Abstract
Objective To analyze bond strength to bleached enamel following application of 10% sodium ascorbate gel. Material and methods Forty third molars were allocated into five groups (n = 8): GP – unbleached specimens restored with composite resin; GN – specimens restored immediately after bleaching; and G15; G30 and G60 (test groups) – bleached specimens treated with 10% sodium ascorbate gel for 15, 30 and 60 min before restoration. The teeth were sectioned and the buccal and lingual faces were restored. After storage in distilled water (37 °C/24 h), sticks of ±0.8 mm2 were tested in tensile (0.5 mm/min). Fractures were observed and classified. Data (in MPa) were analyzed with one-way ANOVA and Tukey tests (α = 0.05). Results No differences were found among GP (26 ± 6.0), G15 (23 ± 7.3), G30 (25 ± 6.1) and G60 (25 ± 5.1), with GN (15 ± 5.5) showing the lowest bond strength (p < 0.0001). Conclusion The application of 10% sodium ascorbate gel for 15 min after bleaching with 37.5% hydrogen peroxide restored the bonding to enamel.
Keywords:
Introduction
Nowadays, the high demand for esthetic treatments has motivated many patients to seek dental offices for tooth-whitening followed by restorative procedures with composite resin materials.[Citation1] During tooth-whitening, oxygen free radicals are delivered from the bleaching agents through the enamel surface. Thus, oxygen, hydroxyl or perhydroxyl ions remain trapped in teeth structures,[Citation2] inhibiting the polymerization of composite resin materials.[Citation3,Citation4] As a result, decreased hardness and fracture toughness, mineral loss and increased roughness have all been observed in enamel just after tooth bleaching.[Citation5]
Waiting periods ranging from 24 h to three weeks after bleaching, to allow for the buffering action of saliva to remove the oxygen present in the dental structures, have been recommended before initiating tooth restorations with composite resins.[Citation6–8] Clinically, however, there are situations that require that adhesive restorations are performed in the same session when, for instance, patients from different places, or have time constraints. This lead time may bring difficulties to the dentist and patient.
In order to shorten the waiting period, 10% sodium ascorbate in its gel form has been applied onto bleached enamel to accelerate the removal of the residual oxygen layer and to re-establish enamel bond strength to levels similar to those of unbleached teet.[Citation2,Citation9,Citation10–13] It has been previously shown that the application of 10% sodium ascorbate gel for 60 min was capable of recovering enamel bond strength in vitro,[Citation10] in situ,[Citation13] and also clinically.[Citation9] Although a waiting period of 60 min is clinically feasible, it is still considered too long for both patients and clinicians. Nevertheless, no studies evaluating the application of 10% sodium ascorbate gel for periods of time shorter than 60 min, especially following bleaching with 37.5% hydrogen peroxide, have been performed.
Therefore, the aim of this study was to evaluate the effect of different application times of 10% sodium ascorbate gel immediately after bleaching with 37.5% hydrogen peroxide on composite bond strength to enamel. The tested hypothesis was that no differences would be found among the different tested application times.
Materials and methods
This study was approved by the local Institutional Review Board (Protocol 07110012.4.0000.0108).
Group allocation
Forty sound extracted third molars without cracks, fractures or stains were used in the study. The teeth were cleaned with periodontal curettes and ultrasound, submitted to prophylaxis with pumice and water, and disinfected in 0.5% chloramine T solution for seven days. The teeth were randomly allocated to five experimental groups (n =8): GP (positive control group) – unbleached specimens restored with composite resin; GN (negative control group) – specimens restored immediately after bleaching; and G15; G30 and G60 (test groups) – bleached specimens treated with sodium ascorbate gel for 15, 30 and 60 min before restoration, respectively. Until the present study application times less than 60 min was not described for 10% sodium ascorbate gel.
