Abstract
Background: Respiratory syncytial virus (RSV) is a common pathogen that is the leading cause of lower respiratory tract infections in young children. High-risk children are at risk of severe infection, which may require hospitalisation. RSV is also associated with a high risk for respiratory morbidity and mortality, which may have long-term clinical and economic consequences.
Objective: To assess the cost effectiveness of palivizumab, a humanised monoclonal antibody, used as prevention against severe respiratory syncytial virus (RSV) infection requiring hospitalisation, in the indication of preterm infants and infants with preterm/bronchopulmonary dysplasia and in the second indication of children with congenital heart disease in the Dutch healthcare setting.
Methods: A decision-tree model was used to estimate the cost effectiveness of palivizumab, used as a preventative treatment against severe respiratory syncytial virus (RSV) infection, in high-risk groups of children in the Netherlands. The analysis was based on a lifetime follow-up period in order to capture the impact of palivizumab on long-term morbidity and mortality resulting from an RSV infection. Data sources included published literature, the palivizumab pivotal trials, official price/tariff lists and national population statistics. The study was conducted from the perspective of society in the Netherlands.
Results: The use of palivizumab results in undiscounted incremental cost-effectiveness ratios of €12,728/QALY and €4,256/QALY in the in preterm/bronchopulmonary dysplasia and congenital heart disease indications, respectively. Inclusion of indirect costs leads to even more favourable cost-effectiveness outcomes. The study is limited by a number of conservative assumptions. It was assumed that palivizumab only affects the occurrence of RSV hospitalisation and does not influence the severity of the RSV infection. Another assumption was that international clinical trial data and data on utilities could be applied to the Dutch healthcare setting.
Conclusion: Palivizumab provides cost-effective prophylaxis against RSV in high-risk infants. The use of palivizumab in these children results in positive short- and long-term health-economic benefits.
Introduction
Respiratory syncytial virus (RSV) is a common pathogen that is the leading cause of lower respiratory tract infections in young childrenCitation1–3. Almost all children are infected at least once by the time they are 2years oldCitation4. Symptoms associated with RSV infection are generally mild and transient in otherwise healthy individuals, although certain groups, including children born prematurely, infants with bronchopulmonary dysplasia (BPD) and those with congenital heart disease (CHD), are at risk of severe infection, which may require hospitalisation, mechanical ventilation and intensive careCitation3. Hospitalisation rates in these high-risk patients may be as high as 20%Citation5.
Annual outbreaks are consistent and predictable, generally occurring within the winter months in European countriesCitation6. In the Netherlands, approximately 2,000 children are admitted to hospital due to RSV each winter seasonCitation7. In a study spanning two RSV seasons, the rate of admission to the intensive care unit (ICU) at Sofia Children’s Hospital, Rotterdam was 28.1%, with 7.3% of children requiring mechanical ventilation. The median duration of hospitalisation was 9.1 days, range 1–29Citation8.
Investigations of the long-term prognosis of patients with severe RSV disease in infancy have shown measurable respiratory abnormalities, such as asthma and recurrent wheezing, which may persist for several years following infectionCitation9. Severe RSV pneumonia or bronchiolitis in infancy has been identified as a possible risk factor for childhood asthma in genetically predisposed childrenCitation10,11. Among young school children, previous RSV infection may increase the risk of asthma tenfoldCitation11. Sampalis etal investigated the morbidity and mortality following RSV-associated hospitalisation among premature (32–35weeks’ gestational age) Canadian infantsCitation12 and observed that infants with a history of RSV hospitalisation were at least 8 times more likely to be hospitalised for respiratory conditions during the 2-year follow-up period than matched controls. A similar study in the UK that reviewed the healthcare resource use in infants with BPD in the first 2years after birth found that infants hospitalised due to proven RSV infection required more frequent and longer admissions to general paediatric wards and ICUs, more outpatient attendances and physician consultations for respiratory related disorders, and had a higher total cost of care than non-RSV hospitalised infantsCitation13. These results demonstrate that severe RSV infection is associated with clinical and healthcare resource-use implications beyond the initial period of hospitalisation. The long-term morbidity resulting from severe RSV infection requiring hospitalisation has also been shown to have a negative impact on quality of lifeCitation14,15.
