Abstract
The aim of this study was to develop nanofibrous gelatin substrates for eyelid fat stem cell (EFSC) expansion that can serve as a potential alternative substrate to replace human amniotic membrane. Biocompatibility results indicated that all substrates were highly biocompatible, as EFSCs could favorably attach and proliferate on the nanofibrous surfaces. Microscopic figures showed that the EFSC were firmly anchored to the substrates and were able to retain a normal stem cell phenotype. Immunocytochemistry (ICC) and real time-PCR results revealed change in the expression profile of EFSCs grown on nanofibrous substrates when compared to those grown on control in epithelial induction condition. In addition, electrospun gelatin mats especially oriented scaffold provides not only a milieu supporting EFSCs expansion, but also serves as a useful alternative carrier for ocular surface tissue engineering and could be used as an alternative substrate to amniotic membrane.
Introduction
Corneal transplantation is a way to replace scar tissue using the full or partial thickness of a donor’s central cornea (Tan et al. Citation2012), but it fails to regenerate limbal stem cells. Moreover, the donor source and graft rejection are major limitations of corneal transplantations (Pleyer and Schlickeiser Citation2009). Stem cell transplantation is a new therapeutic strategy for corneal tissue regeneration that relies on their multipotency. Among stem cells, only mesenchymal stem cells (MSCs) possess the immunomodulatory ability and are well-tolerated during allogenic transplantation (English and Mahon Citation2011, Kolli et al. Citation2010, Rama et al. Citation2010, Satake et al. Citation2011, Tanioka et al. Citation2006, Tseng and Tsai Citation1991). Orbital fat-derived stem cells (OFSCs) are MSCs isolated from human orbital fat tissue (Dominici et al. Citation2006, Ho et al. Citation2011). The safety and immunomodulatory ability of systemic OFSC transplantation has been demonstrated in previously xenotransplant model (Chien et al. Citation2012, Lee et al. Citation2004). One of the major problems associated with stem cell therapy remains the absence of a suitable carrier for the transfer of stem cells to precise tissue locations. So far, various materials and scaffolds have been tested for the transportation of stem cells. To treat severe ocular surface damage and a deficiency in limbal stem cells (LSCs), which are irreplaceable for corneal healing, various carriers for the culturing of LSCs and for their transplantation onto the recipient eye have been tested (Biazar Citation2013, Hartgerink et al. Citation2002, Rama et al. Citation2001, Schwab et al. Citation2006, Tsai et al. Citation2000, Zhang et al. Citation2013). In natural tissues, cells are surrounded by extracellular matrix (ECM), which has physical structural features ranging from nanometer to micrometer scale. Hence, a nano-structured porous structure and a large surface area are needed as an alternative to natural ECM. To mimic the natural ECM, many research groups tried to fabricate nanofibrous scaffold by different methods (Biazar et al. Citation2015) like electrospinning (Agrawal and Ray Citation2001). Gelatin is biodegradable, biocompatible and displays many integrin binding sites for cell adhesion and differentiation. For these reasons, gelatin is widely used in the pharmaceutical and medical fields in a variety of applications, including tissue engineering, wound dressing, drug delivery, and gene therapy. Electrospinning processes can fabricate nanofibers with a diameter ranging from a few tens to hundreds of nanometers and with a defined porosity. The three-dimensional structure of nanofibrous materials has an extremely large surface area, and nanofibers can mimic the structure of ECM proteins, which provide support for cell growth and function (Biazar and Heidari Citation2013a, Citation2013b, Citation2015, Biazar and Sahebalzamanil Citation2014, Biazar et al. Citation2010, Heidari et al. Citation2014, Hosseinkazemi et al. Citation2015, Montazeri et al. Citation2011, Sahebalzamani et al. Citation2015). Eyelid fat derived stem cells (EFDSCs) offer many advantages for tissue engineering strategies over stem cells from other sources and ideal carrier materials have to be identified for them. The aim of this study was to demonstrate and to compare the effects of clinically established biomaterial on proliferation and differentiation activity of human EFDSCs in vitro. In this study, oriented and non-oriented nanofibrous gelatin scaffolds were fabricated by electrospinning method. The samples were evaluated by scanning electron microscope (SEM) analysis and in vitro assays, then loaded with EFDSCs, then investigated by microscopic analyses, cellular investigations, and other markers at the RNA level as well as the expressed proteins.
