Abstract
Objective: This systematic review examines the published evidence on the pharmacoecomonics of Symbicort®. Symbicort is a combination inhaler used in asthma and chronic obstructive pulmonary disease (COPD) that contains budesonide and formoterol. In asthma, Symbicort can be used as fixed or adjustable dose maintenance therapy as well as for both maintenance and reliever therapy (SMART).
* Symbicort is a registered tramdemark of the AstraZeneca group of companies.
Method: A literature search of PubMed was carried out to find all publications on the pharmacoeconomics of Symbicort. Additional studies were searched for in the reference lists of the papers retrieved and by searching tables of contents of relevant journals. A total of 13 studies on Symbicort in asthma and 2 studies on Symbicort in COPD were found.
Results: Total costs were lower with Symbicort than with separate inhalers containing budesonide and formoterol. Adjustable dosing maintained control of asthma using less medication and was associated with lower treatment costs than fixed dosing with Symbicort or the combination of fluticasone/salmeterol. SMART improves asthma control, reduces exacerbations and reduces direct and indirect costs compared to fixed maintenance therapy with either Symbicort or fluticasone/salmeterol. In COPD, Symbicort offers clinical advantages over therapy with the monocomponents and these are achieved at little or no extra cost.
Keywords::
Introduction
Asthma and chronic obstructive pulmonary disease (COPD) are both diseases characterised by airflow obstruction. Symbicort® is a combination inhaler used in both asthma and COPD and it contains the β-agonist bronchodilator formoterol, which has both a rapid onset of action and a long duration of action, and the corticosteroid budesonide.
In asthma the airflow inflammation is caused by constriction of airway smooth muscle, which itself is a response to mucosal inflammation. COPD is also associated with inflammation in the airways, but the cellular nature of this inflammation is different to that seen in asthma and, as well as causing airflow obstruction, there is parenchymal damage leading to emphysema, pulmonary vascular effects leading to pulmonary hypertension and cor pulmonale, and systemic effects including skeletal muscle dysfunction, osteoporosis and cardiac disease.
Burden of asthma
Asthma is one of the most common chronic diseases in the world. It is estimated that around 300 million people in the world currently have asthma and that the disease accounts for about 250,000 deaths per year worldwideCitation1. In many countries, deaths due to asthma have declined recently as a result of better management and in countries where national asthma management plans have been implemented, hospitalisation rates have also decreasedCitation2. In the US, however, the overall death rate has increased, reflecting increased mortality in poor minority groups with inadequate access to healthcareCitation3.
Poorly controlled asthma leads to disturbed sleep, restriction of exercise and interferes with the patients' ability to work. Childhood asthma accounts for many lost school days and may impair academic achievement.
The direct and indirect economic costs of asthma are considerableCitation1,Citation4. Direct costs represent between 1 and 3% of total medical expenditures in most countries. In 2007, the total costs of asthma in the US was estimated to be $19.7 billion, with direct medical costs amounting to $14.7 billionCitation5. The main factors determining the direct costs are medication, hospitalisations and visits to emergency departmentsCitation6,Citation7. The costs of asthma are related to the severity of diseaseCitation8,Citation9 and patients with poorly controlled asthma consume a disproportionate share of asthma healthcare resourcesCitation10, reflecting increased exacerbation and hospitalisation rates.
Symbicort and asthma
The combination of an inhaled steroid (ICS)and a long-acting β-agonist (LABA) is recommended when a medium dose of inhaled glucocorticoid fails to achieve control of asthmaCitation11,Citation12. Combination therapy improves symptom scores, decreases nocturnal asthma, improves lung function, decreases the use of reliever medication and reduces the number of exacerbationsCitation13–15. As well as being used as a fixed-dose maintenance therapy, the dose response of formoterol means that the maintenance dose of Symbicort can be adjusted according to symptoms. This is known as adjustable maintenance dosing. Furthermore, because formoterol has a rapid onset of action, Symbicort can be used for both maintenance and rescue therapy and this is known as SMART therapy
Burden of chronic obstructive pulmonary disease
The burden to the patient with COPD is high, both in terms of symptoms and health-related quality of life. Patients experience poor physical functioning and live with distressing symptoms that require frequent hospital admission as the disease progresses. They are frequently unable to work and become socially isolated and often depressed. COPD also takes its toll on the patient's spouse, carer and family.
