Abstract
Context Adjuvants are compounds used in the preparation of inactive vaccines to enhance the immune response. Aluminum hydroxide (alum) is one of the first compounds approved by the Food and Drug Administration, which is used as adjuvants in vaccine products for humans. Montanide ISA 70 is an oil-emulsion adjuvant and is used in poultry inactive vaccines. Objective In this study, the effects of alum adjuvant on the efficiency and induction of immune response in inactive vaccines of Influenza and Newcastle are compared with those of ISA 70. Materials and methods Six groups of 7-d-old specific-pathogen-free chickens were inoculated with 0.3 ml of the prepared vaccines via the subcutaneous route in the neck. Immune response in each group after 7, 14, 21, 31, 41, and 45 d was evaluated using the technique of hemagglutination inhibition. Results The results were compared using SPSS software. Results showed that vaccines containing adjuvant ISA 70 depicted a higher increase in the immune response and adjuvant of 20% alum is similar to adjuvant of ISA 70 in boosting the immune system. There was no statistically significant difference between 10% and 20% alum, but these adjuvants are visibly different from ISA 70. Conclusion In conclusion, alum can be used as an easily accessible, harmless, and effective adjuvant; however, to increase the immune period using the inactive vaccines for poultry, more research would be necessary.
Introduction
Influenza (AI) and Newcastle (ND) diseases are known to have a significant economic effect on the poultry industry (Arous et al. Citation2013, Brugh et al. Citation1979). Type A Influenza, subtype H9N2, belongs to the enveloped viruses of Orthomyxoviridae family with negative single-stranded and segmented RNA, which usually causes respiratory infections, decrease in egg production, and death in poultry (Lee et al. Citation2011).
Hemagglutinin (HA) and neuraminidase are important proteins on the envelope that play an important role in inducing a humoral immune response (Chauhan et al. Citation2013). ND virus is classified as Rubulavirus and belongs to the family Paramyxoviridae. The genome of this virus is a single-stranded, linear RNA with the size of 16–20 kb, and unlike that of Orthomyxoviruses, it is not a segmented genome (Alexander Citation2001).
The dangers of the infectious spread of the virus and its transmission to mammals, especially to humans, make it very important to render protection against these viruses (Alexander Citation2001). In Iran’s poultry industry, vaccination of birds is the most effective and the optimum method to fight against these infections.
Studies have shown that because of the side effects of using live vaccines, the inactive vaccines are a better alternative. In this regard and for improvement of the formulation and effectiveness of these vaccines, use of a proper adjuvant is necessary (Bowersock and Martin Citation1999).
Adjuvants are added to vaccines to increase the immune response to antigens. They can induce antibody production and potency, increase the duration of antibody response, and decrease the frequency of vaccination and the amount of necessary antigen in each dose of vaccine (Brito and O’Hagan Citation2014).
Nowadays, adjuvant in the treatment of diseases such as cancer are also used (Kazemi et al. Citation2015). Glenny et al. for the first time in Citation1926, showed the effect of adjuvants of aluminum compounds. Because these adjuvants are safe with no side effects, they were the first to be approved for use in human vaccinations in the United States. Today, these adjuvants are used in vaccine formulations (Arthanari et al. Citation2014). Aluminum salts (Alum) are the first and the most used adjuvants in vaccines, for more than 70 years (Lindblad Citation2004).
The first study using emulsion with Freund’s adjuvant go back to 1940 (Brito and O’Hagan Citation2014). This group containing a mixture of two immiscible materials is mixed together in the presence of one or several emulsifiers or surfactants. Emulsions that are used in vaccines can be categorized into two emulsions, water-in-oil (such as Montanide) or oil-in-water (such as MF59). Although water-in-oil emulsions are used for the long-lasting production of humoral immune response in the inactive poultry vaccines, they are not appropriate to be used in human vaccines, because they cause inflammation at the site of injection (Atmar and Keitel Citation2009).
In this study, adjuvants of alum with concentrations of 10% and 20% and ISA 70 were used to make inactive vaccines of AI and ND and were compared by using antibody measurements. With regard to sanctions on Iran and the problems in the import of montanide, we have to replace it with another material. Considering the price of alum, its easy availability, and its safety, it is suggested as an effective substitute for oil adjuvant of montanide.
