Abstract
Background: The cost of the biological drug abatacept may be partly offset by reductions in the cost of productivity losses due to employee absences and reduced effectiveness at work because of rheumatoid arthritis (RA).
Methods: This was a 1-year productivity cost model based on epidemiologic and economic data. The setting was private industry in the US and the primary outcome measure was the difference in the costs of lost productivity and drug treatment with and without abatacept (‘cost difference’).
Results: The lost productivity cost of RA for a firm of 10,000 was $1.69 million, largely due to the cost of RA-related absenteeism ($1.55 million) rather than to worker displacement ($0.12 million) or care-giving for spouses with RA ($0.02 million). In the base case analysis (excluding presenteeism), 37% of the acquisition cost of abatacept was offset by reductions in the cost of RA-related productivity losses. In some industry groups (Utilities and Finance), and in models that included presenteeism, reductions in lost productivity costs exceeded the abatacept cost.
Conclusions: Much of the acquisition cost of abatacept may be offset by reductions in the cost of productivity losses due to RA. Abatacept treatment could be cost saving in some industry groups.
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease that causes chronic inflammation of the joints[Citation1. If uncontrolled, initial inflammation and pain can spread to additional joints and result in irreversible tissue damage. Joint pain and fatigue typically lead to reduced levels of activity and work productivityCitation2. Common treatments for RA include traditional disease-modifying antirheumatic drugs (DMARDs), such as methotrexate, and the newer and more efficacious biological therapiesCitation3. The first biologicals (etanercept, infliximab, adalimumab and anakinra) were tumour necrosis factor (TNF) inhibitors. However, a significant proportion of patients either do not respond to these agents or cannot tolerate prolonged treatmentCitation4,Citation5.
Abatacept is the first in a new class of biologicals that selectively block the signals for full T-cell activation. It has demonstrated efficacy in improving physical function and health-related quality of life in patients with inadequate response to both methotrexate and anti-TNF therapiesCitation6,Citation7. Abatacept also significantly increases patients’ participation in usual activities (work or non-work)Citation8. The increase in activity levels may translate into greater productivity in the work place, so that although the more effective biologicals have higher acquisition costs, these costs may be partly offset by reductions in the cost of productivity losses associated with RA. The objective of this report was to determine the extent to which reductions in the costs of lost productivity offset the acquisition cost of abatacept for treating RA.
Methods
Overview
The model is a 1-year prevalence-based economic model in which the costs of lost productivity and RA treatment are considered from the perspective of the employer. The primary model output is the difference in lost productivity and treatment costs with and without the addition of abatacept. The drug cost includes only abatacept, because treatment with conventional DMARDs and ancillary direct medical costs are assumed to be the same with and without the addition of abatacept. The base case analysis refers to the average costs for large firms in private industry in the US. The effect of uncertainty in study variables is explored in a one-way sensitivity analysis and in an analysis by industry group. Variables in the model are listed in .
Table 1. Variable definitions, values and sources.
Subjects
Subjects are employees with RA who are qualified for abatacept and employees without RA who provide care-giving for a spouse with RA. The model envisions a US firm of n employees (base case n=10,000), made up of n × s men and n(1–s) women, where s is the proportion of men in the firm's workforce. The number of male employees with RA, NRm, is the prevalence of RA among men, pm, multiplied by the number of male employees;
Similarly, the number of female employees with RA is:
The total number of employees with RA is:
The fraction of employees with RA who are qualified for treatment with abatacept is i, and the number of employees with RA qualified for abatacept, NR,i, is:
The number of male employees providing care-giving for a spouse with RA, NSm, was calculated as:
where d is the proportion of employees living with a spouse and g is the fraction of employees with spouses with RA that provide them with care-giving. The number of female employee caregivers, NSw, was calculated as:
(If it is assumed that only employees without RA are caregivers, the functions for NSm and NSw should include the terms (1–pm) and (1–pw), respectively. The value of these terms is approximately 1.0, because pm and pw are of the order of 0.01, and they have been omitted.) The total number of employees providing care-giving for a spouse with RA is:
The analysis considers only active employees. It is assumed that the prevalence of RA among employees’ dependent children is zero and that retirees are covered by Medicare Part D.
Lost productivity cost of RA
Components of lost productivity cost of RA
The RA productivity cost (PC) has two components: one, the cost of productivity lost (PL) because employees with RA are absent from work or are less effective while at work (WLcost), or because healthy employees take time off work to care for a spouse with RA (WGcost); and two, the cost associated with worker displacement (CD), when RA causes workers to change jobs within a firm, leave the firm to find a different job or go onto permanent disability. That is:
PC, PL and CD are expressed in dollars per year. PL, the cost of lost productivity time, is the product of the amount of lost work time in days per year and the unit value of that work time in US dollars per day. The cost of worker displacement, CD, is the cost of hiring and training a replacement for an employee displaced due to RACitation31.
