were found significant concentration into epidermis of psoriatic skin and result signifies it as antipsoriatic drug targeting potential of this novel system by topical route (Kilfoyle et al. Citation2012). Further these all above examples of liposomes in psoriasis are summarized in .
Table 1. Current pharmacotherapy’s in inflammatory disorders.
Table 2. Therapeutic approaches of liposome as drug delivery in effective treatment of inflammatory disorders.
Rheumatoid arthritis
Rheumatoid arthritis (RA) is a dreadful immune based joint disorder, which posse’s synovitis and significant joint destruction. RA progress by imbalance in immune system, i.e., T-cell activation in synovium and ultimately leads to differentiation in CD4 T-cells; it produces cytokines such as IL-1 and TNF-α (Rahman et al. Citation2015b). These are involved to initiate synovial tissue proliferation, metalloproteinase, and adhesion molecular expression (Rahman et al. Citation2015b) which aggravates the disease progression due to angiogenesis, cartilage destruction, and bone erosion. These play a key role in pathogenesis of RA. Therefore, the present treatment options including non-steroidal anti-inflammatory drugs (NSAIDs) (Rahman et al. Citation2015b), glucocorticoids (GCs), disease-modifying anti-rheumatic drugs (DMARDs), and biological agents, they controls the inflammation and prevent joint damage (). However, there are umpteen serious adverse effects including, hypertension, osteoporosis, weight gain, and fluid retention (Rahman et al. Citation2015b). Moreover, they are available as injection only (Rahman et al. Citation2015b). Emergence of liposome gains a spectacular potential and to achieve early diagnosis of disease and efficacious therapy in RA treatment (Rahman et al. Citation2015b). Further developments in liposomes produces second generation liposome which includes stealth liposomes, cationic liposomes, magneto-liposomes, etc (Rahman et al. Citation2015b). Tremendous literature on liposomal based drug delivery has been available onto experimental animal model and some are under clinical developing stage in RA pharmacotherapy (Rahman et al. Citation2015b). Diclofenac sodium (DFN) is widely used in RA therapy by oral administration. However, they have certain limitations like shorter half life and mucosal irritation (Turker et al. Citation2008). Topical administration reduces the oral side effects, but they suffer from limited permeation of DFN. Recently, it is loaded into liposomes by using of soy lecithin, diethyleneglycol monoethyl ether, and propylene glycol. They help to increase skin permeation of DFN (Turker et al. Citation2008). In another study DFN loaded liposomes surface are expressed with hyaluronan, they specifically target the extracellular matrix, integrins, and hyaluronan receptor, it results to mediate higher anti-inflammatory action in RA (Elron-Gross et al. Citation2009). Other NSAIDs is indomethacin reported for RA treatment. To enhance its therapeutic efficacy it is loaded into PEG liposomes, they found to increase circulation half life, clearance time and moreover higher drug concentration achieved to the inflammatory sites as comparative to conventional liposomes (Srinath et al. Citation2000). MTX is common DMARD for RA treatment. Its adverse effect restricts its use (Rahman et al. Citation2015b). To overcome its adverse effect, recently it is encapsulated into liposomes and shows enhanced retention and 10 times higher suppression of inflammation as compared to alone use of MTX (Foong and Green Citation1993). In another study, MTX has loaded into long circulating liposomes; they provides superior efficacy over conventional liposome (Williams et al. Citation1999). GCs is an old drug for RA therapy, it show anti-inflammatory action by inhibitory action on pro-inflammatory cytokines and chemokines (Treadwell et al. Citation1964). However, they have major adverse effects such as osteoporosis, infection, diabetes mellitus, and cataract (Treadwell et al. Citation1964). Lopez-Garcia et al. (Citation1993) demonstrated that loading of triamcinolone acetonide 21-palmitate (TAC-P) into liposomes and administered through i.a. injection in rabbit arthritic model, enhanced the retention in the joint cavity, and gave higher therapeutic efficacy. Dexamethasone palmitate (DXP) has develops into large and small size liposomes and administered by i.a. in arthritic model of rabbits. Large sized liposomes were found to be six times more intact in synovial fluid as compared to small size DXP loaded liposomes, whereas small size DXP loaded were found to have three times more anti-inflammatory effect as compared to alone DXP (Bonanomi et al. Citation1987). Recently, DXP loaded into PEG liposomes, they allow higher encapsulation and targeting efficiency as with respect to DXP loaded conventional liposomes (Metselaar et al. Citation2003). In another study, prednisolone phosphate (PLP) loaded into liposomes at the dose of 10 mg/ml and administered in adjuvant induced arthritis (AIA) rat model, they were found to enhance pharmacodynamic effect as compared to same dose of PLP alone (Van den Hoven et al. Citation2011). Harigai et al. (Citation2007) develop a novel PEG coated cationic liposomes by use of cationic lipid like as 3,5-dipenta decycloxy benzamidine hydrochloride (TRX-20). This has been utilized for delivery of prednisolone phosphate in RA therapy. They were found to have 40 times more selective targeting to the negative charged chondroitin sulfate which is expressed in sub-endothelial membranes and synoviocytes as compared to conventional liposomes without TRX-20 (Harigai et al. Citation2007). Reactive oxygen species (ROS) is required for homeostasis. Their imbalance leads to overproduction of ROS and generate oxidative stress, and they are strongly implicated in RA (Cruz et al. Citation2005). Superoxide dismutase (SOD) has a shorter biological half life and adverse effects make it unacceptable in RA treatment (Cruz et al. Citation2005). To overcome this problem, an acylated SOD derivative has been employed and loaded into conventional liposomes. It is administered in rat arthritis model results to enhanced suppression of inflammatory event (Cruz et al. Citation2005). Recent advancement has developed SOD loaded PEG liposome, which provide higher encapsulation efficiency. Furthermore, i.v. administration in rat adjuvant arthritis model resulted in greater localization at the inflamed tissue and higher specific targeted action is achieved (Corvo et al. 2005). Other than NSAIDs, DMARD, and biological agents, other alternative is gene therapy. It holds a promising approach in RA treatment by delivering the nucleic acid into the cells and to suppress the protein synthesis. Furthermore, they inhibit the expression of proinflammatory cytokines (Khoury et al. Citation2006). Advancement in gene delivery has developed antisense nucleotides and oligonucleotides loaded liposome. Recently employed small interfering RNA loaded liposomes in collagen-induced arthritis (CIA) mouse model, it act to inhibit TNF expression at mRNA and protein levels and is found enhance therapeutic efficacy (Khoury et al. Citation2006). Oligonucleotides (ODN) is a DNA based sequence and wide importance in RA treatment. However, this molecule has a drawback of non-specific interaction with protein. This major drawback is overcome by use of second generation liposome, such as cationic, anionic, and immunoliposomes (Dass Citation2002). The application of various liposomes in treatment of RA are summarizes in .
Inflammatory bowel disease
Inflammatory bowel disease (IBD) includes Crohn’s disease (CD) and ulcerative colitis (UC). Both have a similar symptom which includes diarrohea, bloody stools, abdominal pain, weight loss, fever, and fatigue while pathogenesis behind two diseases is distinct. CD is dreadful inflammatory disorder where bowel wall is expanded to serosal layer of small to large intestine (Fiocchi Citation2015). Whereas UC affects the innermost mucosa and is restricted to colon and rectum only. In the last few years, several studies in animals found that the pathogenesis of IBD involved T cell immune response (Th1 or Th2 cytokines), bacteria, human genetics, and environmental factors which cause onset or reactivation of diseases (Fiocchi Citation2015). Beside this, anti-tumor necrosis factor are also involved in immunological pathways of IBD (Fiocchi Citation2015). Latest nod-2 gene has identified in CD (Salem et al. Citation2015). The available treatments are 5-aminosalicylic acid and corticosteroids in oral and topical form (), which require