Materials and methods
Antigens
AI viruses A/Chicken/Iran/101/1998/H9N2 and ND virus (V4) as antigen were donated by Razi Vaccine and Serum Research Institute.
Adjuvants
Oil adjuvant Montanide ISA 70 product of SEPPIC, Paris, France, was used to make the vaccines. Alum gel was donated by Razi Vaccine and Serum Research institute and levels of 10%, 20%, 30%, and 40% was used in vaccine preparation.
Chickens
In this study, 56 specific-pathogen-free (SPF) 1-week-old chickens were used. The eggs for these chickens were purchased from Venkeys (India) West Hatchery Company.
Preparation of vaccines
Oil adjuvant vaccines
To prepare the oil-in-water emulsion, a homogeny of oil adjuvant of Montanide ISA 70 with the inactive antigen of ND and AI with the ratio of 7:3 is used. The concentration of antigen had a minimum dosage of 108.5 in the aqueous phase (Pour et al. Citation2006).
Aluminum hydroxide gel vaccines
Before the use of alum gel as an adjuvant, first the absorbance capacity of the alum gel for viruses was measured. For this purpose, 10%, 20%, 30%, and 40% concentrations of alum gel in phosphate-buffered saline were prepared with the equal amount of antigen. Hence, after 4 h stirring, the solutions were centrifuged and the supernatants were surveyed by HA technique. All concentrations were of high absorption but in this study, the concentrations of 10% and 20% were used in the preparation of vaccines.
Hemagglutination assay (HA)
This technique has been explained by Masurel and his colleagues in Citation1981 (Rimmelzwaan et al. Citation1998).
Immunization of animals
In the laboratory, the 1-week-old SPF chickens were given 0.3 ml of the vaccines subcutaneously in the neck. Chickens were respectively separated in seven different vaccine groups ().
Table 1. Vaccination groups.
Blood sampling
Blood samples were taken in 7, 14, 21, 31, 41, and 45 d after vaccination. The blood samples were stored in an incubator (37 °C) for 1 h. Serum was separated and stored at 4 °C for HI antibody determination (Kallon et al. Citation2013).
Hemagglutination inhibition assay (HI)
Antibody titer was measured by the HI test. This test was performed as a standard method (Kallon et al. Citation2013).
Statistical analysis
To analyze the data of each group and compare them, Statistical Package for the Social Sciences software (SPSS Inc., Chicago, IL) was used. If P values are less than 0.05, then there is a significant difference between groups.
Results
Optimization of antigens absorption in the aluminum hydroxide gel
Concentrations of 10% and 20% of alum were used to prepare the vaccines. To optimize the time of antigen absorption, supernatants titer were investigated after 4 h by HA test. One hour after stirring, the HA titer of supernatants were zero. Hence, the best time of antigen absorption was 1 h after stirring of 10% and 20% gel for vaccine preparation.
Newcastle vaccines
Immune response of aluminum hydroxide gel vaccines
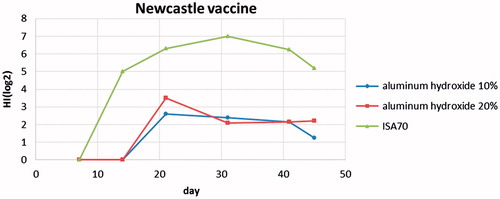
According to the numerical results, comparing the two concentrations of 10% and 20% adjuvant of alum in ND vaccines showed an increase in the immune response [in the test group] after 14 d of inoculation, and there was a sudden increase in the amount of antibody in the blood plasma. In the ND vaccine containing 10% of alum gel, increase in the immune response showed a steady trend and after 41 d of inoculation, this response showed a slight decrease.
In the case of ND vaccine containing 20% of alum, the antibody response was stable even after 21 d of inoculation and also until the last day of blood sampling. In addition, the immune responses at both these time points were slightly more than the immune response elicited by the vaccines containing 10% of alum gel.
Immune response of oil adjuvant vaccine
In the vaccines containing ISA 70 adjuvant, there was a significant increase in the immune response after second blood sampling (day 14 after inoculation). But, this increase was not steady and from day 31 after inoculation, the response decreased ().