As well as the burden imposed by stable disease, COPD patients live with the fear of exacerbations, which are common at all levels of lung function. Exacerbations are associated with significant mortality, lead to frequent hospitalisation and are a major determinant of COPD costs. Recovery from exacerbations is slow, often incomplete and recurrent exacerbations lead to a step-wise decline in lung function and physical statusCitation16.
COPD generates a high societal and economic cost. The major drivers of the direct cost of COPD are hospital care, medication and oxygen therapy. According to the US National Heart Lung and Blood Institute, the total cost of COPD in 2007 was $42.6 billion, with direct costs of $26.7 billion, and hospitalisation accounting for $11.3 billionCitation17. In Europe, the total annual cost of COPD is estimated at €38.7 billion (US$51.2 billion)Citation18. Up to 60% of the total costs of COPD may be attributable to indirect costsCitation19. In Europe, a total of 28.5 million workdays are lost due to COPDCitation18. In the UK, COPD is responsible for 9% of certified sickness absence (i.e. 24 million working days lost), with social security costs and the cost of lost productivity estimated at £600 million and £1.5 billion per year, respectivelyCitation20. Economic analysis of the Confronting COPD in North America and Europe surveyCitation21 estimated the total cost (direct and indirect) of COPD at up to US$4,119 per patient per year, with just over half of the average direct costs (54%) due to hospitalisation and a further 6% attributable to other unscheduled care. Drugs accounted for only 16% of the average annual costs, whilst oxygen accounted for 3% and investigations for 5%Citation22.
Exacerbations and hospitalisations constitute the most important direct healthcare cost associated with COPD, accounting for between 40 and 63% of the total direct costsCitation17,Citation23,Citation24. The mean cost of hospital admission in a cohort of US patients with severe COPD was estimated to be US$7,100Citation25.
Symbicort and chronic obstructive pulmonary disease
Current therapies for COPD, both pharmacological and non-pharmacological, aim to minimise patients symptoms and reduce the risk of exacerbations. In contrast to asthma, COPD guidelines recommend adding inhaled steroids to long-acting β-agonist therapy to reduce exacerbation rates in patients at increased risk of exacerbationsCitation26,Citation27. Symbicort, through its components, leads to sustained bronchodilation, a reduced need for rescue medication, improved health status and reduced exacerbation ratesCitation28,Citation29.
Methods
Pharmacoeconomic evaluations of Symbicort were identified by searching PubMed (indexed for MEDLINE) using the keywords ‘budesonide’, ‘formoterol’, ‘cost’, ‘economics’ and ‘outcomes’. Additional studies were identified by the author from the reference lists of the papers retrieved and by searching tables of contents of recent issues of relevant journals.
A total of 42 publications were identified by these searches and an additional 3 recently published papers were also found. Review articles, studies that did not contain health economic analyses and studies that examined the health economics of budesonide and formoterol in separate inhalers were excluded. This left 13 studies on health economic aspects of Symbicort in asthma and 2 on the health economics of Symbicort in COPD.
Results
Symbicort as fixed-dose maintenance therapy in asthma
One study compared the total treatment costs for patients with asthma followed for 12 months in an open randomised study of 321 patients in SwedenCitation30. Patients were treated with Symbicort 160/4.5 two inhalations twice a day or the same doses of budesonide and formoterol administered via separate inhalers. Patients recorded resource utilisation during the study period. Despite the fact that patients were prescribed the same dose of medication, those receiving them in the single inhaler had fewer emergency room visits, required less additional asthma medication and needed fewer physician visits, resulting in substantial overall cost savings of SEK1,884 per person per year (p=0.043). It was suggested that the better outcomes were the result of better adherence to treatment when using a single inhaler and there were fewer withdrawals from the Symbicort group (9.2 vs. 19.4%, p=0.008). Data from the patients who withdrew were handled using the patient-year approach in which costs are extrapolated to correspond with the study duration, thus potentially overestimating total healthcare costs associated with patients who incurred high costs before premature withdrawal. The sensitivity analysis showed that both direct and indirect costs were lower for Symbicort, but the difference between overall costs was not statistically significant (SEK1,496, p=0.056).