higher daily dose and have limited skin permeation (Singh et al. Citation2015). Thus, liposomes have gained spectacular potential to deliver drugs at the controlled rate with specific targeted action at lower dose (Kesisoglou et al. Citation2005). Further IBD have been found to have more paracellular vascular permeability, this phenomenon allows large accumulation of liposome in the affected area. There are various literatures which reported the presence of macrophages and dendritic cells in IBD, moreover these can lead to capture of liposomes at greater extent as compared to tablet and solutions. Thus, size of liposomes is an important aspect for drug delivery in IBD (Kesisoglou et al. Citation2005). Moreover, they are easily uptaken by the macrophages and dendritic cells and receive significant pharmacodynamic effects (Kesisoglou et al. Citation2005). Tacrolimus is an option when corticosteroid treatment option fails. However, tacrolimus have serious adverse effect (Singh et al. Citation2015). To reduce this problem, recently tacrolimus loaded into cationic and anionic liposomes and administered into colitis induced rats and healthy rats (Jubeh et al. Citation2004). Cationic liposomes were found to have three times more adhesion to colonic mucosa of healthy rats, whereas anionic liposome adhered with inflamed mucosa two times more than cationic liposome (Jubeh et al. Citation2004). In another study, superoxide dismutase, 4-aminotempol and catalase has been loaded into anionic liposomes and administered into rat colitis, it achieved prolong residence time, higher uptake, and greater anti-inflammatory action over alone use of said drug molecules (Jubeh et al. Citation2005, Citation2006). All the above-mentioned examples of liposomes in IBD treatment are summarized in .
Conclusion
An inflammatory disorder is a complex disorder encompasses by multifaceted origin in various organ, i.e., cancer, psoriasis, rheumatoid arthritis, and inflammatory bowel disorders. These all have treated well by several therapies with conventional formulation, but they produced suboptimal response with several significant adverse effects. In this particular domain, liposomes gained a spectacular potential to treat immune based inflammatory disorders by overcome to the problems associated with conventional formulation based therapy. These system provides higher specificity for delivering loaded drug to the desired site of action in inflamed loci by various targeting approaches to received better therapeutic effect at low cost.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Abu Lila AS, Kizuki S, Doi Y, Suzuki T, Ishida T, Kiwada H. 2009. Oxaliplatin encapsulated in PEG-coated cationic liposomes induces significant tumor growth suppression via a dual targeting approach in a murine solid tumor model. J Control Release. 137:8–14.
- Agarwal R, Katare OP, Vyas SP. 2001. Preparation and in vitro evaluation of liposomal/niosomal delivery systems for antipsoriatic drug dithranol. Int J Pharm. 228:43–52.
- Agarwal R, Saraswat A, Kaur I, Katare OP, Kumar B. 2002. A novel liposomal formulation of dithranol for psoriasis: preliminary results. J Dermatol. 29:529–532.
- Ahmad J, Amin S, Rahman M, Rub RA, Singhal M, Ahmad MZ, et al. 2015. Solid matrix based lipidic nanoparticles in oral cancer chemotherapy: applications and pharmacokinetics. Curr Drug Metab. 16:633–644.
- Akhter S, Ahmad I, Ahmad MZ, Ramazani F, Singh A, Rahman Z, et al. 2013. Nanomedicines as cancer therapeutics: current status. Curr Cancer Drug Targets. 13:362–378.
- Akhter S, Ahmad MZ, Ahmad FJ, Storm G, Kok RJ. 2012. Gold nanoparticles in theranostic oncology: current state-of-the-art. Expert Opin Drug Deliv. 9:1225–1243.
- Akhter S, Ahmad Z, Singh A, Ahmad I, Rahman M, Anwar M, et al. 2011. Cancer targeted metallic nanoparticle: targeting overview, recent advancement and toxicity concern. Curr Pharm Des. 17:1834–1850.
- Akira I, Yuko K, Hiroyuki H, Kikkawa H, Horiuchi A, Watanabe Y, Kobayashi T. 2004. Magnetite nanoparticle-loaded anti-HER2 in nunoliposome for combination of antibody therapy with hyperthermia. Cancer Lett. 212:167–175.
- Ali MF, Salah M, Rafea M. 2008. Liposomal methotrexate hydrogel for treatment of localized psoriasis: preparation, characterization and laser targeting. Med Sci Monit. 12:I66–I74.