Lost productivity time of employees with RA
The amount of lost work time (or lost productivity time) per employee with RA includes absenteeism* (w), expressed in days off work per year, and the amount of work loss due to reduced performance while at work (i.e. presenteeism* (r)) expressed in work loss-day equivalents. The lost productivity time per employee with RA is w + r. The lost productivity time to the firm, WL, is w + r multiplied by the number of employees with RA qualified for abatacept, NR,i, so that:
Productivity time loss due to care-giving for spouses with RA
The amount of work time lost due to employees taking time off work to care for a spouse with RA, WG, is the product of the following: the number of employees providing care-giving for spouses with RA, NS; the fraction of the latter that are absent from work to provide RA care-giving, k; and the average days per week of absenteeism, q, multiplied by 50, the default number of working weeks in the year:
Value of lost productivity time
The unit value of lost work is the ‘value added’. Value added is the gross output of an industry minus its intermediate inputs and can be measured as the sum of compensation of employees, taxes on production and imports less subsidies, and gross operating surplus. Employee compensation consists of the wages and supplements to wages, such as pensions, health insurance, paid vacations etc. The employee's hourly compensation is his or her wage, e, increased by a factor b to account for employee supplements (i.e. e × b). Employee compensation is multiplied by a factor f to convert employee compensation to value added. The cost to the firm of lost productivity due to RA (i.e. PL) is the days of lost productivity (WL + WG) multiplied by the value added:
The number 8 converts hourly wages to daily wages. The equation includes an additional variable, m, called the productivity cost modifier. This variable is explained in the sensitivity analysis and its base case value is 1.0.
Cost of worker displacement
The cost to the firm of worker displacement, CD, is a function of the average cost per hire, h, plus the cost of training a replacement, which is the number of hours of training, t, multiplied by the hourly compensation, e × b. The cost per displaced employee is:
The number of employees displaced due to RA is the number of employees with RA eligible for abatacept, NR,i, multiplied by the annual rate of employee displacement, j (i.e. NR,i × j). Hence,
Cost difference of treatment versus no treatment with abatacept
The authors define a as the proportion by which productivity time loss is reduced by treating RA with abatacept instead of conventional DMARD therapy. If it is assumed that abatacept reduces the rate of employee displacement by the same factor, treatment with abatacept reduces the cost of lost productivity under treatment with conventional DMARD therapy (PC) by the amount PC × a. The lost productivity cost of RA treated with abatacept is:
The annual per-patient cost of treatment with abatacept, c, is $17,550. This is the cost in Year 2 of therapy (i.e. once the patient has stabilised on treatment) and represents the cost of 13 treatments of three vials for a person who weighs 60–100 kg. The cost of abatacept to the firm, C, is c multiplied by the number of employees treated with abatacept (i.e. C = NR,i × c). The combined cost to the firm of biological drug treatment and the productivity cost of RA is:
under treatment with abatacept and PC under treatment with conventional DMARDs. The cost difference, D, is:
(i.e. D = C – (PC × a)).
Data sources
The prevalence of RA diagnosed by the American College of Rheumatology criteria, age-adjusted to the US population, was from Gabriel et al Citation10. The proportion of RA patients qualified for treatment with abatacept was calculated from the observed rates of discontinuation of initial DMARD therapy and the average time since onset of RA. Abatacept is indicated for patients with an inadequate response to one or more DMARDs. However, there is no universally accepted definition of ‘inadequate response’Citation33. The authors defined inadequate response operationally as a condition prompting the discontinuation of a drug. It was assumed that patients with RA were initially prescribed methotrexate, the most commonly prescribed initial DMARD Citation34–36. Survival-on-therapy curves indicate that, at 5 years, 36% of patients on methotrexate remained on treatmentCitation11. The mean duration of RA in the US CORRONA registry was 5.3 years (standard deviation (sd) 5.1 years)Citation12. If the mean duration of RA in the US employed population is approximately 5 years, then at any point in time 64% would have discontinued their initial methotrexate treatment and be qualified for abatacept.
Days of absenteeism due to RA during the era of conventional DMARD therapy (prior to the introduction of biologicals) were the average of values from published studies: the median was 38.6 days per year per RA employee (range 7.1–109.2 days)Citation13. Presenteeism is a component of work loss due to arthritisCitation37 but is not included in the base case model because there are relatively few data specific to RACitation38. The proportion of employees with a spouse was approximated by the proportion of adults in the US with a married spouse present in the householdCitation17. The value for the proportion of RA caregivers with absenteeism was the median and range of values from two studiesCitation18,Citation19. The mean of 0.23 and range of 0.015–1.68 for days absenteeism per week by caregivers was taken from Maetzel et alCitation19. The average cost per hire in 2005 was from the Society for Human Resource ManagementCitation20. The number of hours a new worker spends in on-the-job training during the first 3 months of employment was taken from Barron et alCitation21. The annual rate of employee displacement due to RA was from published studiesCitation16, Citation22–25. The base case value of 0.11 represents the annualised rate at which RA employees changed jobs or stopped workCitation16. The upper range of values (0.262) is the rate at which employees changed or lost their job due to RA (the difference of community samples with RA and non-arthritics)Citation22. Three other studies measured only the rate of RA work disability. The annualised rate was 0.036, 0.045 and 0.072; 0.036 was used as the lower end of the range of values for j Citation23–25.