Fixed dosing with Symbicort 160/4.5 was compared to fluticasone 250 μg in an economic evaluation of a multinational, 12-week, randomised, controlled trial involving 373 patientsCitation31. The primary effectiveness variable was the number of episode-free days (defined as a day with no asthma symptoms, no need for rescue medication, no nighttime awakening and morning peak flow of at least 80% baseline). The main analysis concentrated on direct healthcare costs but secondary analyses, one of which included indirect costs and another which was restricted to medication costs, were also undertaken. Healthcare resource utilisation and days when the patient was unable to work were recorded. Data on the 35 patients who withdrew prematurely were again handled using the patient-year approach but sensitivity analyses were also performed using group mean imputation to estimate costs for each day after discontinuation. Differences in resource utilisation patterns in the six countries involved in the study were assessed using a x2 test for the absence of heterogeneity. This showed that physician visits were not homogeneous, with one country accounting for nearly half of all reported visits despite only contributing 65 patients. Sensitivity analyses were therefore carried out excluding the data contributed by this country. Costs were calculated for the whole study population using German and Dutch unit cost data. The costs were presented in Euros. Using German unit costs, Symbicort was associated with lower total healthcare costs per patient than fluticasone (€131 vs. €210, p=0.0043), but when Dutch unit costs were used there was no statistically significant difference (€102 vs. €104). This was due to differences in the price of Symbicort (€0.67 v €0.54) and fluticasone (€0.87 vs. €0.30) in Germany and The Netherlands. Taking into account indirect costs, Symbicort also resulted in significantly lower costs in Germany (€165 vs. €314, p=0.0254) but not in The Netherlands (€128 vs. €186, p>0.05). From a drug budget holder's point of view total medication costs were lower in patients on Symbicort using German costs (€114 vs. €154) but not when using Dutch costs (€96 vs. €61), reflecting the lower price of fluticasone in The Netherlands. Sensitivity analyses excluding the country with the high rate of physician visits and using group mean imputation to handle patients who withdrew prematurely showed results similar to the main analyses.
Fixed dosing with Symbicort 160/4.5 twice daily was compared to fluticasone propionate/salmeterol (SFC) 250/50 Accuhaler in an economic model designed to determine at what point in asthma management ICS/LABA therapy becomes cost effectiveCitation32. The model used meta-analyses of published and unpublished studies reporting symptom-free days as an outcome and the results were presented as cost per quality-adjusted life year ratios. To compare the cost effectiveness of Symbicort with SFC, data from a 12-week, multicentre, randomised, controlled trial were usedCitation33. Although there was no significant difference in the percentage of symptom-free days (incremental percentage for SFC 0.40 95% confidence interval (CI) -3.69, 4.49) SFC was cheaper (incremental treatment costs -£18.26). In a further analysis based purely on medication costs and assuming therapeutic equivalence, SFC was judged to be cheaper in 10 out of 11 dose comparisons; however, this analysis is entirely dependent on the retail costs of the medication.
Symbicort as adjustable maintenance therapy in asthma
shows the trial design, primary outcome variables and summary results of the health economic analyses of Symbicort used as adjustable maintenance dosing (AMD). All studies were open label because of the nature of AMD, but all were randomised and had a parallel group design. Three studies were of 12-week duration and compared AMD to fixed dosing with Symbicort. Two used health-related quality of life as the outcome and looked at total treatment costs. The third used exacerbation frequency and asthma symptoms as the outcome and looked at total direct and indirect costs. In all three studies AMD was as effective as the fixed-dose therapy but patients used less medication and thus total treatment costs favoured Symbicort AMDCitation34–36.
