?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Strong base anion exchange resin (e.g., BL-300) is currently used to eliminate bilirubin from blood in clinical hemoperfusion. However, there is a potential weakness of the resin to remove heparin and activation of coagulation. To overcome this weakness, we prepared a novel non-ionic macroporous polystyrene adsorbent (SZ-9) with rich mesopores and high surface area by suspension polymerization. Test results indicated that SZ-9 performed better adsorption capacity for bilirubin than BL-300. In addition, better blood compatibility was observed during whole blood hemoperfusion. Therefore, adsorbent SZ-9 might be an efficient alternative to anion exchange resin for the removal of bilirubin in hemoperfusion.
Introduction
Liver failure, whether fulminant or acute-on-chronic often is life-threatening and usually associated with high morbidity and mortality (Williams et al. Citation1993). One of the major factors leading to the deaths of patients with acute liver failure (ALF) is a highly reduced detoxification capability of hepatocytes. Toxins and especially protein-bound toxins such as bilirubin significantly accumulate in the blood, and cause further damage to liver, leading to more severe hepatic dysfunction and even death (Zaki et al. Citation1983). Currently, the accepted therapy for end-stage liver failure is liver transplantation (Hartley and Kelly Citation2010). However, the shortage of available donor organs is prompting scientists to look for alternative methods. Therefore, in the past several decades, various liver assist devices have been introduced to either sustain liver function long enough to permit the organ’s regeneration and functional recovery or to bridge patients to transplantation (Morimoto et al. Citation1989, Usami et al. Citation1995).
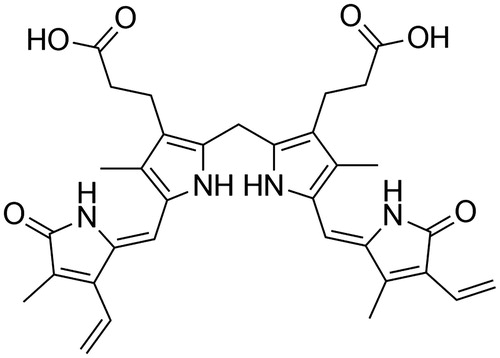
Dialysis techniques can remove small water-soluble metabolites in the extracorporeal circulation system of the patient, but fail to remove albumin-bound toxins efficiently (Duan et al. Citation2006). Adsorption technology is often used to remove protein-bound toxins. Many adsorbents that form the core of the extracorporeal detoxification systems have already been developed, such as activated carbon (Annesini et al. Citation2008, Chirito et al. Citation1977), copolymer of styrene and divinylbenzene, and anion exchange resins (Chen et al. Citation2009, Jia et al. Citation2005, Piemonte et al. Citation2010, Yu Citation2013). Bilirubin is a negatively charged pigment with carboxyl groups linked to the molecule () (Odell Citation1959, Stocker et al. Citation1987). Theoretically, adsorbents with a positive charge should have a better adsorption capability for bilirubin than those without. Many results show that strong base anion exchange resins bind bilirubin with high efficiency. However, there are indications that positively changed adsorbent also remove anticoagulation agents like heparin and citrate (they are anionic), and thus lead to disturbances of the coagulation system (Meijers et al. Citation2007). At the same time, ion exchange resins can also cause disorder of blood electrolytes. Alternatively, non-ionic polymers may have better biocompatible properties than anion exchange resins in liver support therapy.
There are also many studies on the use of non-ionic adsorbents for removing bilirubin in plasma, however, they are not widely used in clinical practice (Annesini et al. Citation2005, Costanzo et al. Citation2010, Weber et al. Citation2008). The reason is that their removal capacity of albumin-bound toxins in plasma is much lower than the ionic adsorbents (i.e. BL-300). Metabolites associated with liver failure such as bilirubin and bile acids are hydrophobic compounds, which are bound to albumin with hydration diameters of about 7 nm (He and Carter Citation1992). It was proven that an appropriate pore size of an adsorbent is a key factor to facilitate the adsorption of albumin-bound toxins and that a large surface area is crucial for achieving a large quantity of adsorbed toxins. For efficient removal of albumin-bound toxins in plasma, an excellent adsorbent should possess a large surface area with a well-developed pore structure (Müller Citation2010).