- Alving CR, Kinsky SC, Haxby JA, Kinsky CB. 1969. Antibody binding and complement fixation by a liposomal model membrane. Biochemistry. 8:1582–1587.
- Bangham AD, Horne RW. 1964. Negative staining of phospholipids and their structural modification by surface active agents as observed in the electron microscope. J Mol Biol. 8:660–668.
- Bangham AD, Standish MM, Watkins JC. 1965. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol. 13:238–252.
- Barenholz Y. 2012. Doxil® – the first FDA-approved nano-drug: lessons learned. J Control Release. 160:117–134.
- Bhatia A, Kumar R, Katare OP. 2004. Tamoxifen in topical liposomes: development, characterization and in-vitro evaluation. J Pharm Pharm Sci. 7:252–259.
- Bhatia A, Singh B, Wadhwa S, Raza K, Katare OP. 2014. Novel phospholipid-based topical formulations of tamoxifen: evaluation for antipsoriatic activity using mouse-tail model. Pharm Dev Technol. 19:160–163.
- Bonanomi MH, Velvart M, Stimpel M, Roos KM, Fehr K, Weder HG. 1987. Studies of pharmacokinetics and therapeutic effects of glucocorticoids entrapped in liposomes after intraarticular application in healthy rabbits and in rabbits with antigen-induced arthritis. Rheumatol Int. 7:203–212.
- Carter P, Smith L, Ryan M. 2004. Identification and validation of cell surface antigens for antibody targeting in oncology. Endocr Relat Cancer. 11:659–687.
- Chiu SJ, Liu S, Perrotti D, Marcucci G, Lee RJ. 2006. Efficient delivery of a Bcl-2-specific antisense oligodeoxyribonucleotide (G3139) via transferrin receptor-targeted liposomes. J Control Release. 112:199–207.
- Cho K, Wang X, Nie S, Chen ZG, Shin DM. 2008. Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res. 14:1310–1316.
- Cleland LG, Shandling M, Percy JS, Poznansky MJ. 1979. Liposomes: a new approach to gold therapy? J Rheumatol Suppl. 5:154–163.
- Corvo ML, Boerman OC, Oyen WJ, Jorge JC, Cruz ME, Crommelin DJ, Storm G. 2000. Subcutaneous administration of superoxide dismutase entrapped in long circulating liposomes: in vivo fate and therapeutic activity in an inflammation model. Pharm Res. 17:600–606.
- Cotran RS, Majno G. 1964. The delayed and prolonged vascular leakage in inflammation. I. Topography of the leaking vessels after thermal injury. Am J Pathol. 45:261–281.
- Coussens LM, Werb Z. 2002. Inflammation and cancer. Nature. 420:860–867.
- Cruz ME, Manuela Gaspar M, Barbara M, Martins F, Corvo ML. 2005. Liposomal superoxide dismutases and their use in the treatment of experimental arthritis. Methods Enzymol. 391:395–413.
- Dass CR. 2002. Liposome-mediated delivery of oligodeoxynucleotides in vivo. Drug Deliv. 9:169–180.
- De Silva M, Thomas DP, Hazleman BL, Wraight P. 1979. Liposomes in arthritis: a new approach. Lancet. 1:1320–1322.
- Dragicevic-Curic N, Scheglmann D, Albrecht V, Fahr A. 2009. Development of liposomes containing ethanol for skin delivery of temoporfin: characterization and in vitro penetration studies. Colloids Surf B Biointerfaces. 74:114–122.
- Dragicevic-Curic N, Winter S, Krajisnik D, Stupar M, Milic J, Graefe S, Fahr A. 2010. Stability evaluation of temoporfin-loaded liposomal gels for topical application. J Liposome Res. 20:38–48.
- Eichhorn ME, Becker S, Strieth S, Werner A, Sauer B, Teifel M, et al. 2006. Paclitaxel encapsulated in cationic lipid complexes (MBT-0206) impairs functional tumor vascular properties as detected by dynamic contrast enhanced magnetic resonance imaging. Cancer Biol Ther. 5:89–96.
- Elron-Gross I, Glucksam Y, Margalit R. 2009. Liposomal dexamethasone diclofenac combinations for local osteoarthritis treatment. Int J Pharm. 376:84–91.