Materials and methods
Materials and scaffold preparation
In this work, we successfully designed nanofibrous gelatin mat. Gelatin (Sigma Co., St. Louis, MO) from porcine skin was dissolved in 60/40 vol.% acetic acid (Sigma Co., St. Louis, MO)/distilled water at a concentration of 30% (w/v). The solution was stirred at 50 °C for 60 min and then 60 mg of genipin (Sigma Co., St. Louis, MO) was dissolved in 0.5 ml of ethanol and 1 ml of 0.75 M phosphate-buffered saline (PBS), pH 7.4, were added to 10 ml of the gelatin solution. About 30 min after genipin addition, the mixture was electrospun (Panzavolta et al. Citation2011). Electrospinning apparatus (Nano-Azma Company, Tehran, Iran) used in this study prepared with two collectors for fabrication of non-oriented and oriented nanofibrous mats. A positive high voltage source through a wire was applied at the tip of a syringe needle and a strong electric field (24 kV) was generated between the solution and the collector. As soon as the electric field reached a critical value with increasing voltage, mutual charge repulsion overcame the surface tension of the polymer solution and an electrically charged jet was ejected from the tip of a conical shape as the Taylor cone. Ultrafine fibers are formed by narrowing the ejected jet fluid as it undergoes increasing surface charge density due to the evaporation of the solvent. Electrospun nanofibrous mats were carefully detached from the collector and dried in vacuum for two days at room temperature to remove the solvent molecules completely. Mats were soaked in 5% (w/v) genipin solution in ethanol for 7 d at 37 °C. Subsequently the mats were dried overnight at 45 °C, then rinsed in ethanol and dried again. The surface characteristics of non-oriented and oriented fibers were studied by SEM (TScan, VEGA, Czech) to analyze the changes in the surface morphology.
Cellular analyses
Human eyelid adipose samples were obtained with informed consent from six patients aged between 25 and 37 years undergoing eyelid cosmetic surgery, at the Labbafinejad Hospital of Beheshti University (Tehran, Iran). All experiments were approved by the Institutional Review Board of Beheshti University. Adipose tissues were surgically dissected from the subcutaneous zone, cut into 1–2 mm3 pieces and washed three times with PBS. The tissue fragments were digested with 0.25% collagenase (Sigma-Aldrich Inc., St. Louis, MO) overnight at 37 °C. Following centrifugation at 400 g for 10 min, cell pellets were washed twice in DMEM-low glucose type (Gibco, Brigg, UK). Cell suspensions were cultured in DMEM/Ham’s F12 (50%:50% v/v), supplemented with 10% fetal bovine serum (FBS; Gibco, Brigg, UK) and 1% penicillin–streptomycin (Gibco, Brigg, UK)at 5% CO2 and 37 °C. Fresh medium was replaced every 3 d (Ho et al. Citation2011).