To the best of our knowledge, non-ionic adsorbents require an appropriate pore size which can efficiently adsorb albumin-bound toxins, has to be 2–6 times the size of hydrated albumin, so that it can be transported freely in the pores of the adsorbent (He Citation1995). At the same time, a high surface area is crucially needed in order to increase the quantity of adsorbed toxins. Based on the above hypothesis, we developed a series of non-ionic adsorbents with well-developed pore structure and high surface area and tested for adsorption of bilirubin and cholic acid. Furthermore, an important aim of this study was to compare systematically the adsorption characteristics of non-ionic resins, for albumin-bound toxins related to liver failure, with that of anion exchange resins. Our experimental results indicate that non-ionic styrene–divinylbenzene copolymer is an excellent adsorbent for albumin-bound toxins and might be an efficient alternative to anion exchange resins in extracorporeal liver support.
Methods
Chemicals and plasma
Bilirubin and cholic acid were purchased from Sigma-Aldrich (St. Louis, MO), and bovine serum albumin fraction V was from Unite Stars (Tianjin, China). Human plasma with high levels of bilirubin from liver failure patients was provided by Tianjin First Central Hospital (Tianjin, China) and used with the patient’s permission. Medisorba BL-300 (Kuraray Medical, Osaka, Japan), a typical commercial anion exchange resin that is currently used to remove bilirubin in clinic, and adopted as a contrast in the study.
Preparation of the adsorbent
Non-ionic styrene divinylbenzene copolymer was prepared by suspension polymerization in the presence of an inert solvent (porogen) (Kun and Kunin Citation1968). Pore size and particle size were controlled by a variation of the porogen and modulator. The resulting adsorbent particles were of spherical shape in the size range from 0.3 to 1.0 mm, as confirmed by optical and scanning electron microscopy. After polymerization, the adsorbent signified as SZ-9 was purified in a chromatographic column by subsequent washing with water, acetone and ethyl alcohol. Then, it was thoroughly washed in distilled water, to remove any remaining solvent and stored in distilled water at 4 °C for further use.
Analysis of specific surface area and pore size distribution
The morphology and microstructure of the adsorbent was analyzed by scanning electron microscope (SEM, QUANTA 200, Oregon). SEM images were obtained by drying the adsorbent beads in vacuum at 120–130 °C for 12 h. The N2 adsorption/desorption isotherms were measured using an Autosorb-Quantachrome NOVA 1200 surface area analyzer (Quantachrome, Boynton Beach, FL) at liquid nitrogen temperature (77 K). Specific surface areas were calculated by Brunauer–Emmett–Teller (BET) method using adsorption isotherms and pore size distributions were calculated by Barrett–Joyner–Halenda (BJH) method using desorption isotherms (Barrett et al. Citation1951, Brunauer et al. Citation1938).
Assessment of adsorption characteristics in static batch experiments
Bilirubin and cholic acid solutions were prepared in phosphate buffer solution (PBS). Due to their poor solubility in water, bilirubin powder was initially dissolved in a small volume of DMSO and 0.1 M NaOH, cholic acid powder was dissolved in 3% ethanol solution, and then added to PBS. In order to simulate the state of bilirubin in plasma, albumin was added to the above PBS-bilirubin solution and stirred for 60 min at room temperature to allow the binding of bilirubin to albumin. Since bilirubin is light-sensitive, all bilirubin adsorption experiments were carried out in a dark room, and the solution was used immediately after preparation.
Prior to use for adsorption experiments, the adsorbent was swelled fully in 0.9% saline solution. Aliquots (wet volume) of adsorbent were incubated with appropriate volumes of the adsorbate solutions at 37 °C with constant shaking for 2 h. In order to study the adsorption properties and reaction mechanism of SZ-9, adsorption kinetics, adsorption isotherms, and the effect of microenvironment on adsorption of bilirubin were studied.
Dynamic adsorption experiments
Dynamic adsorption device was assembled with aliquots of 4 mL of adsorbent (wet beads volume). The adsorbate (human plasma) was pumped through the column using a peristaltic pump. After adding 50 mL of human plasma with high level bilirubin to the device, dynamic adsorption was conducted (Yamazaki et al. Citation1979). Blood chemistry analyses were performed in the Teda Hospital of Tianjin which included electrolytes, proteins, lipids, and enzymes, etc.
Quantitative analysis of bilirubin and cholic acid
Bilirubin analysis was conducted according to the literature (Wang et al. Citation2007). Briefly, test sample was reacted with diazo reagents and the color solution formed was measured photometrically. The cholic acid concentration was determined quantitatively by spectrophotometer at 387 nm after 1 mL of samples reacted with 6.5 mL of 45% sulfuric acid solution in a hot water bath (70 °C) for 1 h (Wang et al. Citation2007).