The Sampalis study was sufficiently powered to show a statistically significant increase in mortality in preterm infants following an RSV hospitalisation. The results showed a more than 5-fold increase in the overall risk of death (8.11 vs 1.58%; p=0.001) during the 2-year follow-up period among infants hospitalised due to RSV versus controlsCitation12. There is also evidence that RSV hospitalisation is associated with a higher rate of mortality in infants with CHD. RSV mortality rates of 4–5% have been reported in this high-risk patient groupCitation16. Rodriguez etal reported that mortality fluctuates between 0.5% and 5.0% in children hospitalised for RSV and that those with underlying conditions such as CHD and BPD are at higher risk for morbidity and mortalityCitation17.
Two studies have attempted to estimate the financial costs associated with severe RSV infection in the NetherlandsCitation18,19. Using a linear regression model, Rietveld etal predicted that the mean direct medical cost resulting from RSV hospitalisation in 2000 was €3,110. Costs were higher for particularly high-risk groups of children, such as infants born at or before 28weeks’ gestational age (€5,555), those with BPD (€5,785) and children less than 1 month old at the time of infection (€4,730)Citation18. Another Dutch study estimated the costs borne by parents of a child hospitalised due to RSV in addition to direct medical costs. Non-medical costs associated with work days lost, travel expenses and consultation of family doctors amounted to $295 per childCitation19.
Palivizumab, a humanised monoclonal antibody, is the only product approved in the Netherlands for the prevention of severe RSV infection requiring hospitalisation. It is indicated for use in children born at 35weeks’ gestation or less who are less than 6 months old at the onset of the RSV season, in children less than 2years old who have received treatment for BPD within the proceeding 6 months and in children less than 2years old with haemodynamically significant CHDCitation20. This indication was granted on the basis of two large, multinational, placebo-controlled trials, which reported a 55% reduction in the risk of hospitalisation in preterm/BPD children and a 45% reduction in the risk of hospitalisation in children with CHD, compared to no prophylaxisCitation21,22.
The seasonal nature of RSV infection allows for forward planning of prophylaxis and healthcare resource management to some extent. An understanding of the cost effectiveness of palivizumab use in high-risk groups is required in order to ensure appropriate use. Therefore, a modelling technique was used to estimate the effectiveness and cost effectiveness of palivizumab versus no prophylaxis in high-risk children in the Netherlands.
Methods
A model was constructed using decision-analytical techniquesCitation23, the structure of which has previously been described for the UK settingCitation24. In order to establish the cost effectiveness of palivizumab from the perspective of society in the Netherlands in 2006, country-specific inputs were derived from the official Dutch costing manual, and other relevant national data sourcesCitation25. When no appropriate costs were available, the Dutch health insurance tariff provided an approximation of the true costCitation26.
Decision-analytical techniques were used to estimate the cost effectiveness of palivizumab, taking into account both the short- and long-term consequences of severe RSV infection, including hospitalisation, wheezing and asthma in later life, and potential mortality.
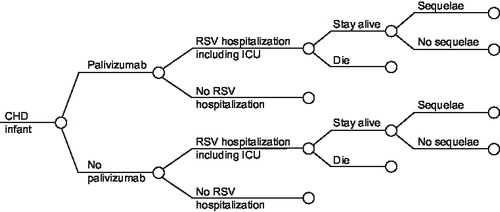
shows the structure of the model for the CHD indication. Infants may develop an RSV infection leading to hospitalisation. The majority of these children will be managed in a paediatric ward, but some will require transfer to a neonatal or paediatric ICU. A small proportion of the children will die. Among the surviving children, a significant proportion may develop long-term sequelae (e.g., wheezing, asthma). The clinical pathway and overall structure of the model is identical for the preterm/BPD population.
Figure 1. Treatment pathway for CHD indication (model structure for the pretem/BPD indication is similar).