Teeth preparation and treatment
Teeth’s roots were grinded 1 mm above the cement-enamel junction using a 180 silicon carbide paper under refrigeration (Politriz, Arotec, Cotia, SP, Brasil). The remaining crowns were then sectioned in the mesial-distal direction using a diamond saw (Blade XL 12235, Extec Corp, Enfield, CT, USA) adapted to a sectioning machine (Isomet 1000, 200 rpm, Buehler, Lake Bluff, IL, USA). In preparation to the experimental procedures, the two halves from the same tooth were ultrasound washed in distilled water for 10 min, and dried with jets of air. For easy handling, teeth halves were fixed onto a glass sheet with pink dental wax (Epoxiglass Ind. Com. de Produtos Químicos Ltda., Diadema-SP, Brazil), exposing the enamel surfaces.[Citation13]
Experimental procedures
All the materials used in this experiment, their compositions and application modes are presented in . Dental bleaching was performed using 37.5% hydrogen peroxide (PollaOffice+, SDI, Limited. Bayswater, Victoria, Australia) in all experimental groups, except for GP. Previously prepared 10% sodium ascorbate gel [Citation11] was applied to the enamel surface and left for 15 min (G15), 30 min (G30) and 60 min (G60). During the bleaching and sodium ascorbate procedures, specimens were kept inside an incubator at 37 °C and 100% humidity. The amount of gel aplied to the enamel surface was standardized using a tray and that was fixed in the amount of 3 mm thick onto the enamel surfaces. Specimens in all experimental groups were then acid etched with 35% H3PO4 (Scotchbond Etchant Gel, 3M ESPE, St. Paul, MN, USA), treated with Adper Single Bond 2 adhesive system (3M Espe, St. Paul, MN, USA), and blocks of composite resin (Filtek Z350 XT, 3M ESPE, St. Paul, MN, USA) 6 mm high were built up on the enamel surface.
Table 1. Materials, compositions and application modes.
The tooth was cut from the mesial to the distal directions thus separating the buccal and ligual surfaces. They were positioned in a plane and the gel applied using a tray in the amount of 3 mm thickness.
Microtensile bond strength
After storage in distilled water at 37 °C for 24 h, the restored teeth were fixed with sticky wax[Citation13] to the acrylic brackets of a sectioning machine (Isomet 1000 machine, Buehler Ltd, Lake Bluff, IL, USA) and serially cut with a diamond saw (Blade XL 12205, 200 rpm, Extec Corp, Enfield, CT, USA) perpendicularly to the enamel/restoration interface into 0.9 mm slices. Each slice was then individually fixed with the same sticky wax to the cutting machine to perform a second sectioning of 0.9 mm, to produce “sticks” with a cross-sectional area of approximately 0.8 mm2. Sticks had their area measured with a digital pachymeter (Zaas Precision, Digital Caliper, Amatools, Piracicaba, SP, Brazil) and were individually observed under a 40X optical microscope (Bel MicroImage Analyzer, Bel Photonics, Monza, Italy). Only sticks from the middle third of teeth mesial and distal halves, showing the flattest resin/enamel interface lines, were selected to be tested in tensile.
Each selected stick was fixed to a specially designed microtensile testing jig (Odeme Biotechnology, Joaçaba, SC, Brazil) with cyanoacrylate gel (Permatex, Odeme Biotechnology, Joaçaba, SC, Brazil) and one drop of monomer (Jet, Clássico Artigos Odontológicos Ltda, Campo Limpo Paulista, SP, Brazil), leaving just the enamel/resin interface exposed. Each stick was tested in tensile at 0.5 mm/min in a universal testing machine (EMIC, São José dos Pinhais, Brazil) until failure. Bond strength was calculated by dividing the tensile force obtained in N by the cross-sectional area (mm2) of the stick, and expressed in Megapascal (MPa).
To determine whether a specimen would be included in the statistical analysis, fragments were observed under a 40× optical microscope (Bel MicroImage Analyzer, Bel Photonics, Monza, Italy), [Citation14] and fracture types were classified as follows: (1) Adhesive: failure in adhesion, with fracture at the interface; (2) Cohesive in enamel; (3) Cohesive in the composite resin and (4) Mixed: fractures in the enamel substrate and resin materials in the same test specimen. Only sticks with adhesive or mixed interface fractures, in which the cohesive part represented ≤10% of the interface area, were included in the statistical analysis.[Citation15] Sticks that presented pretest failures, i.e. broke during preparation for the test, were also discarded from the analysis.[Citation16]
To guarantee independence of data, and at the same time reduce variability, mean bond strength values were calculated for the sticks originating from the same tooth.[Citation16] As the results presented normal distribution they were statistically analyzed with one-way analysis of variance (ANOVA) and Tukey’s post hoc test, at 5% global significance.
Results
and show the mean and standard deviation of the enamel/resin bond strengths (MPa) found with the microtensile testing. A statistically significant difference in mean bond strength was observed between the negative control group GN (dental specimens restored immediately bleaching), and all the other experimental groups (p < 0.0001). No statistically significant differences, however, were found among any of the test groups treated with 10% sodium ascorbate gel (G15, G30, G60) and the positive control group GP (p > 0.05).