Blood compatibility
Hemolysis and blood routine analysis were conducted to evaluate the blood compatibility of the adsorbent. Fresh blood from healthy rabbits, was heparinized by adding 2% potassium oxalate and diluted with 0.9% NaCl solution to produce the red blood cells suspension. For hemolysis assays, the red blood cells(RBC) suspensions (0.1 mL for each sample) were mixed with:(a) 5 mL 0.9% NaCl solution as a negative control; (b) 5 mL deionized water as a positive control; (c) 5 mL 0.9% NaCl solution and 2 g adsorbents. After incubating all the tubes at 37 °C for 60 min, blood cells were removed by centrifugation (2500 rpm) and the supernatants were evaluated at 545 nm for the release of hemoglobin (Guo et al. Citation2010). Experiments were performed in triplicate.
In order to study the influence of adsorbents on blood components, fresh anticoagulant blood from healthy rabbits (2 mL for each sample) were mixed with different adsorbents (200 mg for each sample), respectively (Sideman et al. Citation1983). All the adsorbents were fully swelled overnight in physiological saline. After incubating at 37 °C for 60 min, blood cells were separated. Platelet, erythrocyte, and leukocyte counts were measured using an Automatic Blood Cell Analyzer BC2600 (Shenzhen, China).
Results
Characterization of adsorbents
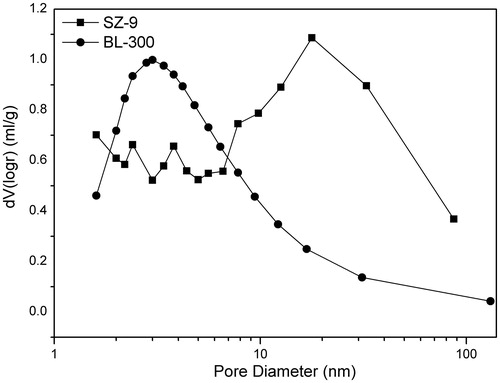
shows SZ-9 has a higher specific surface area (794.9 m2/g) and larger average pore diameter (4.882 nm) than that of BL-300. The BJH pore size distribution of BL-300 () exhibits a concentrated distribution (a relative narrow peak) at about 3 nm, while SZ-9 shows a peak at about 20 nm which is much wider than BL-300. Furthermore, SZ-9 also has a wide distribution of pores smaller than 10 nm, indicating the adsorbent has a medium amount of micropores. A close analysis of the pore distribution curve, we can easily conclude that the SZ-9 adsorbent mainly contains mesopores from a range of 10–50 nm with a medium amount of micropores and some macropores. The high pore volume (0.933 cc/g) and water content (74.38%) of SZ-9 () indicate the adsorbent is highly porous.
Table 1. Physical properties of adsorbents.
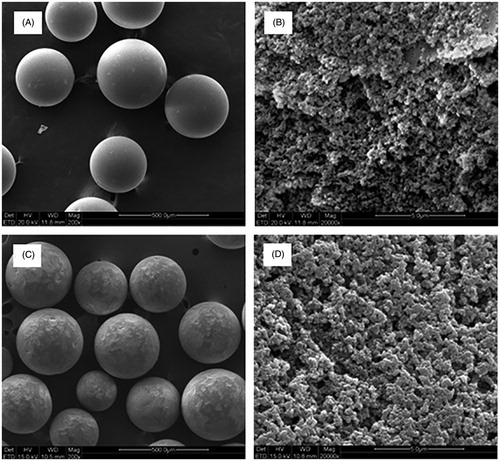
Scanning electron microscopic (SEM) images of adsorbents are shown in . SZ-9 and BL-300 are spherical in shape with a highly porous structure. As these results were derived from the pore size of the adsorbents determined in the dry state, the pores may be larger when swelled in practical use.
Assessment of adsorption characteristics
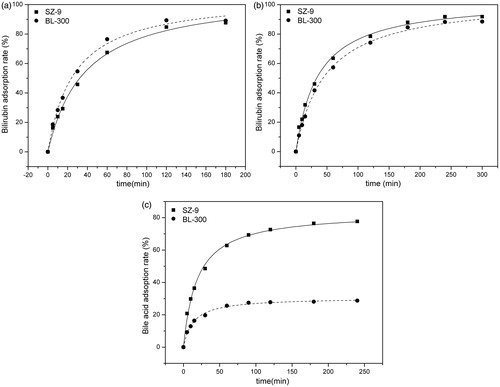
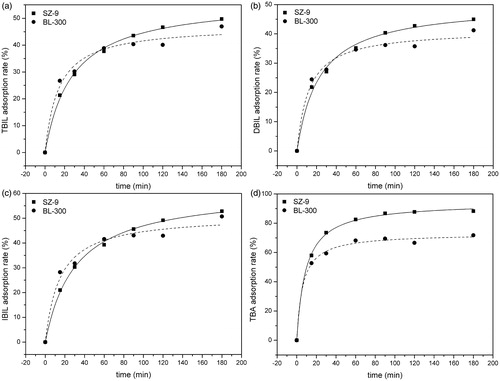
The time-dependent adsorption kinetics of bilirubin and cholic acid are shown in . The curves demonstrate that, in simulated serum solution, the uptake of bilirubin by BL-300 and SZ-9 was quite rapid in the beginning and then slowed down with progress of time. Adsorption equilibrium was reached after 120 min. The bilirubin adsorption rate reached 88%, and adsorption capacity was 4.75 mg/mL. By comparing with BL-300, SZ-9 shows excellent adsorption properties either in PBS or in simulated plasma solution. shows that the adsorption capacity of SZ-9 for cholic acidis much higher than that of BL-300.