After two weeks in culture, adherent cells were obtained and subjected to serial passage. Cells between passages 2 and 7 were utilized for further studies. Flow cytometry analysis was used for evaluation of stem cell surface markers. The cell mixture was passed through a nylon mesh; 100 μl of the mixture was added to each tube besides the following antibodies: anti-CD105, anti-CD166, anti-CD90, and anti-CD34, anti-CD45, anti-CD44, anti-CD73, anti-CD31 (all products from Abcam, Cambridge, UK). Tubes were incubated at 4 °C in a dark room for 45 min. After the washing process, the cells were fixed in 100 μl of 1% paraformaldehyde in PBS before flow cytometric analysis was carried out. Becton Dickenson device was utilized and analysis was performed by flowing software 2.5.1 (BD Company, Franklin Lakes, NJ). Adipogenesis was induced by supplementation with 0.5 mM dexamethasone, 0.5 mM isobutylmethylxanthine, 50 mM indomethacin, and 10 mg/ml insulin to the culture medium. Osteogenesis was induced by supplementation with 100 nM dexamethasone, 10 mM b-glycerophosphate, and 50 mM ascorbat-2-phosphate to the culture medium. The gelatin nanofibrous mats were cut into four pieces with side lengths of 7 mm and placed into a 24-well cell culture plate (Nunc, Germany). EFDSCs of the second passage were seeded onto the gelatin nanofibrous mats (1 × 105 cells/well). Three milliliters of cell culture medium was added to each well. For cell proliferation assay, human EFDSCs were seeded in 96-well cell culture plates (Nunc, Germany) and mats in 20 ml of DMEM at a concentration of 5000 cells/well. After 24 h of culture in a humidified atmosphere with 5% CO2 at 37 °C, medium was removed and replaced with 100 µl of eluate of each biomaterial. Cells cultured in culture medium served as controls. After 24 h incubation, proliferation was assessed with the aid of a MTT Cell Proliferation Kit (Sigma, Germany) according to the manufacturer’s protocol. Ten microliters of the MTT labeling reagent at the final concentration of 0.5 mg/ml was added to each well and incubated for 4 h in a humidified atmosphere with 5% CO2 at 37 °C. Then, 100 µl of solubilization solution was added into each well and kept overnight in incubator in a humidified atmosphere with 5% CO2 at 37 °C. Absorbance was measured at 570 nm. Five samples were performed for each biomaterial. For SEM study, the scaffolds with cells were washed by PBS, and then fixed by glutaraldehyde (2.5%) at 4 °C for 2 h. The samples were dehydrated by methanol (20% [5 min] → 40% [5 min] → 60% [5 min] → 80% [5 min] → 100% [30 s]) and then kept with tetraoxide osmium vapors at 4 °C for 2 h. The samples were kept in desiccator, coated with gold, and investigated by an SEM (TScan, VEGA, Czech).
Total RNA was isolated from the cells using an RNA extraction kit (Fermentas International, Burlington, Canada). RNA samples were treated with DNase I (Fermentas International, Burlington, Canada) in order to avoid the genomic DNA contamination. RNA quantity was assessed by spectrophotometry (NanoDrop; Thermo, Wilmington, DE). For Reverse transcription 2 µg of total RNA was used with the Revert Aid-first strand cDNA synthesis kit (Fermentas International, Burlington, Canada). RNA extracted from limbus tissue was used as a positive control. Real time-PCR (Rotor-Gene Q Real-Time PCR System, Qiagen, Valencia, CA) reaction was performed with SYBR® Premix Ex Taq™ (Takara Bio, Ink, Shiga, Japan) which uses Taq Fast DNA Polymerase, SYBR Green I dye to detect double-stranded DNA. The reaction was performed with following program; 5 min of 95 °C for enzyme activation, initial denaturation for 20 s at 95 °C, annealing temperature for 40 s, and extension at 72 °C for 1 min, followed by 40 cycles with a final extension at 72 °C. The final stage comprises the analysis of the melt curve through a denaturing step (15′′ at 95 °C) followed by annealing (1′ at 60 °C) and ramping to 95 °C with 0.3 °C increment/step. Levels of mRNA for tested genes were quantified using ΔΔCT method and normalized against human β-actin as a housekeeping gene. Data were expressed as Log 10 mean. Statistical analysis was performed using ANOVA. A P value 0.05 was considered to be significant. The level of candidate genes in different sample types was compared by the Fisher LSD test. For immunocytochemistry (ICC) assay, human eyelid adipose-derived stem cells cultured in 24-well chamber slides were washed with PBS. After endogenous peroxidase activity was inactivated by 3% hydrogen peroxide, the slides were incubated in a blocking solution, consisting of 1% bovine serum albumin (BSA; Gibco, Grand Island, NY) for 1 h at room temperature. Between each steps, cells were washed with PBS three times. The cells were then incubated with primary antibodies () overnight at 4 °C. After being labeled with primary antibody, the cells were then incubated with FITC-conjugated goat antimouse or anti-rabbit IgG (BD Pharmingen, San Diego, CA) respectively for 2 h. Immunoreactive cells were visualized by florescent microscopy (Ceti, London, UK). For airlifting cultured test, the second passage cells (P2) were seeded into six-well plates and on the gelatin oriented scaffold, cultured in media (DMEM/Ham’s F12 and 10% FBS) for 14 d and then exposed to air by lowering the medium level (airlifting) for 14 d to promote corneal epithelial differentiation as described (Maurice Citation1970). After airlifting, the EFDSCs were analyzed by real time-PCR for K3, K12 and Connexin 43 was carried out.