Table 1. Trial design, primary outcome variables and summary results of economic analyses of Symbicort used as AMD.
In two longer studies, Symbicort AMD achieved better asthma control than either fixed-dose Symbicort or fluticasone/salmeterol. This was achieved at lower daily medication costs when prices for five European countries were usedCitation37, and resulted in lower total treatment costs in a Canadian settingCitation38.
Price et al. modeled the budget impact of switching all patients in the UK on fixed-dose Symbicort to AMDCitation35. Sensitivity analyses predicted savings of between £1.2 million and £3.9 million. The base case showed savings of £2.6 million.
Symbicort as maintenance and reliever therapy in asthma
shows the trial design, primary outcome variables and summary results of the health economic analyses of Symbicort used as both maintencance and reliever therapy (SMART). Five economic analyses have been published. Two of these use the same efficacy data but apply different resource costs based on either four European countriesCitation39 or Canadian valuesCitation35. The clinical trial data supporting these analyses and one other analysisCitation40 were based on 12-month, open-label, randomised, parallel group studies, whilst the remaining two analyses were based on 6-month, double-blind, randomised studiesCitation41,Citation42.
Table 2. Trial design, primary outcome variables and summary results of economic analyses of Symbicort as SMART.
In all these studies the SMART approach was associated with better asthma control and lower treatment costs than either higher fixed-dose Symbicort or fluticasone/salmeterol. Using UK unit costs the 6-month randomised controlled trial showed mean direct cost savings of £82 (95% CI £56-£107) versus fluticasone/salmeterol and £73 (95% CI £48-£95) versus fixed-dose Symbicort. The trial also showed mean total costs savings of £87 (95% CI £31-£141) versus fluticasone/salmeterol and £91 (95% CI £36-£145) versus fixed-dose Symbicort.
Symbicort in chronic obstructive pulmonary disease
Two studies of the cost effectiveness of Symbicort in patients with COPD have been published. One is a health economic evaluation based on a 12-month, randomised, controlled trialCitation28 using Swedish medication and healthcare utilisation costsCitation44. The other used an analytical model to assess the cost effectiveness of different therapeutic strategies, including the use of Symbicort, for the management of patients with severe and very severe COPD (as defined by the Global Initiative for COPD guidelines) using Italian medication costs and estimates of direct and indirect costsCitation45.
During the 12-month, randomised, controlled trial utilisation of healthcare resources and medication were recorded by all patients on diary cards and these data were used for the economic evaluation. Medication costs were estimated from the Swedish Pharmacopoeia and Swedish unit costs were used to calculate healthcare resource utilisation costs. The results were converted to Euros. The total healthcare costs per patient per year were €2518 (95% CI €1273-€3764) for patients treated with Symbicort, €3194 (95% CI €1943-€4445) for patients treated with budesonide, €3653 (95% CI €2381-€4925) for patients treated with formoterol and €3212 (95% CI €1921-€4504) for patients treated with placeboCitation44. The higher medication costs for Symbicort were offset by the greater reduction in exacerbations and associated hospitalisation costs. The cost effectiveness ratio for Symbicort versus the monocomponents and placebo was estimated using a bootstrap technique involving 500 bootstraps. On a cost effectiveness plane, although the cost and effect pairs for Symbicort versus budesonide centered around the origin, there was a tendency for the pairs to favour Symbicort for both cost and effectiveness (). Compared to formoterol and placebo, Symbicort was consistently more effective and was dominant compared to formoterol regarding cost (). When the cost and effect pairs were plotted as cost effectiveness acceptability curves, the curve for Symbicort versus formoterol was above the 95% proportion line at all values of willingness to pay and the curve versus placebo intersected the 95% proportion line at just above €2 per day.
Figure 1. Cost-effectiveness planes showing the distribution of cost (€, year 2001 values) and effect pairs (based on a bootstrap, n = 500). (A) budesonide/formoterol vs. budesonide (B) budesonide/formoterol vs. formoterol and (C) budesonide/formoterol vs. placebo for patients with chronic obstructive pulmonary disease enrolled in a clinical study.