Figure 4. Adsorption kinetics of absorbents. (a) Bilirubin adsorption in BSA solution: CBIL = 150 mg/L, CBSA = 15 g/L, T = 37 °C; the adsorbent to solution ratio was 1:36 (v/v). (b) Bilirubin adsorption in phosphate buffer solution: CBIL = 150 mg/L, T = 37 °C; the adsorbent to solution ratio was 1:210 (v/v). (c) Bile acid adsorption in phosphate buffer solution: CBA = 150 mg/L, T = 37 °C; the adsorbent to solution ratio was 1:75 (v/v).
The effect of microenvironment on bilirubin adsorption property of different adsorbents is shown in . Temperature has a certain effect on the adsorption property of the two adsorbents, however, the effect on the adsorption performance of SZ-9 is more obvious than that of BL-300. It may reflect that, the adsorption mechanism of BL-300 on bilirubin is mainly electrostatic (Davies et al. Citation1990).
Figure 5. Effect of microenvironment on adsorption of bilirubin. The adsorbent to solution ratio was 1:36 (v/v). (a) Effect of temperature CBIL = 150 mg/L, CBSA = 15 g/L, t = 2 h. (b) Effect of ionic strength in solution. CBIL = 150 mg/L, CBSA = 15 g/L, T = 37 °C, t = 2 h. (c) Effect of albumin in adsorption medium. CBIL = 150 mg/L, T = 37 °C, t = 2 h.
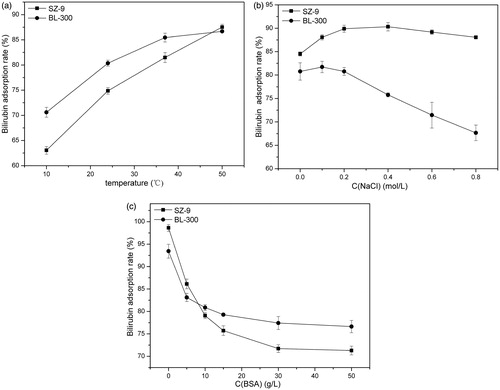
The bilirubin adsorption rate of BL-300 decreased with the increase of ionic strength in solution, while that of SZ-9 shows almost no change or slightly increased (). This result indicates that adsorption property of BL-300 for bilirubin is mainly dependent on the electrostatic interaction. Since bilirubin molecule is amphiphilic, the increase of ionic strength in the solution would promote to a smaller extent the hydrophobic interaction between the bilirubin molecule and the resin (Zangi et al. Citation2007). Therefore, the adsorption rate of SZ-9 for bilirubin increased slightly.
Serum albumin naturally binds to the bilirubin molecule via specific amino-acid residues of albumin. The presence of albumin obviously leads to the decrease of the adsorption capacity of SZ-9 and BL-300 resins for bilirubin () (Davies et al. Citation1990). We speculate that albumin and resin compete for adsorption of bilirubin by occupying the binding sites on the resin. Adsorption rate of the adsorbents for bilirubin levels off when its concentration reached 15 g/L.