Economic data were from the Bureau of Labor Statistics. The proportion of male employees was the mean and range of values for private industry by a major industry group in 2005Citation9. The 10th, 25th, 50th (median), 75th and 90th percentiles of hourly earnings in US private industry in 2005 were $7.26, $9.46, $14.15, $22.20 and $33.74Citation26. The ratio of employee benefits to total compensation was the mean and range of values across major industry groupsCitation27. The ratio of value added to employee compensation was from the 2004 Survey of Current BusinessCitation28. Employee compensation represented 52.9% of the value added for private industry, so that the base case value of f was 100/52.9 or 1.89.
The source for a was the Abatacept in Inadequate Responders to Methotrexate (AIM) trialCitation6. Patients in this trial completed a questionnaire recording the amount of time (number of days) they were unable to perform their ‘usual activities’ because of RA in the past 30 days. The questionnaire defined ‘usual activities’ as ‘work, whether or not you work for pay, and any other activities you do during the day.’ The construct validity and sensitivity to change of the activity question have been reportedCitation39. The questionnaire was completed by patients at baseline and monthly thereafter. The value of a is the mean (95% confidence interval (CI)) proportional reduction in the abatacept group relative to the placebo group at 12 months. Both abatacept (n=432) and placebo (n=214) groups received co-therapy with methotrexate. The mean number of days of limitation in usual activities in the abatacept and placebo groups was 5.4 and 8.8 days, respectively, which yields an a of 0.39.
Model outputs
The primary model output is the productivity cost difference, D, defined as the difference between drug treatment and productivity loss costs with and without abatacept. Intermediate outputs are: the cost of RA-related lost productivity time, WLcost, given by:
the cost of lost productivity time for care-giving, WGcost, given by:
the cost of worker displacement due to RA, CD; the total productivity cost of RA, PC, given by:
and the cost of abatacept, C. The model delivers the productivity loss cost of RA to the employer when all employees with RA are included in the calculation (PC when i=1). Because the focus of the study is the extent to which abatacept cost is offset by reductions in cost of lost productivity, the base case analysis includes the productivity and treatment costs of the subset of RA employees qualified for abatacept (i<1). Two other scenarios deliver model outputs in the case where care-giving is excluded from consideration and when abatacept costs for RA spouses qualified for abatacept are included (this cost is not included in the base case). In the latter scenario, the number of caregivers whose spouse with RA is qualified for abatacept, NS,i, is:
and the cost of abatacept to the firm is:
Sensitivity analysis
A one-way sensitivity analysis explored the relationship between variability in model parameters and the primary study outcome. Ranges of published values or 95% CIs were used. Where these were not available, an arbitrary range (typically from 0.4- to 2.5-fold the base case value) was applied. Employee compensation, e, was varied between the 10th and 90th percentiles for private industry. The range of values for other socioeconomic variables (s, b and f) was the range for private industry groups. The minimum value for presenteeism was zero (the base case value); a hypothetical maximum was set at 4.2-fold the median value for absenteeism, based on ratios of presenteeism to absenteeism observed for self-reported arthritisCitation15,Citation16. The productivity cost modifier, m, was varied with f set at 1.0 rather than the base case value of 1.89 to avoid double counting. The lower range of m was the value under the friction cost approach to productivity costsCitation29 and the upper range was from Nicholson et alCitation30. In addition to the one-way sensitivity analysis, an analysis by private industry group was performed, in which values of the socioeconomic variables (s, e, b and f) corresponding to each industry group were varied in concert.
An analysis of various estimations of presenteeism and absenteeism was also performed. Two studies reported arthritis-related presenteeism. A study of the productive time lost due to self-reported arthritis (‘arthritis’ was not defined) in the US workforce indicated a ratio of presenteeism to absenteeism of 6.7:1.0, implying 4.3 days absenteeism and 28.1 days presenteeism annually (assuming an 8-hour work day and 50-week work year)Citation14. In a Canadian study of self-reported arthritis (predominantly osteoarthritis), the ratio of absenteeism to presenteeism dollar costs (work loss multiplied by wage rate) for the employed population was 4.2:1; the authors inferred values of 38.3 days of presenteeism and 9.1 days of absenteeism, totalling 47.4 days per yearCitation15,Citation16. From the latter ratio of presenteeism to absenteeism of 4.2:1, an RA-related presenteeism value of 162 days was inferred from the base case value of absenteeism of 38.6 for RA.
Results
The total productivity cost of RA for a firm of 10,000 employees was $1.69 million, largely due to the cost of RA-related work loss (). In the base case analysis, 103 of the firm's 10,000 employees had rheumatologist-diagnosed RA and 66 were qualified for abatacept. The annual days of lost work were primarily due to absenteeism by RA patients (2,550 days) rather than by caregivers (56 days). Productivity loss costs were largely due to RA-related absenteeism ($0.99 million) rather than to costs related to time off for care-giving ($0.022 million) or worker displacement ($0.08 million). The productivity cost difference between abatacept and usual DMARD therapy was $0.73 million per year (). If care-giving was excluded from the model, the productivity cost difference was $0.73 million, and if the costs of abatacept use by spouses with RA was covered by employees’ insurance, the productivity cost difference was $1.05 million ().