Table 1. Primer sequences
Results
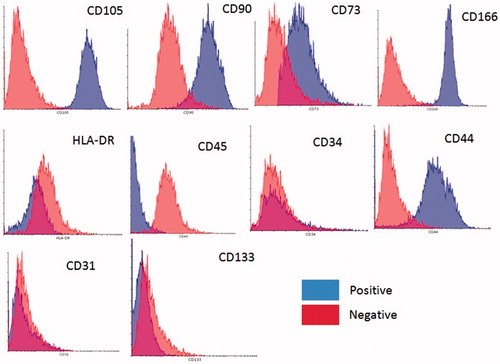
Karyotype analysis was performed on eyelid stem cells at passage 2. All analyzed cells had a normal chromosome karyotype of 46XX. After seven passages continuous culture, the karyotype of EFDSCs that had been cultured on nanofibrous mats were normal 46XX (). The immunophenotype of the isolated stem cell cultures was investigated via flow cytometry. All cells were highly positive for the surface antigens CD166, CD73, CD90, CD44, and CD105. On the contrary, cells were negative for CD34, CD133, CD31, and CD45 ().
Figure 1. Karyotype analysis of eyelid fat derived stem cells (EFDSCs) cultured on nanofibrous substrate. All stem cells at seven passages represented a normal 46XX karyotype.
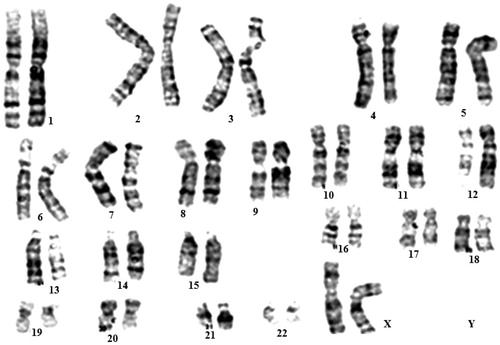
Figure 2. Flow cytometry analysis of eyelid fat derived stem cells (EFDSCs) for surface markers CD34, CD45, CD166, CD73, CD44, CD105, CD90, and CD31. The blue color indicates positives and red one indicates negatives.
Corneal transplantation is a way to replace scar tissue using the full or partial thickness of a donor’s central cornea (Tan et al. Citation2012), but it fails to regenerate LSCs. Moreover, the donor source and graft rejection are major limitations of corneal transplantations (Pleyer and Schlickeiser Citation2009). Recently, replacement of the corneal epithelium under a limbal cell deficiency is achieved by ex vivo cultured cells, including LSCs (Kolli et al. Citation2010, Tseng and Tsai Citation1991), conjunctival epithelial cells (Rama et al. Citation2010, Tanioka et al. Citation2006), and oral mucosal cells (Satake et al. Citation2011). However, long-term graft survival is always a challenge with autologous conjunctival or oral epithelial cell transplantation due to lack of stem cell properties of those cells. Stem cell transplantation is a new therapeutic strategy for corneal tissue regeneration that relies on their multipotency. Wound healing is essential for tissue regeneration. However, the cornea is the window of the eye and the transparency of the cornea determines visual acuity. Corneal tissues are physiologically composed of the corneal epithelium, stroma, and endothelium. Clarity of the cornea depends on an intact corneal epithelium, tight packing of epithelial cells, constant water content, and regular arrangement of keratocytes and keratocyte produced ECM in the stroma (Kao et al. Citation2006, Maurice Citation1970, Müller et al. Citation1995). Advances in tissue engineering techniques now provide an alternative to overcome the limitation of limbal tissue available for transplantation. Pellegrini et al. first showed that the corneal progenitor cells located in the limbus can be cultured to generate cohesive sheets of authentic corneal epithelium, and that cultured corneal epithelium can effectively restore the diseased corneal surface (Pellegrini et al. Citation1997). It is reported that preparation of the human amniotic membrane may influence the phenotype of the cultured limbal epithelial cells. Grueterich et al. (Grueterich et al. Citation2002) have demonstrated that the culture of LESCs on amniotic membrane with an intact amniotic epithelium may result in a more stem-cell-like phenotype than de-epithelialized amnion. Human amniotic membrane is currently the most commonly used substrate for LESCs cultivation and transplantation (Fatimah et al. Citation2010, Nakamura et al. Citation2006, Tosi et al. Citation2005). Although the results are quite promising, amniotic membrane does have some short