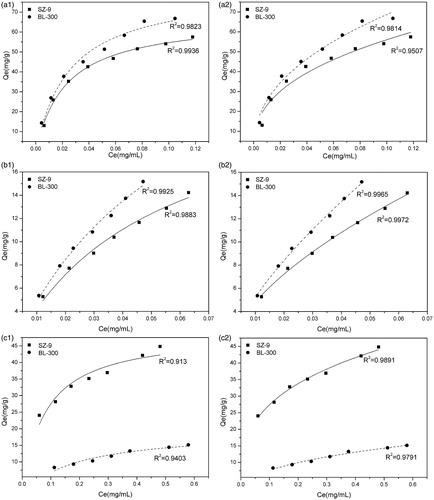
The curves of the equilibrium adsorption amount versus the equilibrium concentration of two adsorbents for bilirubin and cholic acid at 37 °C are shown in . It can be observed that the equilibrium adsorption amount increases with the increasing of the equilibrium concentration of bilirubin and cholic acid, either in phosphate buffer solution or in BSA solution. Two theoretical isotherm models were used to fit the experimental data: the Langmuir and the Freundlich model. The Langmuir model is formulated for monolayer adsorption, the Freundlich isotherm model is usually adopted for multilayer adsorption. Both isotherms are represented in EquationEquations (1)(1)
(1) and Equation(2)
(2)
(2) , respectively.
(1)
(1)
(2)
(2) where Ce stands for the equilibrium concentration (mg/mL), Qe for the adsorption quantity of adsorbents, qm for the maximum adsorption capacity, KL, KF and n are the constants. Non-linear fitting is performed, and R2 (shown in ) stands for the matching degree between the given isotherm models and data. The data fitting with the Langmuir isotherm in suggests that the adsorption of adsorbents towards bilirubin in phosphate buffer solution is a typical monolayer adsorption. In contrarst, the results of data fitting in show that the adsorption of adsorbents towards bilirubin in BSA solution and those towards cholic acid in phosphate buffer solution incline to multilayer adsorption.
Figure 6. Adsorption isotherm of adsorbents. Temperature: 37 °C, a1–c1: fitting data with the Langmuir isotherm, a2–c2: fitting data with the Freundlich isotherm. (a) Adsorption isotherm of adsorbents for bilirubin in phosphate buffer solution. (b) Adsorption isotherm of adsorbents for bilirubin in BSA solution. (c) Adsorption isotherm of adsorbents for bile acid in phosphate buffer solution.
Assessment of adsorption characteristics in plasma
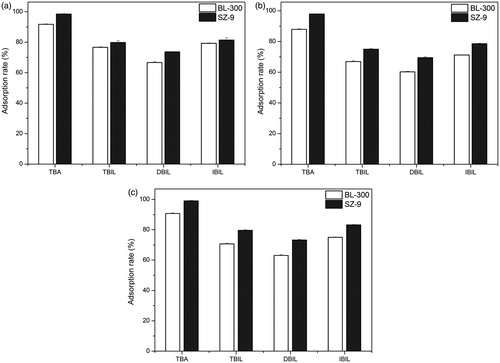
The adsorption performances of the two adsorbents in three high levels (25.3, 178.1, and 258 μmol/L, respectively) of bilirubin are shown in . Results indicate that BL-300 and SZ-9 both have a high adsorption capacity for bilirubin (including direct bilirubin and indirect bilirubin), and SZ-9 performed better results than BL-300, even in very high bilirubin concentrations. Moreover, in terms of total bile acid adsorption, the adsorption rate of SZ-9 was higher than that of BL-300, which is another advantage of SZ-9.
Figure 7. Effect of different concentrations of plasma from patients on adsorption. T = 37 °C, t = 3 h. The adsorbent to plasma ratio was 1:5 (v/v). (a) Initial plasma (L): CTBIL = 25.3 μmol/L, CDBIL = 5.3 μmol/L, CIBIL = 20 μmol/L, CTBA = 25.4 μmol/L. (b) Initial plasma (M): CTBIL = 178.1 μmol/L, CDBIL = 69.1 μmol/L, CIBIL = 109 μmol/L, CTBA = 130.7 μmol/L. (c) Initial plasma (H): CTBIL = 258 μmol/L, CDBIL = 93.1 μmol/L, CIBIL = 164.9 μmol/L, CTBA = 62.6 μmol/L.
Dynamic adsorption experiments
Dynamic adsorption experiments in vitro were conducted to simulate blood perfusion in clinic and to investigate the dynamic adsorption properties of adsorbents. The dynamic adsorption curves () indicate a fast and efficient removal of excess bilirubin and bile acids by SZ-9 and BL-300 in the beginning of perfusion. The total bilirubin adsorption rate of SZ-9 was 2.8% higher than that of BL-300. The adsorption rate of SZ-9 for bile acid was almost 20% higher than that of BL-300, which was consistent with the results in static adsorption experiments. These results are associated with the well-developed pore structure of SZ-9 for efficient removal of serum toxins in liver failure.
Figure 8. Dynamic adsorption in vitro. T = 37 °C; the adsorbent to solution ratio was 1:12.5 (v/v). Initial plasma: CTBIL = 180.8 μmol/L, CDBIL = 71.6 μmol/L, CIBIL = 109.2 μmol/L, CTBA = 117.6 μmol/L.