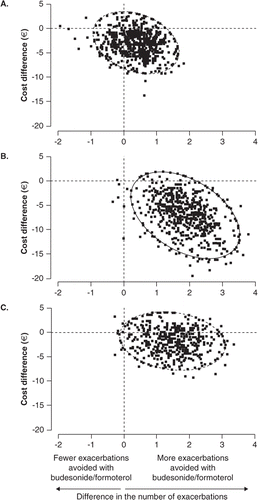
The Italian modeling studyCitation45 combined a 12-month trial of another ICS/LABA combination (fluticasone/salmeterol) versus placebo and its monocomponentsCitation46 and a different 12-month study of Symbicort, which did not include a run-in optimisation phaseCitation29. In addition it also did not use actual healthcare utilisation data from the trials but modeled costs based on differences in exacerbation rates and included an estimate of indirect costs. The model suggested that fluticasone, fluticasone/salmeterol and Symbicort were dominant (i.e. gave a better outcome at smaller cost) compared to placebo, whilst salmeterol alone gave a better outcome compared to placebo, but was more expensive. The incremental cost-effectiveness ratios for Symbicort versus salmeterol were estimated to be €1,392 per exacerbation avoided and €3.48 per symptom-free day. The authors concluded that ICS/LABA combinations had the potential to improve clinical outcomes without increasing healthcare costsCitation47.
Discussion
Asthma and COPD are common chronic diseases that impose major financial burdens on societies and healthcare systems. A number of pharmacological therapies are available for both conditions, and national and international guidelines recommend evidence-based approaches to management. Combination therapy with ICS and LABA is recommended in guidelines for both asthma and COPD but these do not generally differentiate between the different products available.
In asthma the dose response relationship for formoterol means that as well as being used for fixed-dose maintenance therapy, the dose of Symbicort can be increased or decreased to the most appropriate level to control the disease. On average, adjustable dosing resulted in lower medication use whilst maintaining control and was associated with lower treatment costs than fixed dosing with Symbicort or the combination fluticasone and salmeterol. The speed of onset of formoterol also means that Symbicort can be used as reliever therapy as well as maintenance therapy in asthma. This so called SMART approach reduces the frequency of exacerbations leading to reduced direct costs and indirect costs compared to fixed maintenance therapy with either Symbicort or fluticasone/salmeterol, principally as a result of the reduction in exacerbations.
In COPD the clinical differences between Symbicort and combination therapy with fluticasone and salmeterol are less clear. There is, however, good evidence that Symbicort offers clinical advantages over therapy with the monocomponents and this also has health economic benefits.
When considering the economic analyses, medication costs constitute a significant factor in determining cost effectiveness. Symbicort is only formulated in a dry powder inhaler. This is the device that has been used to deliver the drug in the clinical trials and this is the device that is used in routine clinical practice. The combination of fluticasone and salmeterol is available in both a dry powder device and as a metered dose inhaler (MDI). Both devices have been used in the clinical trials and both are used in clinical practice, with the MDI being the most commonly used in most countries (currently 65% in the UK – IMS data). This has considerable impact on cost effectiveness analyses as, in the UK at least, the price of the MDI is substantially more than the dry powder inhaler (£62.29 for the 250/25 Evohaler vs. £40.92 for the 500/50 AccuhalerCitation48).
All the studies of health economic aspects of Symbicort have been sponsored by pharmaceutical companies, either the manufacturers of Symbicort or its competitors. This has the potential to influence publication policies but, when undertaken, the sensitivity analyses have generally supported the advantages of Symbicort. Moreover, the health economic outcomes that have been reported have generally been secondary outcomes of studies principally designed to assess clinical outcomes.
Using Symbicort for patients requiring both a LABA and ICS for their asthma, particularly when used as both maintenance and reliever therapy, would have considerable implications for healthcare budgets. This assessment must be tempered by consideration of the characteristics of the patients enrolled into the clinical studies on which the economic evaluations were based and the duration of these studies. For example, in the clinical trial in which the detailed budget impact of using SMART in the UK was presented, patients entering the study were taking quite high doses of inhaled steroid (equivalent to 740-750 μg beclomethasone) prior to randomisation and this was only a 6-month studyCitation49. Nevertheless, there is no reason to believe that the benefits offered by SMART therapy would reduce over time and thus the long term cost savings could be very large.