comings. One of the major issues is ensuring the biosafety of human amniotic membrane in disease transmission, e.g. HIV, hepatitis B and C as well as from bacteria and fungus which will grow readily on human amniotic membrane. Thus, procuring and storing human amniotic membrane is a serious concern. Nanotechnology has the potential to solve above-mentioned problems by fabricating desired biocompatible materials to construct a functional tissue engineered ocular surface. This technology is based on the same principle as performed with human amniotic membrane as a substrate. Various extracellular matrices have been used previously for stem cell expansion such as fibrin, collagen scaffold, temperature responsive cell culture surfaces, human anterior capsule, natural and synthetic scaffolds, etc. (Baradaran-Rafii et al. Citation2015a, Citation2015b, Selvam et al. Citation2006). In one of the studies (Sharma et al. Citation2011), researchers have proposed poly-ɛ-caprolactone (PCL), which is a synthetic aliphatic polyesters bioresorbable and biocompatible as an excellent and biocompatible scaffold for LSC expansion. In ophthalmic application, poly-ɛ-caprolactone has already been explored as a carrier (Sharma et al. Citation2011) due to its in vivo biocompatibility as it does not induce any immunological reactions after degradation. In the initial part of this test, the EFDSCs were cultured on an oriented and non-oriented gelatin nanofibrous scaffolds (). For potential application in corneal epithelial transplantation, membranes had to be cut out in desired size. We cultivated stem cells on nanofibrous mats because they could adhere and proliferate well on scaffold. Images captured by confocal and SEM microscopic systems in figures, cultured stem cells on nanofibrous mats spread out well and display physiologic phenotype of epithelial cells (). These results suggested that nanofibrous mats, especially oriented nanofibers, were able to support the function of the stem cells in vitro. The major findings in these experiments illustrated that gelatin nanofibrous mats successfully allowed cell adherence and improved phenotypic expressions. Since our final goal is to develop carrier sheet, the establishment of animal model for corneal epithelial transplantation may be required in future studies.
Figure 3. Human EFSCs after osteogenic and adipogenic differentiation in vitro. Control or undifferentiated EFSCs are not reddish, whereas the differentiated EFSCs derived osteoblast show vast extracellular calcium deposits, stained in Alizarin reds (A) and lipid droplets are visible in EFSCs adipogenic test culture (B) but not in control (C) after staining with Oil-Red-O indicating that EFSCs are undergoing adipogenic differentiation. (D) Transcription of the adipocyte-specific markers PPARγ2 and osteoblast specific markers; Osteocalsin, Osteopontin. Relative levels of mRNA expression are shown as means ± SEM of three independent experiments.
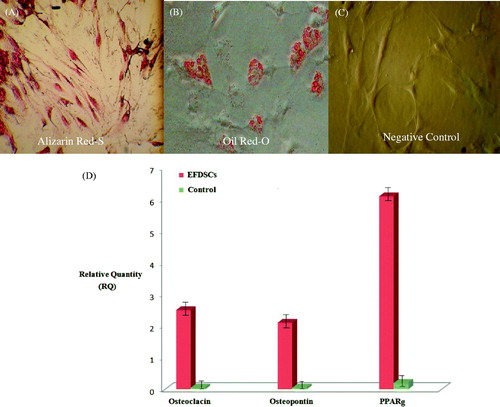
Figure 4. SEM images of designed mats: (A) non-oriented gelatin nanofibers and (B) oriented gelatin nanofibers. Cultured stem cells before induction airlifting on control (TCPS) surface (C); after induction airlifting on oriented gelatin nanofibrous mat (D); and on non-oriented mat (E); inverted microscopic images of cultured stem cells; before induction airlifting on control (TCPS) surface (F); culture on oriented gelatin nanofibrous mat (G); and non-oriented mat (H); after induction airlifting on oriented gelatin nanofibrous mat (I); and on non-oriented (J).