Table 2. Costs under different model assumptions for a firm of 10,000 employees.
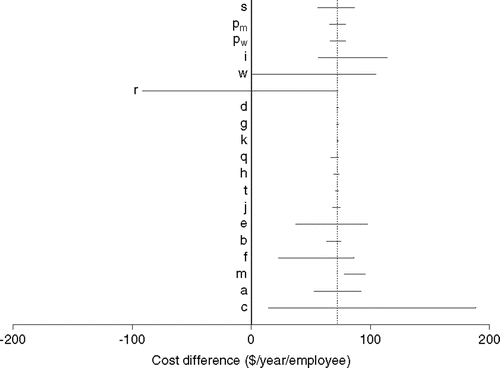
shows the one-way sensitivity analysis, with the cost difference expressed in dollars per employee ($72.6 in the base case analysis). Varying abatacept cost (c) and work loss due to RA (w, r) had the greatest effect on the cost difference. Increasing the amount of presenteeism from the base case value of zero delivered negative (i.e. cost saving) values for the cost difference. Changes in most variables related to worker displacement (h, t, j) or care-giving (d, g, k, q) had a relatively minor effect on the cost difference.
Figure 1. One-way sensitivity analysis of cost difference with abatacept vs. current disease-modifying antirheumatic drugs.
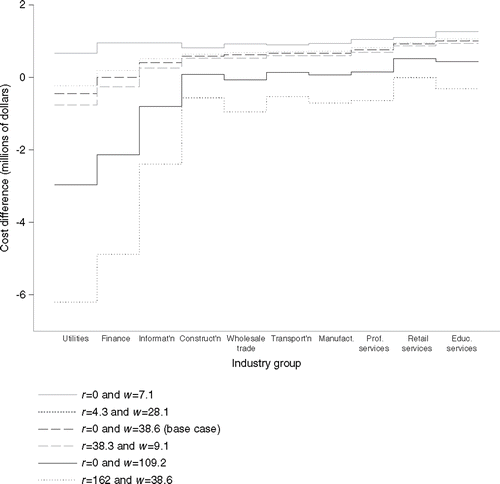
shows the effect of varying estimations of absenteeism and presenteeism by industry group. With base case values of absenteeism (w=38.6 days) and presenteeism (r=0) for RA, the cost difference varied from –$0.45 million for Utilities to $1.01 million for Educational services. The curve for the base case for RA was close to the curves for self-reported arthritis (r=4.3 and w=28.1; r=38.3 and w=9.1). With presenteeism ignored for RA (r=0), the lower range of values for absenteeism (w=7.1) yielded positive cost differences for all industry groups, whereas the upper range of values of absenteeism (w=109.2) yielded negative cost differences for four of the industry groups. With the base case value of absenteeism for RA (w=38.6) and an inferred value for presenteeism (r=162), the cost difference was negative for all industry groups, ranging to approximately –$6.2 million for Utilities.
Discussion
Measurement of presenteeism
In the one-way sensitivity analysis, presenteeism had a potentially decisive effect on the productivity cost difference. It has proven difficult to quantify the amount of work time lost due to arthritis-related presenteeism. In practice, presenteeism is measured subjectively by questionnaire14,32,37,40. There have been a few attempts to validate these questionnaires against an objective measure of ‘widget production’Citation41, although the Work Limitations Questionnaire has been validated against objective measures of work output by customer service representativesCitation42. Nevertheless, estimates of the work loss time due to arthritis-related presenteeismCitation14,Citation16 should be viewed as approximations. Furthermore, studies reporting the value of work loss due to presenteeism did so for self-reported arthritis and might not be representative of the population with RA Citation14–16. The value for absenteeism in the first of these studies (4.3 days annually)Citation14 is outside the range of values for RA reported in the literature (7.1 days)Citation13 and the value in the second study (9.1 days) is just within that rangeCitation15,Citation16.
Valuation of work loss
Whilst absenteeism is relatively easy to measure, it is difficult to quantify the amount of productivity loss resulting from a given duration of absenteeism or the monetary value of that productivity loss. The first point of contention is the relationship between lost work time and lost productivity, captured in the model by the productivity cost modifier m. The main approaches to this are generally labelled the human capital and friction cost approachesCitation29,Citation43,Citation44. In the human capital approach, the amount of productivity lost in a unit of time of work absence equals the amount of productivity that would have been produced had the worker been present (i.e. m equals 1). In the friction cost approach, m is <1 for absences shorter than the friction period, defined as absences shorter than those that lead to replacement, and productivity losses may be considerably less than those computed under the human capital approachCitation44. Nicholson et alCitation30 hypothesised that m could be >1 and that it varied with certain job characteristics—team production, time sensitivity and availability of substitutes. The second point of disagreement is the valuation of production loss. The value of a unit of productivity loss is generally equated with the absent employee's compensation rate. From the perspective of the employer in private industry, however, the value of productivity loss is its value addedCitation31, since the value of the widgets that would have been produced is greater than the compensation paid to the employee necessary to produce those widgets.