Table 2. Immune response of SPF chickens to ND vaccine antibodies measured by HI.
By comparing the results of adjuvants, on a diagram (), it is noticeable that increase of immune response using oil adjuvants is more than the alum adjuvants, but from day 31 of vaccination on, the amount of antibody decreases. On the other hand, in the case of vaccines containing 20% alum adjuvant, immune response stays steady after day 31 of inoculation and until the last blood sampling, there is no decrease in the antibody.
Influenza vaccines
Immune response vaccines with adjuvant of aluminum hydroxide
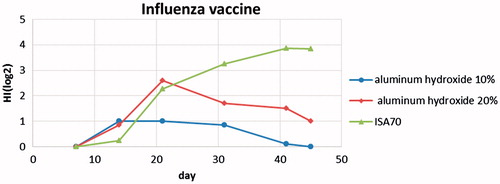
According to the results in , the immune response for AI vaccines containing 10% adjuvant of alum in the second blood sampling (day 14 after inoculation) and in the third blood sampling (day 21 after inoculation) remained the same and in the last blood sampling after day 45, it decreased to zero.
Table 3. Immune response of SPF chickens to AI vaccine antibodies measured by HI.
For AI vaccines containing 20% adjuvant of alum, the level of immune response increased significantly and reached maximum level by day 21 after inoculation, but it decreased after day 31.
Immune response with oil adjuvant vaccine
The AI vaccine containing ISA 70 adjuvant, after second blood sampling (day 14 after inoculation), showed a significant increase in immune response, which remained high until the last blood sampling. Comparison of the results in the figure shows that increase of immune response for ISA 70 adjuvant is similar to that by alum adjuvant of 20% until day 21 after inoculation, but after day 21, the decrease in the antibody was faster for 20% alum adjuvant. Also, the decrease in the antibody for 10% alum adjuvant is more rapid in comparison with 20% alum adjuvant ().
Discussion
AI and ND diseases are very important and every year, they cause heavy financial loss in the poultry industry. To prevent these diseases, it is important to produce inexpensive vaccines.
It has been more than 70 years that adjuvants are being used in vaccines to increase the immune response in host subjects. Adjuvants are additives that increase the antigen’s ability to induce an immune response (Stils Citation2005). In general, one of the important roles of adjuvants is to reduce the necessary amount of antigen in each vaccine dose (Luke and Subbarao Citation2006).
Vogel and Powell had analyzed more than 100 adjuvants in the year Citation1995 (Arous et al. Citation2013). Because most of these adjuvants are hard to produce, expensive, and have poisonous effects, their usage is limited, which were explained in the year Citation2000 by Stewart-Tull (Atmar and Keitel Citation2009).
Alum is one of the first and oldest adjuvants, which is used in producing vaccines for humans and is approved by the Food and Drug Administration (FDA) (Arous et al. Citation2013, Arthanari et al. Citation2014, Luke and Subbarao Citation2006, Vasievich and Huang Citation2011). In the year Citation2000, Gupta and Rost reported that aluminum compounds, especially aluminum phosphate and alum, were used as adjuvants in the production of human vaccines. Adjuvants of aluminum, unlike emulsions, interact with antigens using electrostatic forces in the aqueous and non-aqueous phases. They also interact with antigens by hydrophobic reactions, van der Waals forces, and hydrogen bonds (Stils Citation2005). Another report said alum may not be able to effectively induce the immune system (Arthanari et al. Citation2014).
Mishra et al. investigated that alum-HBsAg formulation induced humoral immunity significantly because the IgG1 was increased in serum (Mishra et al. Citation2014).
In the year Citation1962, Perez-Rebelo used alum in their study on ND virus. In Citation1980, Larghi and Nebel used a concentration of 50% alum gel to absorb Rabies virus. Reid and Blackall in 1987 suggested the use of 25% alum gel for Haemophilus paragallinarum (Gupta et al. Citation1995, Perez-Rebelo Citation1962, Reid and Blackall Citation1987).
In the present study, for antigen absorption of alum, one should bear in mind that all samples of 10%, 20%, 30%, and 40% of alum showed complete absorption in the first hour. Based on the results of this study and previous studies and to reduce the chance of tissue damage at the site of injection, the concentrations of 10% and 20% alum gel were selected for use (Stils Citation2005).