- Fiocchi C. 2015. Inflammatory bowel disease pathogenesis: where are we? J Gastroenterol Hepatol. 1:12–18.
- Foong WC, Green KL. 1993. Treatment of antigen-induced arthritis in rabbits with liposome-entrapped methotrexate injected intraarticularly. J Pharm Pharmacol. 45:204–209.
- Fritze A, Hens F, Kimpfler A, Schubert R, Peschka-Süss R. 2006. Remote loading of doxorubicin into liposomes driven by a transmembrane phosphate gradient. Biochim Biophys Acta. 1758:1633–1640.
- Funk M, Schulze B, Guenzi E, Michaelis U, Bohnenkamp H, Eichhorn M, Schmitt-Sody M. 2007. Cationic liposomal preparations for the treatment of rheumatoid arthritis. WO/2007/134819; PCT/EP2007/004467.
- Goren D, Horowitz AT, Zalipsky S, Woodle MC, Yarden Y, Gabizon A. 1996. Targeting of stealth liposomes to erbB-2 (Her/2) receptor: in vitro and in vivo studies. Br J Cancer. 74:1749–1756.
- Harigai T, Hagiwara H, Ogawa Y, Ishizuka T, Kaneda S, Kimura J. 2007. Prednisolone phosphate-containing TRX-20 liposomes inhibit cytokine and chemokine production in human fibroblast-like synovial cells: a novel approach to rheumatoid arthritis therapy. J Pharm Pharmacol. 59:137–143.
- Iancu L, Shneur A, Cohen H. 1979. Trials with xanthine derivatives in systemic treatment of psoriasis. Dermatologica. 159:55–61.
- Immordino ML, Dosio F, Cattel L. 2006. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomed. 1:297–315.
- Jiang L, Li L, He X, Yi Q, He B, Cao J, Pan W, Gu Z. 2015. Overcoming drug-resistant lung cancer by paclitaxel loaded dual-functional liposomes with mitochondrial targeting and pH-response. Biomaterials. 52:126–139.
- Jubeh TT, Antler S, Haupt S, Barenholz Y, Rubinstein A. 2005. Local prevention of oxidative stress in the intestinal epithelium of the rat by adhesive liposomes of superoxide dismutase and tempamine. Mol Pharm. 2:2–11.
- Jubeh TT, Barenholz Y, Rubinstein A. 2004. Differential adhesion of normal and inflamed rat colonic mucosa by charged liposomes. Pharm Res. 21:447–453.
- Jubeh TT, Nadler-Milbauer M, Barenholz Y, Rubinstein A. 2006. Local treatment of experimental colitis in the rat by negatively charged liposomes of catalase, TMN and SOD. J Drug Target 14:155–163.
- Kaasgaard T, Andresen TL. 2010. Liposomal cancer therapy: exploiting tumor characteristics. Expert Opin Drug Deliv. 7:225–243.
- Kesisoglou F, Zhou SY, Niemiec S, Lee JW, Zimmermann EM, Fleisher D. 2005. Liposomal formulations of inflammatory bowel disease drugs: local versus systemic drug delivery in a rat model. Pharm Res. 22:1320–1330.
- Khoury M, Louis-Plence P, Escriou V, Noel D, Largeau C, Cantos C, et al. 2006. Efficient new cationic liposome formulation for systemic delivery of small interfering RNA silencing tumor necrosis factor alpha in experimental arthritis. Arthritis Rheum. 54:1867–1877.
- Kilfoyle BE, Sheihet L, Zhang Z, Laohoo M, Kohn J, Michniak-Kohn BB. 2012. Development of paclitaxel-TyroSpheres for topical skin treatment. J Control Release. 163:18–24.
- Kirpotin D, Park JW, Hong K, Zalipsky S, Li WL, Carter P, Benz CC, Papahadjopoulos D. 1977. Sterically stabilized Anti-HER2 immunoliposomes: design and targeting to human breast cancer cells in vitro. Biochemistry. 36:66–75.
- Lian T, Ho R. 2001. Trends and developments in liposome drug delivery systems. J Pharm Sci. 90:667–680.