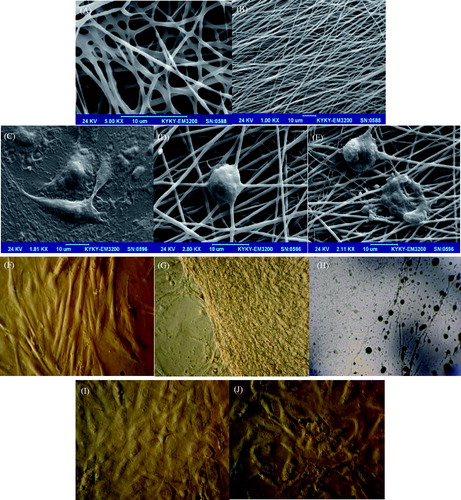
Figure 5. The live/dead assay result for rate of proliferation of EFSCs on nanofibrous mats at 24, 48 and 72 h continuous culture.
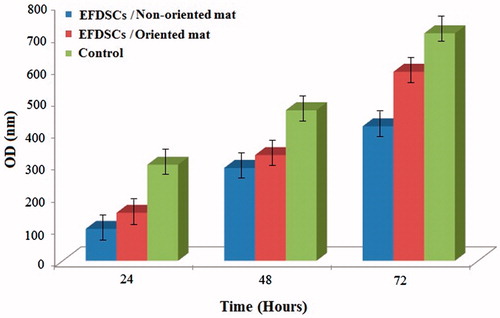
Figure 6. Immunocytochemistry of KRT3, KRT12, Connexin 43, DAPI and negative control (NC) for eyelid fat derived stem cells (EFDSCs) after culture on the (A) non-oriented nanofibrous gelatin mat, (B) oriented nanofibrous gelatin mat, and (C) control (TCPS).
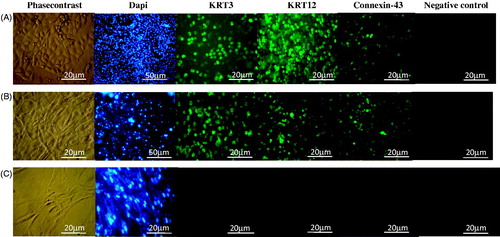
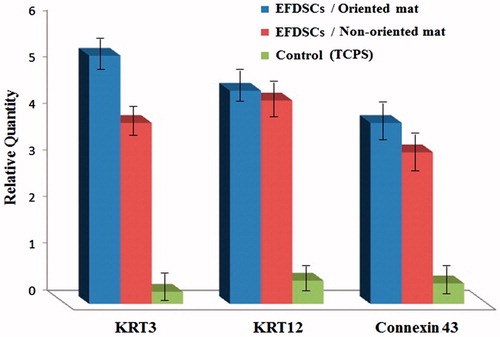
Figure 7. Expression level of KRT3, KRT12, Connexin 43, negative control (NC) for eyelid fat derived stem cells (EFDSCs) after culture on the (A) non-oriented nanofibrous gelatin mat, (B) oriented nanofibrous gelatin mat, and (C) control (TCPS) after 15 d induction by air lifting method (P < 0.001).
Conclusion
In conclusion, our study showed that EFDSCs were able to proliferate in vitro. These cells, when cultured with oriented and non-oriented gelatin nanofibrous scaffolds all cells maintained the features of stem cells. It is further suggested that this culture system would be useful for the clinical application of cell culture as well as the study of stem cell mechanisms.
Funding information
This study was funded by Ophthalmic Research Center grant.
Acknowledgements
We are grateful to the Labbafinejad Hospital for providing the human limbal tissue.
Disclosure statement
The authors state no conflict of interest and have received no payment in preparation of this manuscript.
References
- Agrawal CM, Ray RB. 2001. Biodegradable polymeric scaffolds for musculoskeletal tissue engineering. J Biomed Mater Res. 55:141–150.
- Baradaran-Rafii A, Biazar E, Keshel SH. 2015a. Cellular response of limbal stem cells on poly(hydroxybuthyrate-co-hydroxyvalerate) porous scaffolds for ocular surface bioengineering. Int J Polym Mater Polym Biomater. 64:815–821.