A significant effect of antigen depot was explained by Herbert (Citation1967). The amount of antibody after inoculation of antigen in an emulsion of water-in-oil was compared by him with a daily injection of antigen in saline for over a 1-month period. Although in both groups the amount of antibody was high, in the case of injection of the emulsion, the amount of antibody remained high, unlike the saline group, in which the antibody decreased after the daily injections were stopped (n = 14).
It is said that antigen exists at the site of inoculation up to 7 weeks after the injection. This opinion was experimented by Harrison in the year Citation1935. Considering that all vaccines in this study contained the same dose of vaccines, which means that they had the same amount of antigen, a significant difference in the antibody response for the two vaccinated groups of 10% and 20% alum is visible. It means that increase in the amount of alum increases the immune response. Therefore, according to previous studies and the results of this study, we can assume that the performance of vaccines containing 20% adjuvant of alum is better than the ones containing 10% of alum. This higher performance is based on the increase in the immune response related to a longer period of antigen depot.
Stone et al. compared the immunity of three commercial oil-based vaccines with one vaccine prepared using alum against the ND poultry disease. The immunity produced by oil-based vaccines, in all cases, was significantly higher than that induced by the vaccine containing alum (Stone et al. Citation1980). In addition, in the year 2011, it was reported that oil adjuvants result in better performance compared with alum (Liu et al. Citation2011).
Box and Furminger reported that the HI titer of ND inactivated vaccines containing alum resulted in the maximum in the 4th week after vaccination. Then, the titer was decreased, insofar decline to minimum titer in 12th week after vaccination (Box and Furminger Citation1975).
ND disease vaccines in this study produced consistent results with the concentration of 10% serum-grade alum at 21 d after vaccination (3 weeks), but at day 45 after vaccination, the immune response slightly reduced.
The ND vaccine with 20% of alum gel adjuvant produced antibody titers that were constant from 3 weeks to 45 d after inoculation. Yamanaka in 1993 reported that 2 weeks after vaccination with inactivated ND vaccine containing montanide produced an immune response that is more than eight times the response elicited by the inactivated ND disease vaccine at the same time period. Stone et al. (Citation1980) also reported such consistent results (Yamanaka et al. Citation1993).
In the present study, oil-emulsion of ND vaccine enhanced the immune response in 14 d after inoculation. This response was decreased in 45 d after inoculation. The results show that immune response stimulation rate of oil vaccine of ND disease is more rapid compared with alum gel. However, the titers decreased in both groups at day 45.
Use of the different volume of adjuvant is the reason for this difference. In other words, the alum vaccines containing up to 20% of adjuvant in a vicinal dose, whereas, in oil-emulsion vaccines, the oil-emulsion volume could be up to 70%. Perhaps, one of the reasons for the effectiveness of oil vaccines to stimulate and durability of immune responses is the use of the higher volume of adjuvant in the vaccine combination.
The AI vaccine adjuvant alum (10%) increased the antibody titer at 14 d after inoculation and remained stable until 31 d after inoculation. The flu vaccine adjuvant alum (20%) showed antibody titer at 21 d after inoculation and reached its maximum level. Immune response of AI oil vaccine continued to increase between 14 and 45 d.
According to results, both vaccines simultaneously increased antibody titers but the persistence of titers in the group what vaccinated with AI alum vaccine was lower than oil vaccine. It is another reason for the superiority of oil vaccines.
Statistical analysis of the results showed no significant difference in AI and ND disease vaccine adjuvants alum, 10%, 20%, and ISA 70. However, there was a statistically significant difference between alum and montanide, but as the results show, alum is also able to induce a high antibody response.
The AI vaccine for ND disease vaccine showed similar results, the difference being a weaker immune response to ND vaccine, which is likely due to the lower quality of the AI antigen. The stability of the immune response to AI vaccine oil was seen in all groups. Hence, given the vaccine immune response and the increased longevity is concerned, this can replace the ISA 70 adjuvant alum for use in certain critical times.