- Liu GX, Fang GQ, Xu W. 2014. Dual targeting biomimetic liposomes for paclitaxel/DNA combination cancer treatment. Int J Mol Sci. 15:15287–15303.
- Lopez-Garcia F, Vazquez-Auton JM, Gil F, Latoore R, Moreno F, Villalaín J, Gómez-Fernández JC. 1993. Intra-articular therapy of experimental arthritis with a derivative of triamcinolone acetonide incorporated in liposomes. J Pharm Pharmacol. 45:576–578.
- Lukyanov AN, Elbayoumi TA, Chakilam AR, Torchilin VP. 2004. Tumor-targeted liposomes: doxorubicin-loaded long-circulating liposomes modified with anticancer antibody. J Control Release. 100:135–144.
- Madni A, Sarfraz M, Rehman M, Ahmad M, Akhtar N, Ahmad S, et al. 2014. Liposomal drug delivery: a versatile platform for challenging clinical applications. J Pharm Pharm Sci. 17:401–426.
- Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. 2000. Tumour vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 65:271–284.
- Maeda P. 2001. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul. 41:189–207.
- Metselaar JM, Wauben MH, Wagenaar-Hilbers JP, Boerman OC, Storm G. 2003. Complete remission of experimental arthritis by joint targeting of glucocorticoids with long-circulating liposomes. Arthritis Rheum. 48:2059–2066.
- Mornet S, Vasseur F, Grasset P, Veverka P, Goglio G, Demourgues A. 2006. Magnetic nanoparticle design for medical applications. J Prog Solid State Chem. 34:237–247.
- Nakamura K, Abu Lila AS, Matsunaga M, Doi Y, Ishida T, Kiwada H. 2011. A double-modulation strategy in cancer treatment with a chemotherapeutic agent and siRNA. Mol Ther. 19:2040–2047.
- Noble GT, Stefanick JF, Ashley JD, Kiziltepe T, Bilgicer B. 2014. Ligand-targeted liposome design: challenges and fundamental considerations. Trends Biotechnol. 32:32–45.
- O'Byrne KJ, Thomas AL, Sharma RA, DeCatris M, Shields F, Beare S, Steward WP. 2002. A phase I dose-escalating study of DaunoXome, liposomal daunorubicin, in metastatic breast cancer. Br J Cancer. 87:15–20.
- Olayioye MA, Neve RM, Lane HA, Hynes NE. 2000. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 19:3159–3167.
- Pople PV, Singh KK. 2011. Development and evaluation of colloidal modified nanolipid carrier: application to topical delivery of tacrolimus. Eur J Pharm Biopharm. 79:82–94.
- Rahman M, Ahmad MZ, Kazmi I, Akhter S, Afzal M, Gupta G, et al. 2012a. Advancement in multifunctional nanoparticles for the effective treatment of cancer. Expert Opin Drug Deliv. 9:367–381.
- Rahman M, Ahmad MZ, Kazmi I, Akhter S, Afzal M, Gupta G, Sinha VR. 2012b. Emergence of nanomedicine as cancer targeted magic bullets: recent development and need to address the toxicity apprehension. Curr Drug Discov Technol. 9:319–329.
- Rahman M, Akhter S, Ahmad J, Ahmad MZ, Beg S, Ahmad FJ. 2015a. Nanomedicine-based drug targeting for psoriasis: potentials and emerging trends in nanoscale pharmacotherapy. Expert Opin Drug Deliv. 12:635–652.
- Rahman M, Beg S, Sharma G, Anwar F, Kumar V. 2015b. Emergence of lipid-based vesicular carriers as nanoscale pharmacotherapy in rheumatoid arthritis. Recent Pat Nanomed. 5:111–121.
- Rahman M, Zaki Ahmad M, Kazmi I, Akhter S, Beg S, Gupta G, et al. 2012c. Insight into the biomarkers as the novel anti-psoriatic drug discovery tool: a contemporary viewpoint. Curr Drug Discov Technol. 9:48–62.