COPD is a progressive disease and thus the potential for long-term benefits to patients of combination therapy are less, and it is likely that the frequency of exacerbations would increase over time. However, the economic analyses suggest that benefits to patients in terms of reducing exacerbation rates, and thereby potentially delaying disease progression, and the improved health status that they enjoy are achieved at very little or possibly no additional cost.
Differences in the delivery of care and hospitalisation costs make generalisation of the economic assessments to countries other than those whose unit costs have been modeled difficultCitation50. Despite this, analyses have looked at unit costs in a number of Western European countries as well as in Canada and Australia. In this review costs have been quoted in the original currencies and have not been updated to 2007 values. Although well-established methods such as purchasing price parity can be used to compare costs, differences in unit costs between countries and models of healthcare for asthma and COPD make these comparisons of limited value. However, because Symbicort seems dominant in most of the analyses it is likely that it will offer the economic advantages irrespective of country.
In conclusion, in asthma Symbicort is more cost effective than using the components in separate inhalers. When used flexibly for maintenance therapy Symbicort achieves equivalent control at lower doses and lower direct costs and when used as a single inhaler for both maintenance and relief it offers better control than fixed dosing at a lower cost. In COPD, Symbicort offers better outcomes than therapy with formoterol or budesonide and achieves this at little or no extra total cost.
Acknowledgements
Declaration of interest: The authors has received sponsorship to attend international meetings, and honoraria for lecturing, attending advisory boards and preparing educational materials from Altana, AstraZeneca, GlaxoSmithKline and Boehringer Ingelheim. He has received no payment in the preparation of this manuscript.
Notes
* Symbicort is a registered tramdemark of the AstraZeneca group of companies.
References
- Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee Report. Allergy 2004; 59: 469–478.
- Haahtela T, Laitinen LA. Asthma programme in Finland 1994-2004. Report of a Working Group. Clinical & Experimental Allergy 1996; 26((Suppl. 1))1–24.
- Federico MJ, Liu AH. Overcoming childhood asthma disparities of the innercity poor. Pediatric Clinics of North America 2003; 50: 655–675.
- Weiss KB, Sullivan SD. The health economics of asthma and rhinitis. I. Assessing the economic impact. Journal of Allergy and Clinical Immunology 2001; 107((1))3–8.
- National Heart Lung and Blood Institute. Morbidity and Mortality: 2007 Chartbook on Cardiovascular, Lung and Blood Diseases. Bethesda, MD: National Institutes of Health 2007.
- Birnbaum HG, Berger WE, Greenberg PE, et al. Direct and indirect costs of asthma to an employer. Journal of Allergy and Clinical Immunology 2002; 109: 264–270.
- Schwenkglenks M, Lowy A, Anderhub H, Szucs TD. Costs of asthma in a cohort of Swiss adults: associations with exacerbation status and severity. Value in Health 2003; 6: 75–83.
- Van Ganse E, Laforest L, Pietri G, et al. Persistent asthma: disease control, resource utilisation and direct costs. European Respiratory Journal 2002; 20: 260–267.
- Godard P, Chanez P, Siraudin L, Nicoloyannis N, Duru G. Costs of asthma are correlated with severity: a 1-year prospective study. European Respiratory Journal 2002; 19: 61–67.
- Bootman JL, Crown WH, Luskin AT. Clinical and economic effects of suboptimally controlled asthma. Managed Care Interface 2004; 17: 31–36.
- Global Initiative for Asthma. Global strategy for asthma management and prevention. 2006 [cited 2007 Dec]; Available from: http://www.ginasthma.com
- British guideline on the management of asthma. Thorax 2003; 58((Suppl. 1))i1–i94.
- Pauwels RA, Lofdahl CG, Postma DS, et al. Effect of inhaled formoterol and budesonide on exacerbations of asthma. Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group. New England Journal of Medicine 1997; 337: 1405–1411.