The analysis was performed for the high-risk population: children born prematurely, children with BPD and children with CHD, in line with the populations studied in the pivotal palivizumab trialsCitation21,22. As logistic regression analysis reported for the IMpact study showed that gestational age is not a significant predictor of RSV hospitalisationCitation21, children born between 32 and 35weeks’ gestation, and those born before 32weeks’ gestation were considered as one preterm population.
Dutch guidelines for pharmacoeconomic research recommend analysis of long-term clinical effectiveness in order to evaluate healthcare technologiesCitation27. Thus, the base-case analysis assumes a lifetime follow-up period in order to capture the impact of palivizumab on long-term morbidity and mortality resulting from an RSV infection. A reduction in the rate of RSV infections was limited to prophylaxis during a single RSV season, corresponding to data available from the palivizumab clinical trials, in which children were followed for 150days from the point of randomisation.
The patients in the international, phase III trials were followed over a period of 150days. The model extrapolated the efficacy data from the palivizumab clinical trials (reduction in the rate of RSV hospitalisations) to calculate the likely number of life-years gained (LYG) and quality-adjusted life-years (QALYs) from use of palivizumab prophylaxis. RSV hospitalisations are associated with clinical and economic consequences beyond the clinical trial period. A proportion of the children may develop long-term sequelae (e.g., wheezing, asthma) leading to a reduction of QALYs and additional medical costs. A small proportion of the children will die, which will lead to a life-time loss of productivity costs. The analyses were based on life-time horizon in order to capture long-term costs and morbidity beyond the RSV hospitalisation period, which include the medical costs for management of wheezing and future lost productivity of a child resulting from mortality.
Cost analyses included estimates of the cost of palivizumab acquisition and administration, cost of RSV hospitalisation and costs associated with longer-term clinical complications of severe RSV infection, such as asthma. Future outcomes and costs were discounted when the time horizon of the model extended beyond 1year. The base-case analysis assumed a discount rate of 4.0% for economic outcomes and 1.5% for clinical outcomes, which was varied in a sensitivity analysis.
Hospitalisation rates
shows the rates of hospitalisation observed in the palivizumab clinical trials, which form the primary input data for the base-case analysisCitation21,22. As stratification of the CHD data did not yield statistically significant differences between palivizumab and placebo in cyanotic patients, the analysis was based on the overall CHD probability for hospitalisation. The literature shows that the hospitalisation rates for the placebo arms in the palivizumab trials were lower than those for the control groups in other clinical studies. Scenario analyses were therefore conducted based on hospitalisation rates observed in the control groups of trials using RSV immune globulin, adjusted using the odds ratios from the palivizumab trials to provide a likely rate of hospitalisation for palivizumab-treated children Citation28–30.
Table 1. Hospitalisation inputs.
Mortality
Mortality is an important consideration from a clinical and societal perspective, particularly in children, as a small reduction in the rate of mortality can lead to a large increase in life expectancy. Although the palivizumab trials were not powered to detect statistically significant differences in mortality, the absolute mortality in both trials was lower in the palivizumab groups than in controlsCitation21,22. For children born prematurely and those with BPD, the base-case analysis is based on the observational study by Sampalis etal, which reported an overall mortality rate of 8.11% in the 2years following hospitalisation for severe RSV infection, compared to just 1.58% in matched controls (p=0.001)Citation12. The patient population for this study consisted only of preterm infants (32–35weeks’ gestational age), therefore this mortality rate is probably an underestimation of the mortality for children with BPD or more preterm infants (<32 weeks)Citation12. Children with CHD represent a group with a much higher risk of morbidity and mortality resulting from complications of RSV infectionCitation31. To minimise uncertainty in this high-risk group, the base-case analysis was performed using mortality rates estimated for the CHD sub-population of a previous meta-analysis, as outlined in the recently published UK analysisCitation24. Calculated mortality rates were 2.3% for children with CHD receiving palivizumab and 4.0% for the no prophylaxis group.