Figure 1. Mean bond strength values (in Mpa) for the experimental groups; *statistically different (p < 0.05).
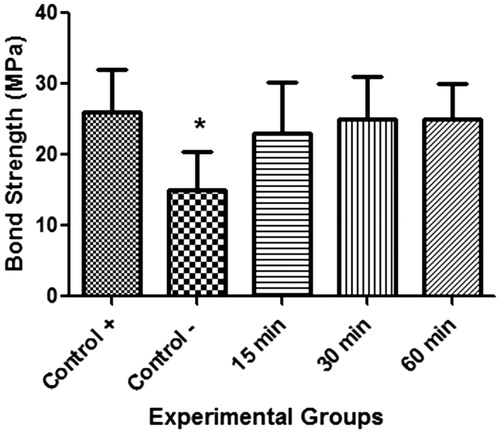
Table 2. Mean microtensile bond strength (standard deviations) in MPa for the different experimental conditions .
The percentage of specimens with premature debonded failures, and the frequency of each fracture mode are shown in . The highest number of premature failures was observed for the group restored immediately after bleaching (GN). It was recommended not to use premature failures for statistical purposes according to Roulet et al., 2007.[Citation16]
Table 3. Fracture modes of specimens and their representative percentages (%).
Discussion
This study demonstrated that 10% sodium ascorbate gel applied for 15 min immediately after the completion of the bleaching treatment with 37.5% hydrogen peroxide was sufficient to restore composite resin bond strength to human enamel to values statistically similar to unbleached teeth. The lack of statistical significance in bond strength obtained among the three tested groups support the null hypothesis set forth by this study.
Bonded specimens in this study were tested in microtensile, which has been shown to be an effective method to analyze bond strength between restorative materials and tooth structures. Among the main advantages of this test are the possibility of determining the location of the fracture, obtain several specimens of a single material, and evaluate bond strength in small áreas.[Citation17] Tensile tests produce a more uniform distribution of stress at the bonded interface and show a more reliable correlation with the clinical loss of retention than shear tests.[Citation18] In this study, only those sticks presenting adhesive or predominantly adhesive (mixed) failures were considered in the statistical analysis, while sticks presenting cohesive failures either in the resin or enamel, or broke apart before testing (pretest failures), were disconsidered.[Citation16] Additionally, mean microtensile bond strength was calculated for the sticks originating from the same dental specimen in order to minimize possible differences in adhesion that occur in different parts of the specimen.
The positive and negative control groups used in this study clearly defined the difference in bond strength between unbleached (GP) and bleached enamel (GN) immediately restored with composite resin. The highest bond strength, as expected, was observed in the positive control group (GP).[Citation6,Citation7] In this untreated condition, during polymerization, resin tags are formed within the enamel substrate conditioned with phosphoric acid, enhancing enamel-resin adhesion.[Citation19] The lowest bond strength, on the other hand, was observed in the negative control group (GN). Once again, an expected result, which was observed previously,[Citation20] and confirmed here by the high prevalence of premature fractures. Just after the bleaching treatment, free radicals, such as oxygen, generated by the degradation of hydrogen peroxide remain trapped within the dental structures, interfering with the polymerization of the composite resin and inhibiting the formation of resin tags.[Citation21]
The application of 10% sodium ascorbate gel for 60 min has already been found to be effective to restore bond strength to enamel following bleaching with 37.5% hydrogen peroxide both in situ[Citation13] and in vivo.[Citation9] These results have great clinical significance, as many patients need to have restorations replaced or undergo esthetic procedures soon after in-office dental bleaching. However, in clinical terms, the shorter the chair time, the more convenient it is for both patients and clinicians, optimizing the conclusion of the esthetic restorative treatment. It was suggested[Citation3] that enamel should be exposed to sodium ascorbate for approximately a third of the time necessary for the bleaching treatment. Following this rationale, and taking into consideration that the bleaching procedure with 37.5% hydrogen peroxide took 40 min in the present study, the exposition time to the antioxidant should be around 12 min. The results found here seems to agree with their assumption, as 10% sodium ascorbate gel applied for 15 min demonstrated to be sufficient to restore bond strength to levels similar to unbleached enamel. This is a promising result, as it represents a significant reduction in terms of the waiting time required to initiate the restorative treatment. Nonetheless, despite this improvement, the results of this study also warrant some more careful examination on the use of the antioxidant.