A vaccination plan was employed in poultry flocks according to a study conducted in Citation2011 by Lee et al. It was shown that two-dose vaccination program with H9N2 vaccine with alum as adjuvant and adjuvant oil could enhance the immune response, compared with the vaccination program with one dose of vaccination, thus improving the immune response (Lee et al. Citation2011).
This strategy can greatly protect against bird flu in the poultry industry and is also economically effective. Thus, it can be suggested that alum adjuvant and inactivated adjuvant vaccine oil can be used together in poultry.
It can be due to the high price of oil in the adjuvant alum gel vaccine emulsion and low price, it is recommended gel use of vaccines in vaccination programs and to earn higher grades, the percentage increased gel or a booster dose was administered.
Due to the similarity of the numerical results obtained with 20% alum adjuvant and ISA 70 to increase immune, it is suggested that the amount of aluminum in the vaccine formulation is used more than 20%.
Considering that 70% of oil vaccines is adjuvant, so to reduce the dose of 0.3 to 0.1 in under a week-old chickens, suggested ISA 70 replaced that alum. ISA 70 that used in poultry vaccines is an expensive, rare, and imported raw material in Iran. In addition, hi-tech equipment was needed to homogenize it. Therefore, according to the results and with regard to the sanctions against Iran, replacing the adjuvant alum gel adjuvant with cheaper, acceptably efficient, accessible, and non-risk adjuvants (approved by the FDA) is essential.
Conclusion
With regard to sanctions on Iran and the lack of import montanide, alum can be used as an easily accessible, harmless, and effective adjuvant substitute to ISA 70; however, to increase the immune period using the inactive vaccines for poultry, more research would be necessary.
Declaration of interest
This study has been supported by a grant provided from Razi Vaccine and Serum Research Institute, Karaj, Iran. The authors report no declarations of interest.
Acknowledgements
The authors express their gratitude to Dr. Alireza Bahonar and Mahnaz Haddadi who supported the development of this work.
References
- Alexander DJ. 2001. Gordon Memorial Lecture. Newcastle disease. Br Poult Sci. 42:5–22.
- Arous JB, Deville S, Pal J, Baksi S, Bertrand F, Dupuis L. 2013. Reduction of Newcastle disease vaccine dose using a novel adjuvant for cellular immune response in poultry. Proc Vaccinol. 7:28–33.
- Arthanari S, Mani G, Peng MM, Jang HT. 2014. Chitosan-HPMC-blended microspheres as a vaccine carrier for the delivery of tetanus toxoid. Artif Cells Nanomed Biotechnol. [Epub ahead of print]. DOI: 10.3109/21691401.2014.966193.
- Atmar RL, Keitel WA. 2009. Adjuvants for pandemic influenza vaccines. In: Compans RW, Orenstein WA, Eds. Vaccines for Pandemic Influenza, vol. 333. Heidelberg: Springer, pp. 323–344.
- Bowersock TL, Martin S. 1999. Vaccine delivery to animals. Adv Drug Deliv Rev. 38:167–194.
- Box PG, Furminger IG. 1975. Newcastle disease antibody levels in chickens after vaccination with oil emulsion adjuvant killed vaccine. Vet Rec. 96:108–111.
- Brito LA, O’Hagan DT. 2014. Designing and building the next generation of improved vaccine adjuvants. J Control Release. 190:563–579.
- Brugh M, Beard CW, Stone HD. 1979. Immunization of chickens and turkeys against avian influenza with monovalent and polyvalent oil emulsion vaccines. Am J Vet Res. 40:165–169.
- Chauhan N, Narang J, Pundir S, Singh S, Pundir CS. 2013. Laboratory diagnosis of swine flu: a review. Artif Cells Nanomed Biotechnol. 41:189–195.
- Glenny A, Pope C, Waddington H, Wallace U. 1926. Immunological notes. XVII–XXIV. J Pathol Bacteriol. 29:31–40.
- Gupta RK, Rost BE. 2000. Aluminum compounds as vaccine adjuvants. In: O'Hagan DT, Ed. Vaccine Adjuvants, vol. 42. New York: Springer, pp. 65–89.
- Gupta RK, Rost BE, Relyveld E, Siber GR. 1995. Adjuvant properties of aluminum and calcium compounds. In: Powell MF, Newman MJ, Eds. Vaccine Design, vol. 6. New York: Springer US, pp. 229–248.