Measurements of the effects of biologicals on productivity loss
In pharmacoeconomic modelling studies, the effects of biological DMARDs on productivity losses have generally been inferred from their effects on the Health Assessment Questionnaire (HAQ) score Citation45–48, and the measure of productivity loss has been employment status (employed or unemployed)Citation46,Citation49–52. Employment status is a measure of productivity loss from the perspective of society but not from the perspective of the private employer. There are several reports that infliximab reduces work loss in patients with RA47,48,53,54. Other studies of infliximab included absenteeism as an outcome, but not in patients with RA. In the Infliximab Multinational Psoriatic Arthritis Clinical Trial II (IMPACT 2), infliximab significantly increased perceived productivity (patients’ general productivity at work, at home and/or at school, measured on a visual analogue scaleCitation55) and reduced the percentage of patients with limitations in their work and other daily activities, measured using items from the Short Form 36 (SF-36), by a factor of 0.38Citation56. In the Ankylosing Spondylitis Study for the Evaluation of Recombinant Infliximab Therapy (ASSERT) of patients with ankylosing spondylitis, the number of lost work days was an annualised 13.6 days in the placebo group and 5.6 days in the infliximab group, a proportional reduction of 0.59Citation55. At 6 months, 64% of patients in the infliximab group reported limitations in work and daily activities compared with 79% in the placebo group, a proportional reduction of 0.19. These proportional effects of biologicals on activity limitation and work loss are comparable with the range of 0.22–0.57 for the value for a in this analysis (a, the proportion by which abatacept reduces work loss). It should be noted, however, that there was no evident positive effect of therapy with anti-TNFs on work disability in a recent cohort studyCitation57.
Limitations
This analysis has several limitations. The effect of abatacept on RA-related absenteeism was modelled from its effects on usual activities rather than specifically on work loss, and it was assumed that the effects of abatacept on work loss, worker displacement and time off for care-giving were all equivalent. It is not known whether these assumptions are accurate. Employee age was not included because the source data for RA-related absenteeism are averages across age groups. In addition, there is little information about variation in the prevalence of RA by age categoryCitation10, Citation58–60, and the prevalence rates applied here might differ from those among the employed population. Similarly, RA-related absenteeism is not reported by sex in the literature. The effect of abatacept on usual activities, however, did not vary statistically significantly by age or by sex (unpublished observation).
This productivity cost analysis applies to the large employer in US private industry. Applying the model to small firms or to other sectors of the US economy would entail changes in the values of some input variables. In 2006, 60% of workers in private industry worked for large firms (≥200 employees) and 47% worked for firms of ≥1,000 employeesCitation61. Employee compensation is lower for small firms. In 2006, employee compensation (sum of e and b) was $20.24 for firms of 1–49 workers compared with $35.94 for firms of ≥500 workersCitation27. Thus, the value of the productivity loss due to RA would be less for smaller firms.
Among other approximations and simplifications in the productivity cost model, the authors assumed that all employees of large firms were covered by employer-provided health insurance. In fact, 63% of employees of large firms were covered by their employers’ health benefits in 2006 compared with 53% of employees of small firms (although 98% of all large firms and 60% of small firms offered health insurance)Citation61. The remaining employees included those covered through their spouse's employer, and others who were ineligible or refused healthcare coverage. Part-time employees were excluded from consideration. Part-time employees are less likely to have employer-provided health insurance: as of 2006, only 31% of all firms that offered health insurance offered it to part-time workers, although this value was 40% for firms of 200–999 workers and 55% for firms of 1,000–4,999 workersCitation61. The costs of RA-related disability in the model did not include disability payments. Permanent disability payments come from an insurance fund, not directly from the employer.
An analysis by job type was not included. The RA literature does not report absenteeism by job type. Several studies have shown that there is an independent relationship between RA-related work disability and a manual work type variously reported as service industry work or work with more physical and fewer discretionary activitiesCitation62, non-professional or non-administrative workCitation63, non-professional or non-managerial workCitation64, manual or semi-manual workCitation23 and blue collar workCitation65. First, it is difficult to translate these job types to the specific job descriptions in a given industry. Second, the one-way sensitivity analysis showed that the rate of worker displacement due to RA disability is not critical. And third, the range of rates of RA-related work disability reported among workers overall is much greater than the difference in rates between manual and non-manual work types in a given study (e.g. the difference in the annual rates of RA-related job displacement reported by Young et alCitation23 for manual and sedentary workers (0.098 and 0.064, respectively) is small compared with the range of 0.036–0.262 reported overall ()).
The productivity cost model described here is not directly applicable to countries in Europe and elsewhere that have national health insurance systems. In addition to requiring changes in the values for data inputs (e.g. the default number of working weeks in the year and the range of hourly wage rates), changes in the model structure would be required. Adaptation to the societal perspective would enlist variables such as employment status, which was not considered here.