The results for various plasma samples before and after dynamic adsorption are presented in . The concentrations of Na+ and K+ changed slightly, and the concentration of chloride ions in the plasma in contact with BL-300 increased significantly. The slight depletion of uric acid, urea and creatinine has no clinical mal effects and is definitely tolerable. In terms of the treatment effectiveness of adsorbents for acute liver failure, SZ-9 can remove 49.72% of bilirubin and 88.27% of bile acid, which is about 4 and 7% respectively higher than BL-300.
Table 2. Blood chemistry of plasma before and after dynamic adsorption in vitro.
Blood compatibility
The extent of hemolysis is manifested as hemolysis rate (%) using the following equation.
(3)
(3)
The mean optical density (OD) values of negative and positive controls were 0.0273 ± 0.0015 and 0.9307 ± 0.0015, respectively, and the hemolysis rates of adsorbent samples are shown in . Hemolysis rate of BL-300 is about 11%, and that of SZ-9 is very low. After 60 min contact with blood, ion exchange microspheres cling together and the blood platelet depletion was evidently high. On the other hand, there was almost no platelet adhesion on SZ-9, which indicates no clotting problem for whole blood perfusion. No significant changes were observed for WBC and RBC for SZ-9 adsorbent.
Table 3. Blood routine and hemolysis assay after 60 min contact with adsorbents.
Discussion
Extracorporeal liver support systems have been developed rapidly in the past decades. Polymeric adsorbents that form the core of these systems are used to remove hydrophobic, especially albumin-bound substances that accumulate in the blood during liver failure. Adsorbents used in liver support systems are selective and eliminate substances which have the same physicochemical properties. For example, the anion exchange resin with a large number of quarternary ammonium groups (positively charged) can remove bilirubin via two carboxyl groups (negatively charged) in its molecule. Therefore, adsorbents with positive charge although can eliminate efficiently negatively charged toxic substances like bilirubin, but at the same time, it can remove other valuable substances with negative charge, for example most coagulation factors with negative characteristic at physiological pH, can also be removed during perfusion which may result in a disturbed equilibrium in the body. In the context of liver failure, alterations of the coagulation cascade are particularly relevant, as blood coagulation is impaired in patients with liver failure due to reduced synthesis of coagulation factors in the liver. This is one of the disadvantages or shortcoming of using ionic adsorbent in liver failure treatment, so non-ionic adsorbent is preferred. Beside the functional groups on the adsorbent that play an important role in liver failure detoxification, the physical structure of the adsorbent is also crucially important.
We assumed that adsorbents should have an appropriate pore size to allow albumin-bound substances like bilirubin to move freely in the pores of the adsorbent. At the same time, the adsorbent should possess a high surface area in order to adsorb a large quantity of toxins. In this study, we aimed at the modulation of pore structure and specific area of non-ionic styrene divinylbenzene copolymers by monitoring the degree of crosslinking and using different kinds of porogenic agents. A serious of styrene divinylbenzene copolymers (data not shown) were prepared, and found that SZ-9 represents a new family of non-ionic styrene divinylbenzene copolymers which not only possess a well-developed micro-, meso-, and macropore structure, but also have a large specific surface area. As we showed that SZ-9 is an excellent adsorbent for removal of albumin-bound toxins, moreover, it has higher removal efficiency for less tightly bound albumin toxins such as bile acids.
From the preparation point of view, SZ-9 could be used directly after suspension polymerization while anion exchange resin with functional groups still need a series of chemical reactions after polymerization, i.e., chloromethylation and amination reactions (Nakaji and Hayashi Citation2003). In order to reduce the non-specific adsorption and to improve the blood compatibility of the adsorbent, BL-300 also need coating with a hydrophilic polymer, like poly(2-hydroxyethyl) methacrylate (Nakaji and Hayashi Citation2003). Therefore, the complicated production process of ion exchange resins result in its high cost, and not all patients can afford its expensive treatments. SZ-9 has a much lower production costs than BL-300, which would benefit more to liver failure patients if it is applied to clinics in future.
Bilirubin is bound to albumin with an association constant of 9.5 × 107 M − 1, and the albumin molecule in solution is an ellipsoid of 4 × 14 nm (Jacobsen and Brodersen Citation1983). Hence, bilirubin adsorption kinetics in BSA solution and in phosphate buffer solution was investigated, respectively. In vitro, adsorption kinetic data demonstrated that albumin-bound bilirubin or free bilirubin could be efficiently removed by SZ-9 and BL-300. Adsorption rate of SZ-9 for bilirubin reached 88% which is 3.3% higher than that of BL-300, and its adsorption capacity is 4.75 mg/mL. SZ-9 reached adsorption equilibrium within 120 min, which is in line with the clinical treatment time of hemoperfusion. Furthermore, SZ-9 shows a significantly higher adsorption capacity for bile acids than BL-300 which is attributed to the higher specific surface area of SZ-9.