Life expectancy
As there is no clinical evidence for a lower life expectancy for the preterm/BPD indications, it was assumed that life expectancy following an RSV episode is similar to population life expectancy of cohort children at age 1 in the Netherlands (80.05 years), derived from national data on population statisticsCitation32. A correction was made for a small reduction in life expectancy in children with CHDCitation33.
Utilities
A study by Greenough etal reported utilities in children with a history of RSV infection using the Health Utility Index (HUI), a multi-attribute score that includes measures for sensation, mobility, emotion, cognition, self-care, pain, vision, hearing, speech, ambulation and dexterityCitation15. The HUI 2 questionnaire has been designed to capture utility scores in the range –0.03 to 1.00 while the HUI 3 questionnaire captures scores from –0.36 to 1.00. For the base-case analysis it was assumed that high-risk children who did not experience a hospitalisation due to RSV infection would not have perfect health but would have a utility corresponding with a median HUI 2 for non-RSV infected children (u=0.95); high-risk children who experienced a hospitalisation due to a RSV infection would have a utility corresponding with a median HUI 2 for RSV-infected children (u=0.88); all patients above 16years of age would have perfect health (u=1). As the median HUI 3 multi-attribute score did not differ significantly with RSV infection, a scenario analysis was conducted based on a utility of 0.93 (no RSV hospitalisation) for all children up to the age of 16years.
Palivizumab use
At the recommended dose of 15 mg/kg body weight, one 100-mg vial of palivizumab can be used for a single administration for an infant weighing up to 6.6 kg. In the Netherlands population, some at-risk children will weigh more than this and some will weigh less, permitting use of a 50-mg vial. In the base-case analysis, use of 50-mg (€590) and 100-mg (€986) vials of palivizumab was based on average vial use observed in the two phase III trialsCitation21,22. Similarly, the base-case assumes that each preterm/BPD child receives 4.87 monthly doses of palivizumab over the winter/spring season and that each child with CHD receives 4.93 doses, as seen in the clinical trials. A scenario analysis was performed based on resource-use data from a Dutch cost-effectiveness study performed by Rietveld etal, which assumed that a 100-mg vial would be used for each administrationCitation34. Under trial conditions, physicians were required to use a second vial even if only a fraction of the contents were to be used. In clinical practice, physicians may choose not to open a second vial to avoid waste. Therefore, an alternative scenario considered vial use reduced by 10% and 20% to reflect realistic treatment patterns.
Palivizumab can be administered in the community without the need to refer children to hospital. Each administration was assumed to equal the cost of a general practitioner visit (€20.49)Citation34. A fixed prescription fee (€6.11) was added for each monthly dose, except for the first dose which is administered during a hospital visit unrelated to RSV.
Cost of RSV hospitalisation
The base-case analysis assumed a mean hospitalisation cost of €3,468 for the preterm/BPD population, based on Rietveld etal’s linear regression model and adjusted for inflationCitation18. Sensitivity analysis varied the cost of hospitalisation between the minimum and maximum values reported in sub-group analyses (€3,323 for children with a gestational age exceeding 37weeks and €6,450 for infants with BPD). As no specific data were available for the CHD indication, the base-case analysis assumed a hospitalisation cost equivalent to that for the BPD indication, although this may be an underestimation of the true cost in this relatively high-risk group. This cost was varied in sensitivity analysis between 80% and 120% of the input value.
Costs associated with asthma
A recent report presents information on healthcare use and costs related to asthma and chronic obstructive pulmonary disease (COPD) in the NetherlandsCitation35. Total costs for treating asthma in 2000 were estimated to be €141 million. Annual costs per patient in the 0–14-year age group came to approximately €233. The base-case analysis combined this cost information with data from a study by Simoes etal, which showed that palivizumab use decreased the risk of recurrent wheezing in preterm children from 19.1% to 6.8%36.
Non-medical costs
A study by Miedema etal showed that parents had on average two telephone contacts with the family doctor and each child was seen twice prior to hospitalisation. Parents lost half a working day before hospitalisation and 1.5 workdays during hospitalisation and drove an average of 118 miles to visit their childCitation19. Workdays lost, travel costs and consultation of family doctors resulted in a total cost of $295 per child, or €306 after correction for inflation.