The proportion by which abatacept reduces work loss, a, was the only variable in the model that was sourced in a clinical trial (AIM), and so questions regarding the representativeness of the AIM trial population are only relevant as they pertain to the value of a. Patients in AIM seem to have had more severe functional impairment than RA patients in general: patients in AIM had a mean score on the HAQ of 1.7 (sd 0.6), whereas a median HAQ score of 1.1 (range 0.6–1.3) has been reported in studies of RA (the HAQ is scored on a scale from 0–3)Citation13. Whether or not the value of a varies with RA severity or functional impairment is not known. In addition, in AIM conventional DMARD therapy was defined as methotrexate, but in clinical practice a variety of conventional DMARDs are used. A cross-section of the drugs used by patients in the CORRONA database indicated that 59.6% used methotrexate, 33.1% used biologicals and 40.5% used combination DMARD therapyCitation12. The source for a, the proportion by which abatacept reduces work loss, was the AIM trial, where methotrexate was the comparator6. The point value of a calculated using the 6-month data from the Abatacept Trial in Treatment of Anti-TNF Inadequate Responders (ATTAIN), in which patients took methotrexate, hydroxychloroquine, sulfasalazine and leflunomide etc., was 0.31, not statistically significantly different from the value from the AIM trialCitation7. The proportion of patients qualified for abatacept, i, was calculated for patients not responding to methotrexate. Factoring in other conventional DMARDs would tend to increase this proportion because the discontinuation rate of other DMARDs is greater than that for methotrexateCitation4,Citation11,Citation66. In addition, drug treatment costs with usual DMARDs should have included a certain proportion of patients using biologicals, which are unlikely to be used in combination with abatacept. This would reduce the incremental cost of drug treatment in the abatacept population and hence decrease the cost difference.
Conclusions
In conclusion, a model based on epidemiological and economic data indicates that the cost of RA-related productivity losses for a US firm of 10,000 employees is $1.69 million per year. Much of the acquisition cost of abatacept of $1.16 million is offset by reductions in lost productivity costs: the productivity cost reduction of $0.43 million (productivity cost of RA $1.09 million without abatacept minus $0.66 with abatacept) represents approximately 37% of the abatacept cost. These figures are averages across US private industry. In some industry groups (Utilities and Finance), the reductions in the costs of lost productivity (i.e. the costs savings) exceed the abatacept cost. Scenarios that include presenteeism reduce the cost difference and could make abatacept cost saving across all industry groups. Future research should be aimed at objectively measuring the contribution of presenteeism to RA-related productivity loss and should also directly address the effects of abatacept and other biologicals on presenteeism, absenteeism and work disability.
Acknowledgements
Declaration of interest: Wayne Burton and Alan Morrison received funding from Bristol Myers Squibb for the work carried out in this study. Riccardo Marioni has received sponsorship from Bristol Myers Squibb, and Yong Yuan, Tracy Li and Ross Maclean are employees of the company.
The authors are grateful to Stephen J Gange PhD, for providing unpublished data and to Victoria Stern for assisting in the preparation of this manuscript.
Notes
References
- Scott DL, Kingsley GH. Tu mor necrosis factor inhibitors for rheumatoid arthritis. New England Journal of Medicine 2006; 355, 704–712
- Akil M, Amos RS. ABC of rheumatology. Rheumatoid arthritis—I: clinical features and diagnosis. British Medical Journal 1995; 310: 587–590
- O’Dell JR. Therapeutic strategies for rheumatoid arthritis. New England Journal of Medicine 2004; 350: 2591–2602
- Aletaha D, Smolen JS. Effectiveness profiles and dose dependent retention of traditional disease modifying antirheumatic drugs for rheumatoid arthritis. An observational study. Journal of Rheumatology 2002; 29: 1631–1638
- Criscione LG, St Clair EW. Tumor necrosis factor-alpha antagonists for the treatment of rheumatic diseases. Current Opinion in Rheumatology 2002; 14: 204–211
- Kremer JM, Genant HK, Moreland LW, et al. Effects of abatacept in patients with methotrexate-resistant active rheumatoid arthritis: a randomized trial. Annals of Internal Medicine 2006; 144: 865–876
- Genovese MC, Becker JC, Schiff M, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. New England Journal of Medicine 2005; 353: 1114–1123
- Westhovens R, Schiff M, Dougados M, et al. Abatacept increases activity levels in rheumatoid arthritis patients with inadequate responses to methotrexate or anti-TNF therapy. Annals of the Rheumatic Diseases 2005; 64((S3))398
- U.S. Department of Labor. Bureau of Labor Statistics. Employment and earnings. Annual average data;. January 2006.
- Gabriel SE, Crowson CS, O’Fallon WM. The epidemiology of rheumatoid arthritis in Rochester, Minnesota, 1955–1985. Arthritis and Rheumatism 1999; 42: 415–420
- Maetzel A, Wong A, Strand V, et al. Meta-analysis of treatment termination rates among rheumatoid arthritis patients receiving disease-modifying anti-rheumatic drugs. Rheumatology (Oxford, England) 2000; 39: 975–981
- Tutuncu Z, Reed G, Kremer J, et al. Do patients with older-onset rheumatoid arthritis receive less aggressive treatment? Annals of the Rheumatic Diseases 2006; 65: 1226–1229
- Burton W, Morrison A, Maclean R, et al. Systematic review of studies of productivity loss due to rheumatoid arthritis. Occupational Medicine (Oxford, England) 2006; 56: 18–27
- Stewart WF, Ricci JA, Chee E, et al. Lost productive time and cost due to common pain conditions in the US workforce. Journal of the American Medical Association 2003; 290: 2443–2454
- Gignac MA, Badley EM, Lacaille D, et al. Managing arthritis and employment: making arthritis-related work changes as a means of adaptation. Arthritis and Rheumatism 2004; 51: 909–916
- Li X, Gignac MA, Anis AH. The indirect costs of arthritis resulting from unemployment, reduced performance, and occupational changes while at work. Medical Care 2006; 44: 304–310
- U.S. Census Bureau. America's Families and Living Arrangements: 2005. Available at: http://www.census.gov/population/www/socdemo/hh-fam/cps2005.html [accessed 5 September 2006].