Considering that complex components in blood have a great influence on the adsorption of adsorbents for serum toxins, adsorption experiments in human plasma with high bilirubin levels were conducted. Results indicated that SZ-9 performed better adsorption of bilirubin than BL-300, and the superiority of SZ-9 were more obvious in high bilirubin concentrations of human plasma. Compare with HA-330 (made in China), a typical commercial non-ionic adsorbent currently used to remove bilirubin in clinic, SZ-9 has a much higher adsorption capacity for bilirubin, which is about 2.5-fold higher than that of HA-330 (data not shown). In addition, SZ-9 also has excellent adsorption properties for bile acids in plasma solutions.
Bilirubin adsorption rate of BL-300 increased with temperature increasing, which reflected that its adsorption mechanism is not only electrostatic interaction but also hydrophobic. On the other hand, non-ionic SZ-9 resin has a high specific surface area and well-developed pore structure, adsorbs bilirubin solely by hydrophobic interaction and is not affected by ionic concentrations.
To assess blood compatibility of the adsorbents, we studied blood routine and hemolysis assay after contacting the polymers with blood for 60 min. Hemolysis rate of BL-300 was about 11%, and that of SZ-9 was negligible. In addition, SZ-9 had little influence on blood cells. All the above findings indicate that SZ-9 has a high potential to be applied in clinical performance of whole blood perfusion. Whole blood perfusion is used clinically with some limitations because the adsorbents may damage or activate blood cells, so usually plasma perfusion is used for hemoperfusion (Takenaka Citation1998). Newly synthesized SZ-9 adsorbent may be a good candidate to solve the above problem.
Conclusion
According to the above findings, we conclude that the newly synthesized non-ionic styrene–divinylbenzene copolymer of bead type adsorbent (SZ-9), has a relatively simple preparation process without any functionalization reactions. It has a well-developed, mainly of meso-porous structure, with a high surface area and high pore volume which showed good adsorption performance for albumin bound bilirubin and cholic acid. Furthermore, the adsorbent has a negligible hemolytic activity and non-mal effects on blood cells, good blood compatible properties in hemoperfusion. Therefore, it is a well-defined, unique adsorbent for the removal of protein-bound and middle molecule toxins, having a high potential in clinical application for the treatment of acute liver failure by whole blood hemoperfusion.
Funding information
This work was supported by grants from National Natural Science Foundation of China (No.81271710), the Natural Science Foundation of Tianjin (No. 12ZCDZSY20000, 14ZCZDSY00011, 14JCTPJC00487, 13JCQNJC14200).
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Annesini MC, Di Carlo C, Piemonte V, Turchetti L. 2008. Bilirubin and tryptophan adsorption in albumin-containing solutions: I. Equilibrium isotherms on activated carbon. Biochem Eng J. 40:205–210.
- Annesini MC, Di Paola L, Marrelli L, Piemonte V, Turchetti L. 2005. Bilirubin removal from albumin-containing solution by adsorption on polymer resin. Int J Artif Organs. 28:686–693.
- Barrett EP, Joyner LG, Halenda PP. 1951. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J Am Chem Soc. 73:373–380.
- Brunauer S, Emmett PH, Teller E. 1938. Adsorption of gases in multimolecular layers. J Am Chem Soc. 60:309–319.
- Chen Z, Gao B, Yang X. 2009. Preparation and adsorption property of aminated cross linking microbeads of GMA/EGDMA for bilirubin. J Chem Sci. 121:1061–1068.
- Chirito E, Reiter B, Lister C, Chang TM. 1977. Artificial liver: the effect of ACAC microencapsulated charcoal hemoperfusion on fulminant hepatic failure. Artif Organs. 1:76–83.
- Costanzo JA, Ober CA, Black R, Carta G, Fernandez EJ. 2010. Evaluation of polymer matrices for an adsorptive approach to plasma detoxification. Biomaterials. 31:2857–2865.
- Davies CR, Malchesky PS, Saidel GM. 1990. Temperature and albumin effects on adsorption of bilirubin from standard solution using anion-exchange resin. Artif Organs. 14:14–19.
- Duan ZJ, Li LL, Ju J, Gao ZH, He GH. 2006. Treatment of hyperbilirubinemia with blood purification in China. World J Gastroenterol. 12:7467–7471.