- Lalloo UG, Malolepszy J, Kozma D, et al. Budesonide and formoterol in a single inhaler improves asthma control compared with increasing the dose of corticosteroid in adults with mild-to-moderate asthma. Chest 2003; 123((5))1480–1487.
- Kips JC, O'Connor BJ, Inman MD, Svensson K, Pauwels RA, O'Byrne PM. A long-term study of the antiinflammatory effect of low-dose budesonide plus formoterol versus high-dose budesonide in asthma. American Journal of Respiratory Critical Care Medicine 2000; 161: 996–1001.
- Donaldson GC, Wedzicha JA. COPD exacerbations. 1: Epidemiology. Thorax 2006; 61((2))164–168.
- Hilleman DE, Dewan N, Malesker M, Friedman M. Pharmacoeconomic evaluation of COPD. Chest 2000; 118((5))1278–1285.
- European Respiratory Society and European Lung Foundation. European Lung White Book. Lausanne: European Respiratory Society 2003.
- Ruchlin HS, Dasbach EJ. An economic overview of chronic obstructive pulmonary disease. Pharmacoeconomics 2001; 19((6))623–642.
- Calverley P, Sondhi S. The burden of obstructive lung disease in the UK - COPD and asthma. Thorax 1998; 53((Suppl. 4))A83.
- Wouters EF. Economic analysis of the Confronting COPD survey: an overview of results. Respiratory Medicine 2003; 97((Suppl. C))S3–S14.
- Britton M. The burden of COPD in the U.K.: results from the Confronting COPD survey. Respiratory Medicine 2003; 97((Suppl. C))S71–S79.
- Miravitlles M, Murio C, Guerrero T, Gisbert R. Costs of chronic bronchitis and COPD: a 1-year follow-up study. Chest 2003; 123((3))784–791.
- Andersson F, Borg S, Jansson SA, et al. The costs of exacerbations in chronic obstructive pulmonary disease (COPD). Respiratory Medicine 2002; 96((9))700–708.
- Connors AF Jr, Dawson NV, Thomas C, et al. Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments) [published erratum appears in American Journal of Respiratory Critical Care Medicine 1997; 155(1): 386]. American Journal of Respiratory Critical Care Medicine 1996; 154(4 Pt 1): 959–967.
- Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. American Journal of Respiratory Critical Care Medicine 2007; 176((6))532–555.
- National Institute for Clinical Excellence (NICE). Chronic obstructive pulmonary disease. National clinical guideline for management of chronic obstructive pulmonary disease in adults in primary and secondary care. Thorax 2004; 59((Suppl. 1))1–232.
- Calverley PM, Boonsawat W, Cseke Z, Zhong N, Peterson S, Olsson H. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. European Respiratory Journal 2003; 22((6))912–919.
- Szafranski W, Cukier A, Ramirez A, et al. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. European Respiratory Journal 2003; 21((1))74–81.
- Rosenhall L, Borg S, Andersson F, Ericsson K. Budesonide/formoterol in a single inhaler (Symbicort) reduces healthcare costs compared with separate inhalers in the treatment of asthma over 12 months. International. Journal of Clinical Practice 2003; 57((8))662–667.
- Ericsson K, Bantje TA, Huber RM, Borg S, Bateman ED. Cost-effectiveness analysis of budesonide/formoterol compared with fluticasone in moderate-persistent asthma. Respiratory Medicine 2006; 100((4))586–594.
- Doull I, Price D, Thomas M, et al. Cost-effectiveness of salmeterol xinafoate/fluticasone propionate combination inhaler in chronic asthma. Current Medical Research and Opinion 2007; 23((5))1147–1159.
- Dahl R, Chuchalin A, Gor D, Yoxall S, Sharma R. EXCEL: A randomised trial comparing salmeterol/fluticasone propionate and formoterol/budesonide combinations in adults with persistent asthma. Respiratory Medicine 2006; 100((7))1152–1162.