- Brouwer WB, van Exel NJ, van de Berg B, et al. Burden of caregiving: evidence of objective burden, subjective burden, and quality of life impacts on informal caregivers of patients with rheumatoid arthritis. Arthritis and Rheumatism 2004; 51: 570–577
- Maetzel A, Li LC, Pencharz J, et al. The economic burden associated with osteoarthritis, rheumatoid arthritis, and hypertension: a comparative study. Annals of the Rheumatic Diseases 2004; 63: 395–401
- Society for Human Resource Management.Human Capital Benchmarking Study,. 2005
- Barron JM, Black DA, Loewenstein MA. Job matching and on-the-job training. Journal of Labor Economics 1989; 7: 1–19
- Gabriel SE, Crowson CS, Campion ME, et al. Indirect and nonmedical costs among people with rheumatoid arthritis and osteoarthritis compared with nonarthritic controls. Journal of Rheumatology 1997; 24: 43–48
- Young A, Dixey J, Kulinskaya E, et al. Which patients stop working because of rheumatoid arthritis? Results of five years’ follow up in 732 patients from the Early RA Study (ERAS). Annals of the Rheumatic Diseases 2002; 61: 335–340
- Reisine S, Fifield J, Walsh SJ, et al. Factors associated with continued employment among patients with rheumatoid arthritis: a survival model. Journal of Rheumatology 2001; 28: 2400–2408
- Albers JM, Kuper HH, van Riel PL, et al. Socio-economic consequences of rheumatoid arthritis in the first years of the disease. Rheumatology (Oxford, England) 1999; 38: 423–430
- U.S. Department of Labor. Bureau of Labor Statistics. May 2005 occupational employment and wage estimates: national cross-industry estimates. Available at: http://www.bls.gov/oes/oes_dl.htm [accessed 16 October 2007].
- U.S. Department of Labor. Bureau of Labor Statistics. Employer costs for employee compensation;. March 2006.
- Smith RE, Lum SK. Annual industry accounts. Revised estimates for 2002–2004. Survey of Current Business 2005; 85: 18–69
- Koopmanschap MA, Rutten FF. A practical guide for calculating indirect costs of disease. PharmacoEconomics 1996; 10: 460–466
- Nicholson S, Pauly MV, Polsky D, et al. Measuring the effects of work loss on productivity with team production. Health Economics 2006; 15: 111–123
- Guico-Pabia CJ, Murray JF, Teutsch SM, et al. Indirect cost of ischemic heart disease to employers. American Journal of Managed Care 2001; 7: 27–34
- Burton WN, Pransky G, Conti DJ, et al. The association of medical conditions and presenteeism. Journal of Occupational and Environmental Medicine 2004; 46((6 Suppl))S38–S45
- Hider SL, Bruce IN, Silman AJ, et al. Defining response to disease modifying antirheumatic drugs in patients with rheumatoid arthritis. Journal of Rheumatology 2005; 32: 6–10
- Mikuls TR, O’Dell J. The changing face of rheumatoid arthritis therapy: results of serial surveys. Arthritis and Rheumatism 2000; 43: 464–465
- Sokka T, Willoughby J, Yazici Y, et al. Databases of patients with early rheumatoid arthritis in the USA. Clinical and Experimental Rheumatology 2003; 21((5 Suppl 31))S146–S153
- Gibofsky A, Palmer WR, Goldman JA, et al. Real-world utilization of DMARDs and biologics in rheumatoid arthritis: the RADIUS (Rheumatoid Arthritis Disease-Modifying Anti-Rheumatic Drug Intervention and Utilization Study) study. Current Medical Research and Opinion 2006; 22: 169–183
- Burton WN, Chen CY, Schultz AB, et al. Worker productivity loss associated with arthritis. Disease Management 2006; 9: 131–143
- Lerner D, Reed JI, Massarotti E, et al. The Work Limitations Questionnaire's validity and reliability among patients with osteoarthritis. Journal of Clinical Epidemiology 2002; 55: 197–208
- Wells GA, Li T, Maxwell L, et al. Responsiveness of patient reported outcomes including fatigue, sleep quality and activity limitation and quality of life following treatment with abatacept in patients with rheumatoid arthritis. Annals of the Rheumatic Diseases 2007., Epub ahead of print]
- Wang PS, Beck A, Berglund P, et al. Chronic medical conditions and work performance in the health and work performance questionnaire calibration surveys. Journal of Occupational and Environmental Medicine 2003; 45: 1303–1311
- Kessler RC, Barber C, Beck A, et al. The World Health Organization Health and Work Performance Questionnaire (HPQ). Journal of Occupational and Environmental Medicine 2003; 45: 156–174
- Lerner D, Amick 3rd BC, Lee JC, et al. Relationship of employee-reported work limitations to work productivity. Medical Care 2003; 41: 649–659