- Guo L, Zhang J, He Q, Zhang L, Zhao J, Zhu Z, et al. 2010. Preparation of millimetre-sized mesoporous carbon spheres as an effective bilirubin adsorbent and their blood compatibility. Chem Commun. 46:7127–7129.
- Hartley JL, Kelly DA. 2010. End stage liver failure. Paediatr Child Health. 20:30–35.
- He B. 1995. The application of adsorption resin for separation and purification of substances. In: He B, Huang W, Eds. Ion Exchange and Adsorption Resin. China: SSTEPH, pp. 402–405.
- He XM, Carter DC. 1992. Atomic structure and chemistry of human serum albumin. Nature. 358:209–215.
- Jacobsen J, Brodersen R. 1983. Albumin-bilirubin binding mechanism. J Biol Chem. 258:6319–6326.
- Jia LY, Guo R, Zhang XO, Zhou CY. 2005. Synthesis and characterization of novel adsorbents with different ligand composition for bilirubin conjugated with albumin. Separat Sci Technol. 40:1387–1399.
- Kun KA, Kunin R. 1968. Macroreticular resins. III. Formation of macroreticular styrene–divinylbenzene copolymers. J Polym Sci a-1 Polym Chem. 6:2689–2701.
- Müller BR. 2010. Effect of particle size and surface area on the adsorption of albumin-bonded bilirubin on activated carbon. Carbon. 48:3607–3615.
- Meijers BKI, Verhamme P, Nevens F, Hoylaerts MF, Bammens B, Wilmer A, et al. 2007. Major coagulation disturbances during fractionated plasma separation and adsorption. Am J Transpl. 7:2195–2199.
- Morimoto T, Matsushima M, Sowa N, Ide K, Sawanishi K. 1989. Plasma adsorption using bilirubin-adsorbent materials as a treatment for patients with hepatic failure. Artif Organs. 13:447–452.
- Nakaji S, Hayashi N. 2003. Bilirubin adsorption column Medisorba BL-300. Ther Apher Dial. 7:98–103.
- Odell GB. 1959. The dissociation of bilirubin from albumin and its clinical implications. J Pediatr. 55:268–279.
- Piemonte V, Turchetti L, Annesini MC. 2010. Bilirubin removal from albumin-containing solutions: dynamic adsorption on anionic resin. Asia Pacif J Chem Eng. 5:708–713.
- Sideman S, Mor L, Brandes JM, Lupovitch S. 1983. Preparation of a biocompatible albumin-coated ion exchange resin for bilirubin removal from the blood of jaundiced newborns. J Biomed Mater Res. 17:91–107.
- Stocker R, Glazer AN, Ames BN. 1987. Antioxidant activity of albumin-bound bilirubin. Proc Natl Acad Sci USA. 84:5918–5922.
- Takenaka Y. 1998. Bilirubin adsorbent column for plasma perfusion. Ther Apher. 2:129–133.
- Usami M, Nomura H, Nishimatsu S, Shiroiwa H, Kasahara H, Takeyama Y, Saitoh Y. 1995. In vivo and in vitro evaluation of bilirubin removal in postoperative hyperbilirubinemia patients. Artif Organs. 19:102–105.
- Wang Y, Zhang J, Zhu XX, Yu A. 2007. Specific binding of cholic acid by cross-linked polymers prepared by the hybrid imprinting method. Polymer. 48:5565–5571.
- Weber V, Linsberger I, Hauner M, Leistner A, Leistner A, Falkenhagen D. 2008. Neutral styrene divinylbenzene copolymers for adsorption of toxins in liver failure. Biomacromolecules. 9:1322–1328.
- Williams R, Schalm SW, O'Grady JG. 1993. Acute liver failure: redefining the syndromes. Lancet. 342:273–275.
- Yamazaki Z, Inoue N, Wada T, Oda T, Atsumi K, Kataoka K, Fujisaki Y. 1979. Use of an AR-1 resin column to reduce bilirubin-level in modified ascitic fluid. Trans Am Soc Artif Intern Organs. 25:480–486.
- Yu Y. 2013. Adsorbents in blood purification: from lab search to clinical therapy. Chin Sci Bull. 58:4357–4361.
- Zaki AEO, Wardle EN, Canalese J, Ede RJ, Williams R. 1983. Potential toxins of acute liver failure and their effects on blood-brain barrier permeability. Experientia. 39:988–991.
- Zangi R, Hagen M, Berne BJ. 2007. Effect of ions on the hydrophobic interaction between two plates. J Am Chem Soc. 129:4678–4686.