- Bruggenjurgen B, Selim D, Kardos P, et al. Economic assessment of adjustable maintenance treatment with budesonide/formoterol in a single inhaler versus fixed treatment in asthma. Pharmacoeconomics 2005; 23((7))723–731.
- Price D, Haughney J, Lloyd A, Hutchinson J, Plumb J. An economic evaluation of adjustable and fixed dosing with budesonide/formoterol via a single inhaler in asthma patients: the ASSURE study. Current Medical Research and Opinion 2004; 20((10))1671–1679.
- Canonica GW, Castellani P, Cazzola M, et al. Adjustable maintenance dosing with budesonide/formoterol in a single inhaler provides effective asthma symptom control at a lower dose than fixed maintenance dosing. Pulmonary Pharmacology & Therapeutics 2004; 17((4))239–247.
- Aalbers R, Backer V, Kava TT, et al. Adjustable maintenance dosing with budesonide/formoterol compared with fixed-dose salmeterol/fluticasone in moderate to severe asthma. Current Medical Research and Opinion 2004; 20((2))225–240.
- FitzGerald JM, Sears MR, Boulet LP, et al. Adjustable maintenance dosing with budesonide/formoterol reduces asthma exacerbations compared with traditional fixed dosing: a five-month multicentre Canadian study. Canadian Respiratory Journal 2003; 10((8))427–434.
- Johansson G, Andreasson EB, Larsson PE, Vogelmeier CF. Cost effectiveness of budesonide/formoterol for maintenance and reliever therapy versus salmeterol/fluticasone plus salbutamol in the treatment of asthma. Pharmacoeconomics 2006; 24((7))695–708.
- Lundborg M, Wille S, Bjermer L, et al. Maintenance plus reliever budesonide/formoterol compared with a higher maintenance dose of budesonide/formoterol plus formoterol as reliever in asthma: an efficacy and cost-effectiveness study. Current Medical Research and Opinion 2006; 22((5))809–821.
- Bousquet J, Boulet L, Peters MJ, et al. Budesonide/formoterol for maintenance and relief in uncontrolled asthma vs. high-dose salmeterol/fluticasone. Respiratory Medicine 2007; 101: 2437–2446.
- Price D, Wiren A, Kuna P. Cost-effectiveness of budesonide/formoterol for maintenance and reliever asthma therapy. Allergy 2007; 62((10))1189–1198.
- Miller E, Sears MR, McIvor A, Liovas A. Canadian economic evaluation of budesonide-formoterol as maintenance and reliever treatment in patients with moderate to severe asthma. Canadian Respiratory Journal 2007; 14((5))269–275.
- Lofdahl CG, Ericsson A, Svensson K, Andreasson E. Cost effectiveness of budesonide/formoterol in a single inhaler for COPD compared with each monocomponent used alone. Pharmacoeconomics 2005; 23((4))365–375.
- Dal Negro R, Eandi M, Pradelli L, Iannazzo S. Cost-effectiveness and healthcare budget impact in Italy of inhaled corticosteroids and bronchodilators for severe and very severe COPD patients. International Journal of Chronic Obstructive Pulmonary Disease 2007; 2((2))169–176.
- Calverley P, Pauwels R, Vestbo J, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2003; 361((9356))449–456.
- Dal Negro RW, Tognella S, Tosatto R, Dionisi M, Turco P, Donner CF. Costs of chronic obstructive pulmonary disease (COPD) in Italy: The SIRIO study (Social Impact of Respiratory Integrated Outcomes). Respiratory Medicine 2008; 102((1))92–101.
- British National Formulary 54. 2007.
- Kuna P, Peters MJ, Manjra AI, Jorup C, Naya IP, Martinez-Jimenez NE, et al. Effect of budesonide/formoterol maintenance and reliever therapy on asthma exacerbations. International Journal of Clinical Practice 2007; 61((5))725–736.
- Drummond MF, Pang F. Trasferability of economic evaluation results. In:. Economic Evaluation in Health Care: Merging Theory with Practice., MF Drummond, A, eds. McGuire. Oxford: Oxford University Press. 2001; 256–276.