- Harrison W. 1935. Some observations on the use of alum precipitated diphtheria toxoid. Am J Public Health Nations Health. 25:298–300.
- Herbert W. 1967. Some investigations into the mode of action of the water-in mineral-oil emulsion antigen adjuvants. Proc Int Symp Adjuvants Immun. 6:213–220.
- Kallon S, Li X, Ji J, Chen C, Xi Q, Chang S, et al. 2013. Astragalus polysaccharide enhances immunity and inhibits H9N2 avian influenza virus in vitro and in vivo. J Anim Sci Biotechnol. 4:22.
- Kazemi T, Younesi V, Jadidi-Niaragh F, Yousefi M. 2015. Immunotherapeutic approaches for cancer therapy: an updated review. Artif Cells Nanomed Biotechnol. [Epub ahead of print]. DOI: 10.3109/21691401.2015.1019669.
- Larghi OP, Nebel A. 1980. Rabies virus inactivation by binary ethylenimine: new method for inactivated vaccine production. J Clin Microbiol. 11:120–122.
- Lee DH, Kwon JS, Lee HJ, Lee YN, Hur W, Hong YH, et al. 2011. Inactivated H9N2 avian influenza virus vaccine with gel-primed and mineral oil-boosted regimen could produce improved immune response in broiler breeders. Poult Sci. 90:1020–1022.
- Lindblad EB. 2004. Aluminium compounds for use in vaccines. Immunol Cell Biol. 82:497.
- Liu CG, Liu M, Liu F, Liu da DF, Zhang Y, Pan WQ, et al. 2011. Evaluation of several adjuvants in avian influenza vaccine to chickens and ducks. Virol J. 8:321.
- Luke CJ, Subbarao K. 2006. Vaccines for pandemic influenza. Emerg Infect Dis. 12:66.
- Masurel N, Ophof P, De Jong P. 1981. Antibody response to immunization with influenza A/USSR/77 (H1N1) virus in young individuals primed or unprimed for A/New Jersey/76 (H1N1) virus. J Hygiene. 87:201–209.
- Mishra N, Khatri K, Gupta M, Vyas SP. 2014. Development and characterization of LTA-appended chitosan nanoparticles for mucosal immunization against hepatitis B. Artif Cells Nanomed Biotechnol. 42:245–255.
- Perez-Rebelo R. 1962. Immunity study of a new diluted formalin-inactivated Newcastle disease vaccine. Avian Dis. 6:284–289.
- Pour M, Momayez R, Akhavizadegan M. 2006. The efficacy of inactivated oil-emulsion H9N2 avian influenza vaccine. Iran J Vet Res. 7:85–88.
- Reid GG, Blackall PJ. 1987. Comparison of adjuvants for an inactivated infectious coryza vaccine. Avian Dis. 31:59–63.
- Rimmelzwaan GF, Baars M, Claas EC, Osterhaus AD. 1998. Comparison of RNA hybridization, hemagglutination assay, titration of infectious virus and immunofluorescence as methods for monitoring influenza virus replication in vitro. J Virol Methods. 74:57–66.
- Stewart-Tull DE. 2000. Harmful and beneficial activities of immunological adjuvants. In: O'Hagan DT, Ed. Vaccine Adjuvants, vol. 42. New York: Springer, pp. 29–48.
- Stils HF Jr. 2005. Adjuvants and antibody production: dispelling the myths associated with Freund’s complete and other adjuvants. ILAR J. 46:280–293.
- Stone H, Brugh M, Erickson G, Beard C. 1980. Evaluation of inactivated Newcastle disease oil-emulsion vaccines. Avian Dis. 24:99–111.
- Vasievich EA, Huang L. 2011. The suppressive tumor microenvironment: a challenge in cancer immunotherapy. Mol Pharma. 8:635–641.
- Vogel FR, Powell MF, Alving CR. 1995. A compendium of vaccine adjuvants and excipients. Vaccine Design: Subunit Adjuvant Approach. 6:141–228.
- Yamanaka M, Okabe T, Nakai M, Goto N. 1993. Local pathological reactions and immune response of chickens to ISA-70 and other adjuvants containing Newcastle disease virus antigen. Avian Dis. 73:459–466.