- Baradaran-Rafii A, Biazar E, Keshel SH. 2015b. Cellular response of limbal stem cells on polycaprolactone nanofibrous scaffolds for ocular epithelial regeneration. Curr Eye Res. [Epub ahead of print]. doi: 10.3109/02713683.2015.1019004.
- Biazar E. 2013. Use of umbilical cord and cord blood-derived stem cells for tissue repair and regeneration. Expert Opin Biol Ther. 13:1653–1662.
- Biazar E, Heidari SK. 2013a. Chitosan-cross-linked nanofibrous PHBV nerve guide for rat sciatic nerve regeneration across a defect bridge. ASAIO J. 59:651–659.
- Biazar E, Heidari SK. 2013b. The healing effect of stem cells loaded in nanofibrous scaffolds on full thickness skin defects. J Biomed Nanotechnol. 9:1471–1482.
- Biazar E, Heidari SK. 2015. Electrospun poly (3-hydroxybutyrate-co-3-hydroxyvalerate)/hydroxyapatite scaffold with unrestricted somatic stem cells for bone regeneration. ASAIO J. 61:357–365.
- Biazar E, Sahebalzamanil A. 2014. Modification of poly caprolactone nanofibrous mat by laminin protein and its cellular study. J Biomater Tissue Eng. 4:20–27.
- Biazar E, Zhang Z, Heidari S. 2010. Cellular orientation on micro-patterned biocompatible PHBV film. J Paramed Sci. 1:74–77.
- Biazar E, Heidari S, Rezaei M, Jahandideh R. 2015. Bone reconstruction in rat calvarial defects by chitosan/hydroxyapatite nanoparticles scaffold loaded with unrestricted somatic stem cells. Artif Cells Nanomed Biotechnol. 43:112–116.
- Chien MH, Bien MY, Ku CC, Chang YC, Pao HY, Yang YL, et al. 2012. Systemic human orbital fat-derived stem/stromal cell transplantation ameliorates acute inflammation in lipopolysaccharide induced acute lung injury. Crit Care Med. 40:1245–1253.
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. 2006. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 8:315–317.
- English K, Mahon BP. 2011. Allogeneic mesenchymal stem cells: agents of immune modulation. J Cell Biochem. 112:1963–1968.
- Fatimah SS, Ng SL, Chua KH, Hayati AR, Tan AE, Chin Tan GC. 2010. Value of human amniotic epithelial cells in tissue engineering for cornea. Hum Cell. 23:141–151.
- Grueterich M, Espana EM, Touhami A, Ti SE, Tseng SC. 2002. Phenotypic study of a case with successful transplantation of ex vivo expanded human limbal epithelium for unilateral total limbal stem cell deficiency. Ophthalmology. 109:1547–1552.
- Hartgerink JD, Beniash E, Stupp SI. 2002. Peptide-amphiphile nanofibers: a versatile scaffold for the preparation of selfassembling materials. Proc Natl Acad Sci. 99:5133–5138.
- Heidari S, Biazar E, Rezaei M, Rahmati M, Ronaghi A, Ebrahimi M, et al. 2014. The healing effect of unrestricted somatic stem cells loaded in collagen-modified nanofibrous PHBV scaffold on full-thickness skin defects. Artif Cells Nanomed Biotechnol. 42:210–216.
- Ho JH, Ma WH, Tseng TC, Chen YF, Chen MH, Lee OK. 2011. Isolation and characterization of multi-potent stem cells from human orbital fat tissues. Tissue Eng Part A. 17:255–266.
- Hosseinkazemi H, Biazar E, Bonakdar S, Ebadi MT, Shokrgozar MA, Rabiee M. 2015. Modification of PCL electrospun nanofibrous mat with Calendula officinalis extract for improved interaction with cells. Int J Polym Mater Polym Biomater. 64:459–464.
- Kao WW, Funderburgh JL, Xia Y, Liu CY, Conrad GW. 2006. Focus on molecules: lumican. Exp Eye Res. 82:3–4.
- Kolli S, Ahmad S, Lako M, Figueiredo F. 2010. Successful clinical implementation of corneal epithelial stem cell therapy for treatment of unilateral limbal stem cell deficiency. Stem Cells. 28:597–610.