The fact that no statistical differences concerning bond strength were found among the three tested groups raises the issue of the oxidizing nature of the antioxidant agent. Although sodium ascorbate is an ascorbic acid salt with powerful antioxidant activity, it degrades rapidly when in contact with the oxygen present in the air, significantly losing its effectiveness along time.[Citation22] In this study, a layer of 10% sodium ascorbate gel was applied onto the enamel surface immediately after the bleaching treatment had finished and left untouched for their respective tested times. Bond strength only slightly increased over the experimental time, which seems to suggest that most of the antioxidant effect of the 10% sodium ascorbate gel occurred within the first few minutes of its application, and that leaving the gel for longer periods of time (30 and 60 min) did not to bring much contribution to final bond strength. This assumption is agreement with a previous study in which the oxidant/antioxidant reaction kinetics demonstrated that the application of sodium ascorbate for longer periods of time did not influence the effectiveness of the reaction.[Citation23]
The same authors also concluded that the application of a 25% sodium ascorbate solution for 5 min was sufficient for the ascorbate to exert an antioxidant effect.[Citation23] Some other previous authors have also proposed the use of sodium ascorbate solutions for 10 min,[Citation21] saying that this time was sufficient to eliminate the oxygen present in the dental structures. Another recent study demonstrated that two 1 min applications of a 35% sodium ascorbate solution immediately after bleaching eliminated residual peroxide.[Citation24] However, sodium ascorbate solutions lose their antioxidant effect very quickly, must be prepared right at the time of use, and reapplied frequently.[Citation21,Citation24,Citation25] Although these studies are important for asserting the effectiveness of sodium ascorbate as an antioxidant, the use of the agent in its solution form is clinically cumbersome.
The gel format used in this study has several advantages over the solution. The gel is less fluid and, therefore, provides a more adequate control during its application, as the gel stays in position on the dental surface for the defined exposition period.[Citation9–11,Citation17,Citation18,Citation23–25] Moreover, when chemical substances are converted into a gel form, the rate the active ingredient is released is much slower than that when they are in the form of a solution, extending their effectiveness over time.[Citation11] Nonetheless, due to the inherent characteristics of sodium ascorbate, the repeated application of the antioxidant agent in its gel form might be an alternative strategy to reduce the time of the procedure even further. Future studies comparing single and multi applications of 10% sodium ascorbate gel over bleached enamel may be able to indicate what the optimum procedure should be.
The results of this study are clinically relevant as it demonstrate that the use of 10% sodium ascorbate gel for 15 min is sufficient to recover bond strength. An important step was taken in the development of a commercial sodium ascorbate product for dental use. Nonetheless, new studies assessing the chemical stability of the gel need to be performed before its use may be considered clinically. The right product, besides effective, should also have an acceptable shelf life to be reused along time.
The manipulation of sodium ascorbate gel was performed using carbopol as espessant because carbopol is a high molecular polymer currently used in pharmaceutical formulations (liquid, gel, solid) as espessant.[Citation26] In this study the bleaching and bonding procedures were performed in enamel without abrasion to simulate the clinical scenario of dental bleaching that do not need to cut enamel. Appart from this, the acid etching showed ability to prepare the surface for bonding, apart from the numerical values of bonding observed in this study.
Taking into consideration the results of this study, the following conclusions may be drawn: 1. Bleaching with 37.5% hydrogen peroxide reduces composite resin bond strength to human enamel; 2. The application of 10% sodium ascorbate gel on recently bleached enamel was capable of restoring composite resin bond strength to the same level of unbleached enamel and 3. Keeping 10% sodium ascorbate gel for 15 min on bleached enamel was enough to restore composite resin bond strength to levels that would permit immediate adhesive procedures.
Acknowledgements
The authors would like to thank 3M do Brasil for kindly supplying the adhesive system (Adper Single Bond 2) and the composite resin (Filtek Z350 XT), and Mr Antonio Carlos Correa for the English version of the paper.
Disclosure statement
The authors report no conflict of interest. All the content and writing of this manuscript is the responsibility of the authors.
References
- Kunt GE, Yimaz N, Sen S, et al. Effect of antioxidant treatment on the shear bond strength of composite resin to bleached enamel. Acta Odontol Scand. 2011;69:287–291.
- Comlekoglu ME, Gokce B, Kaya AD, et al. Reversal of reduced bond strength after bleaching. Gen Dent. 2010;58:258–263.