- Jacob-Tacken KH, Koopmanschap MA, Meerding WJ, et al. Correcting for compensating mechanisms related to productivity costs in economic evaluations of health care programmes. Health Economics 2005; 14: 435–443
- Lofland JH, Locklear JC, Frick KD. Different approaches to valuing the lost productivity of patients with migraine. PharmacoEconomics 2001; 19: 917–925
- Doan QV, Chiou CF, Dubois RW. Review of eight pharmacoeconomic studies of the value of biologic DMARDs (adalimumab, etanercept, and infliximab) in the management of rheumatoid arthritis. Journal of Managed Care Pharmacy 2006; 12: 555–569
- Kobelt G, Eberhardt K, Jonsson L, et al. Economic consequences of the progression of rheumatoid arthritis in Sweden. Arthritis and Rheumatism 1999; 42: 347–356
- Kavanaugh A, Han C, Bala M. Functional status and radiographic joint damage are associated with health economic outcomes in patients with rheumatoid arthritis. Journal of Rheumatology 2004; 31: 849–855
- Smolen JS, Han C, van der Heijde D, et al. Infliximab treatment maintains employability in patients with early rheumatoid arthritis. Arthritis and Rheumatism 2006; 54: 716–722
- Choi HK, Seeger JD, Kuntz KM. A cost-effectiveness analysis of treatment options for patients with methotrexate-resistant rheumatoid arthritis. Arthritis and Rheumatism 2000; 43: 2316–2327
- Choi HK, Seeger JD, Kuntz KM. A cost effectiveness analysis of treatment options for methotrexate-naive rheumatoid arthritis. Journal of Rheumatology 2002; 29: 1156–1165
- Kobelt G, Jonsson L, Young A, et al. The cost-effectiveness of infliximab (Remicade) in the treatment of rheumatoid arthritis in Sweden and the United Kingdom based on the ATTRACT study. Rheumatology (Oxford, England) 2003; 42: 326–335
- Wong JB, Singh G, Kavanaugh A. Estimating the cost-effectiveness of 54 weeks of infliximab for rheumatoid arthritis. American Journal of Medicine 2002; 113: 400–408
- Yelin E, Trupin L, Katz P, et al. Association between etanercept use and employment outcomes among patients with rheumatoid arthritis. Arthritis and Rheumatism 2003; 48: 3046–3054
- Kobelt G, Eberhardt K, Geborek P. TNF inhibitors in the treatment of rheumatoid arthritis in clinical practice: costs and outcomes in a follow up study of patients with RA treated with etanercept or infliximab in southern Sweden. Annals of the Rheumatic Diseases 2004; 63: 4–10
- van der Heijde D, Han C, Devlam K, et al. Infliximab improves productivity and reduces workday loss in patients with ankylosing spondylitis: results from a randomized, placebo-controlled trial. Arthritis and Rheumatism 2006; 55: 569–574
- Kavanaugh A, Antoni C, Mease P, et al. Effect of infliximab therapy on employment, time lost from work, and productivity in patients with psoriatic arthritis. Journal of Rheumatology 2006; 33: 2254–2259
- Wolfe F, Allaire S, Michaud K. The prevalence and incidence of work disability in rheumatoid arthritis, and the effect of anti-tumor necrosis factor on work disability. Journal of Rheumatology 2007, [Epub ahead of print]
- Jacobson DL, Gange SJ, Rose NR, et al. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clinical Immunology and Immunopathology 1997; 84: 223–243
- Gabriel SE. The epidemiology of rheumatoid arthritis. Rheumatic Diseases Clinics of North America 2001; 27: 269–281
- Kvien TK. Epidemiology and burden of illness of rheumatoid arthritis. PharmacoEconomics. 2004; 22((2 Suppl))1–12
- Kaiser Family Foundation.Employer Health Benefits. 2006 annual survey. 2006 Kaiser Family Foundation
- Yelin E, Henke C, Epstein W. The work dynamics of the person with rheumatoid arthritis. Arthritis and Rheumatism 1987; 30: 507–512
- Allaire SH, Anderson JJ, Meenan RF. Reducing work disability associated with rheumatoid arthritis: identification of additional risk factors and persons likely to benefit from intervention. Arthritis Care and Research 1996; 9: 349–357
- De Roos AJ, Callahan LF. Differences by sex in correlates of work status in rheumatoid arthritis patients. Arthritis Care and Research 1999; 12: 381–391
- Verstappen SM, Boonen A, Bijlsma JW, et al. Working status among Dutch patients with rheumatoid arthritis: work disability and working conditions. Rheumatology (Oxford, England) 2005; 44: 202–206
- Maradit-Kremers H, Nicola PJ, Crowson CS, et al. Patient, disease, and therapy-related factors that influence discontinuation of disease-modifying antirheumatic drugs: a population-based incidence cohort of patients with rheumatoid arthritis. Journal of Rheumatology 2006; 33: 248–255