- Lee KD, Kuo TK, Whang-Peng J, Chung YF, Lin CT, Chou SH, et al. 2004. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology. 40:1275–1284.
- Maurice DM. 1970. The transparency of the corneal stroma. Vision Res. 10:107–108.
- Montazeri M, Rashidi N, Biazar E, Rad H, Sahebalzamani M, Heidari S, Majdi A. 2011. Compatibility of cardiac muscle cells on coated-gelatin electro-spun polyhydroxybutyrate-valerate nano fibrous film. Biosci Biotech Res Asia. 8:515–521.
- Müller LJ, Pels L, Vrensen GF. 1995. Novel aspects of the ultrastructural organization of human corneal keratocytes. Invest Ophthalmol Vis Sci. 36:2557–2567.
- Nakamura T, Inatomi T, Sotozono C. 2006. Transplantation of autologous serum-derived cultivated corneal epithelial equivalents for the treatment of severe ocular surface disease. Ophthalmology. 113:1765–1772.
- Panzavolta S, Gioffrè M, Focarete ML, Gualandi C, Foroni L, Bigi A. 2011. Electrospun gelatin nanofibers: optimization of genipin cross-linking to preserve fiber morphology after exposure to water. Acta Biomater. 7:1702–1709.
- Pellegrini G, Traverso CE, Franzi AT. 1997. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 349:990–993.
- Pleyer U, Schlickeiser S. 2009. The taming of the shrew? The immunology of corneal transplantation. Acta Ophthalmol. 87:488–497.
- Rama P, Bonini S, Lambiase A, Golisano O, Paterna P, De Luca M, Pellegrini G. 2001. Autologous fibrin-cultured limbal stem cells permanently restore the corneal surface of patients with total limbal stem cell deficiency. Transplantation. 72:1478–1485.
- Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, Pellegrini G. 2010. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 363:147–155.
- Sahebalzamani M, Biazar E, Shahrezaei M, Hosseinkazemi H, Rahiminavaie H. 2015. Surface modification of PHBV nanofibrous mat by laminin protein and its cellular study. Int J Polym Mater Polym Biomater. 64:149–154.
- Satake Y, Higa K, Tsubota K, Shimazaki J. 2011. Long-term outcome of cultivated oral mucosal epithelial sheet transplantation in treatment of total limbal stem cell deficiency. Ophthalmology. 118:1524–1530.
- Schwab IR, Johnson NT, Harkim DG. 2006. Inherent risks associated with manufacture of bioengineered ocular surface tissue. Arch Ophthalmol. 124:1734–1740.
- Selvam S, Thomas PB, Yiu SC. 2006. Tissue engineering: current and future approaches to ocular surface reconstruction. Ocul Surf. 4:120–136.
- Sharma S, Mohanty S, Gupta D, Jassal M, Agrawal AK, Tandon R. 2011. Cellular response of limbal epithelial cells on electrospun poly-ɛ-caprolactone nanofibrous scaffolds for ocular surface bioengineering: a preliminary in vitro study. Mol Vis. 17:2898–2910.
- Tan DT, Dart JK, Holland EJ, Kinoshita S. 2012. Corneal transplantation. Lancet. 379:1749–1761.
- Tanioka H, Kawasaki S, Yamasaki K, Ang LP, Koizumi N, Nakamura T, et al. 2006. Establishment of a cultivated human conjunctival epithelium as an alternative tissue source for Autologous corneal epithelial transplantation. Invest Ophthalmol Vis Sci. 47:3820–3827.
- Tosi GM, Massaro-Giordano M, Caporossi A, Toti P. 2005. Amniotic membrane transplantation in ocular surface disorders. J Cell Physiol. 202:849–851.
- Tsai RJ, Li LM, Chen JK. 2000. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N Engl J Med. 343:86–93.
- Tseng SCG, Tsai RJF. 1991. Limbal transplantation for ocular surface reconstruction – a review. Fortschr Ophthalmol. 88:236–242.
- Zhang W, Wang P, Wang Y, Fu W, Pua X, Zhang F, et al. 2013. Development of a cross-linked polysaccharide of Ligusticum wallichii – squid skin collagen scaffold fabrication; and property studies for tissue-engineering applications. Int J Polym Mater Polym Biomater. 63:65–72.