- Lai SCN, Tay FR, Cheung GS, et al. Reversal of compromised bonding in bleached enamel. J Dent Res. 2002;81:477–481.
- Spyrides GM, Perdigão J, Pagani C, et al. Effect of whitening agents on dentin bonding. J Esthet Dent. 2000;12:264–270.
- Attin T, Schmidlin PR, Wegehaupt F, et al. Influence of study design on the impact of bleaching agents on dental enamel microhardness: a review. Dent Mater. 2009;25:143–157.
- Cavalli V, Giannini M, Carvalho RM. Effect of carbamide peroxide bleaching agents on tensile strength of human enamel. Dent Mater. 2004;20:733–739.
- Titley KC, Torneck CD, Smith DC, et al. Scanning electron microscopy observations on the penetration and structure of resin tags in bleached and unbleached bovine enamel. J Endod. 1991;17:72–75.
- Uysal T, Basciftci FA, Uflümez S, et al. Can previously bleached teeth be bonded safely? Am J Orthod Dentofacial Orthop. 2003;123:628–632.
- Garcia EG, Mena-Serrano A, Andrade AM, et al. Immediate bonding to bleached enamel treated with 10% sodium ascorbate gel: a case report with one-year follow-up. Eur J Esthet Dent. 2012;7:154–162.
- Kaya AD, Türkün M, Arici M. Reversal of compromised bonding in bleached enamel using antioxidant gel. Oper Dent. 2008;33:441–444.
- Kimyai S, Valizadeh H. The effect of hydrogel and solution of sodium ascorbate on bond strength in bleached enamel. Oper Dent. 2006;31:496–499.
- Lima AF, Fonseca FMS, Freitas MS, et al. Effect of bleaching treatment and reduced application time of an antioxidant on bond strength to bleached enamel and subjacent dentin. J Adhes Dent. 2011;13:537–542.
- Miranda TAM, Moura SK, Amorim VHO, et al. Influence of exposure time to saliva and antioxidant treatment on bond strength to enamel after tooth bleaching: an in situ study. J Appl Oral Sci. 2013;21:567–574.
- EL Zohairya AA, Sabera MH, Abdallab AI, et al. Efficacy of microtensile versus microshear bond testing for evaluation of bond strength of dental adhesive systems to enamel. Dent Mater. 2010;26:848–854.
- Scherer SS, Cesarb PF, Swainc MV. Direct comparison of the bond strength results of the different test methods: a critical literature review. Dent Mater. 2010;26:e78–e93.
- Roulet JF, Van Meerbeek B. Editorial: Statistics: a nuisance, a tool or a must? J Adhes Dent. 2007;9:287–288.
- Da Silva JMG, Botta AC, Barcellos DC, et al. Effect of antioxidant agents on bond strength of composite to bleached enamel with 38% hydrogen peroxide. Mat Res. 2011;14:235–238.
- Heintze SD. Clinical relevance of tests on bond strength, microleakage and marginal adaptation. Dent Mater. 2013;29:59–84.
- Pashley DH, Tay FR, Breschi L, et al. State of the art etch-and-rinse adhesives. Dent Mater. 2011;27:1–16.
- McGuckin RS, Thurmond BA, Osovitz S. Enamel shear bond strengths after vital bleaching. Am J Dent. 1992;5:216–222.
- Briso ALF, Toseto RM, Rahal V, et al. Effect of sodium ascorbate on tag formation in bleached enamel. J Adhes Dent. 2012;14:19–23.
- Rowe RC, Sheskey PJ, Owen SC. Handbook of pharmaceutical excipients. London: Pharmaceutical Press; 2006.
- Freire A, Souza EM, de Menezes Caldas DB, et al. Reaction kinetics of sodium ascorbate and dental bleaching gel. J Dent. 2009;37:932–936.
- Freire A, Durski MT, Ingberman M, et al. Assessing the use of 35 percent sodium ascorbate for removal of residual hydrogen peroxide after in-office tooth bleaching. J Am Dent Assoc. 2011;142:836–841.
- Garcia EJ, Ordoni TL, Alencar SM, et al. Antioxidant activity by DPPH assay of potential solutions to be applied on bleached teeth. Braz Dent J. 2012;23:22–27.
- Garala K, Joshi P, Shah M, et al. Formulation and evaluation of periodontal in situ gel. Int J Pharm Investigv. 2013;3:29–41.