- Remitz A, Reitamo S, Erkko P, Granlund H, Lauerma AI. 1999. Tacrolimus ointment improves psoriasis in a microplaque assay. Br J Dermatol. 141:103–107.
- Salem M, Seidelin JB, Eickhardt S, Alhede M, Rogler G, Nielsen OH. 2015. Species-specific engagement of human nucleotide oligomerization domain 2 (NOD)2 and Toll-like receptor (TLR) signalling upon intracellular bacterial infection: role of Crohn's associated NOD2 gene variants. Clin Exp Immunol. 179:426–434.
- Sen K, Mahitosh M. 2013. Second generation liposomal cancer therapeutics: transition from laboratory to clinic. Int J Pharm. 448:28–43.
- Singh D, Srivastava S, Pradhan M, Kanwar JR, Singh MR. 2015. Inflammatory bowel disease: pathogenesis, causative factors, issues, drug treatment strategies, and delivery approaches. Crit Rev Ther Drug Carrier Syst. 32:181–214.
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. 1989. Studies of the HER2/neu protooncogene in human breast and ovarian cancer. Science. 244:707–712.
- Srinath P, Vyas SP, Diwan PV. 2000. Preparation and pharmacodynamic evaluation of liposomes of indomethacin. Drug Dev Ind Pharm. 26:313–321.
- Suzuki R, Takizawa T, Kuwata Y, Mutoh M, Ishiguro N, Utoguchi N, et al. 2008. Effective anti-tumor activity of oxaliplatin encapsulated in transferrin-PEG-liposome. Int J Pharm. 346:143–150.
- Ta T, Porter TM. 2013. Thermosensitive liposomes for localized delivery and triggered release of chemotherapy. J Control Release. 169:112–125.
- Touitou E, Shaco-Ezra N, Dayan N, Jushynski M, Rafaeloff R, Azoury R. 1992. Dyphylline liposomes for delivery to the skin. J Pharm Sci. 81:131–134.
- Treadwell BL, Sever ED, Savage O, Copeman WS. 1964. Side-effects of long-term treatment with corticosteroids and corticotrophin. Lancet. 1:1121–1233.
- Trotta M, Peira E, Debernardi FM, Gallarate M. 2002. Elastic liposomes for skin delivery of dipotassium glycyrrhizinate. Int J Pharm. 241:319–327.
- Turker S, Erdogan S, Ozer Y, Bilgili H, Deveci S. 2008. Enhanced efficacy of diclofenac sodium loaded lipogelosome formulation in intra-articular treatment of rheumatoid arthritis. J Drug Targeting. 16:51–57.
- Van den Hoven JM, Van Tomme SR, Metselaar JM, Nuijen B, Beijnen JH, Storm G. 2011. Liposomal drug formulations in the treatment of rheumatoid arthritis. Mol Pharm. 8:1002–1015.
- Williams AS, Jones SG, Goodfellow RM. 1999. Interleukin-1 (IL-1) inhibition: a possible mechanism for the anti-inflammatory potency of liposomally conjugated methotrexate formulations in arthritis. Br J Pharmacol. 128:234–240.
- Yoo HS, Park TG. 2004. Folate receptor targeted biodegradable polymeric doxorubicin micelles. J Control Release. 96:273–283.
- Yuba E, Tajima N, Yoshizaki Y, Harada A, Hayashi H, Kono K. 2014. Dextran derivative-based pH-sensitive liposomes for cancer immunotherapy. Biomaterials. 35:3091–3101.
- Zhang Y, Kohler N, Zhang MQ. 2002. Surface modification of superparamagnetic magnetite nanoparticles and their intracellular uptake. Biomaterials. 23:1553–1561.
- Zhao YZ, Dai DD, Lu CT, Chen LJ, Lin M, Shen XT, et al. 2013. Epirubicin loaded with propylene glycol liposomes significantly overcomes multidrug resistance in breast cancer. Cancer Lett. 330:74–83.
- Zhou J, Zhao WY, Ma X, Ju RJ, Li XY, Li N, et al. 2013. The anticancer efficacy of paclitaxel liposomes modified with mitochondrial targeting conjugate in resistant lung cancer. Biomaterials. 34:3626–3638.