Long-term indirect costs
The human capital method was applied to estimate potential future indirect costs due to lost productivity following the death of a child. Assuming a unit cost of €34.90 per hour of manufacturing labour (which represents the average Dutch wage), 1,540 working hours per year and 73.6% employment, the annual indirect cost resulting from lost productivity was estimated to be €40,133 after correction for inflationCitation25.
The costs were based on 2006 data. Costing data from previous years were updated to 2006 using an inflation correction.
Results
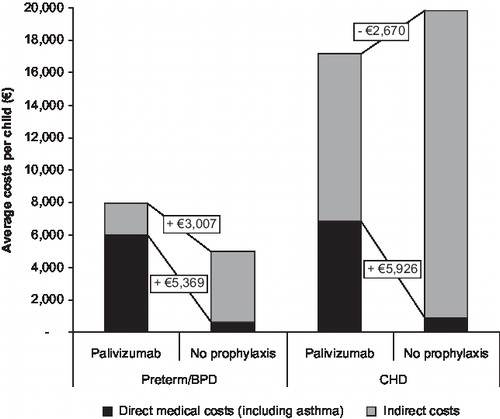
shows the breakdown of direct and indirect costs for the average child. In the preterm/BPD indication, use of palivizumab leads to an additional direct medical cost of €5,547 vs. no prophylaxis, including palivizumab acquisition and administration costs and costs associated with RSV hospitalisation. When the costs of asthma treatment are taken into consideration, the incremental cost of palivizumab falls slightly to €5,369. The inclusion of indirect costs further reduces the additional costs for palivizumab to €3,007. For the CHD indication, use of palivizumab leads to an additional direct medical cost of €5,926, including the impact of costs associated with asthma. However, inclusion of indirect costs leads to a cost saving of €2,670 versus no prophylaxis.
In terms of clinical outcomes, use of palivizumab in the preterm/BPD group results in 0.37 life-years gained (0.21 years discounted) or 0.42 QALYs (0.27 discounted). Equivalent gains associated with palivizumab use in the CHD population are 1.36 life-years or 1.39 QALYs (0.81 life-years or 0.84 QALYs after discounting).
shows the cost-effectiveness outcomes for the preterm/BPD population and shows that, in the base-case analysis, the use of palivizumab results in an undiscounted incremental cost-effectiveness ratio (ICER) of €12,728/QALY, taking into account direct medical costs, including those associated with asthma treatment. When discounted, the ICER increases to €20,236/QALY. The cost per life-year gained is slightly higher: €24,977/LYG and €14,701/LYG, with and without discounting, respectively. The discounted ICERs for the preterm and BPD sub-populations are €18,563/QALY and €23,461/QALY, respectively. also shows the impact of including indirect costs. The ICER resulting from use of palivizumab improves to €7,130/QALY (undiscounted) and €11,336/QALY (discounted) in the base-case analysis.
Table 2. Cost-effectiveness results (QALYs) – preterm/BPD population.
Scenario analyses based on results from the Groothuis etal and PREVENT studies, in which higher rates of hospitalisation were observed in the control groups than in the palivizumab trial, produce more favourable cost-effectiveness outcomes for palivizumab. These results show that the model outcomes are sensitive to changes in the rate of hospitalisation.
shows the cost-effectiveness outcomes for the CHD population. In the base-case analysis, taking into account total direct costs including those associated with asthma, use of palivizumab results in an undiscounted ICER of €4,256/QALY, which rises to €7,067/QALY after discounting. As for the preterm/BPD population, the cost per life-year gained is slightly higher: €7,310/LYG and €4,353/LYG with and without discounting, respectively. When indirect costs are included, palivizumab becomes the dominant strategy, producing both health benefits and cost savings.
Table 3. Cost-effectiveness results (QALYs) – CHD population.
Scenario analysis based on results from the CARDIAC study, in which a higher rate of hospitalisation was observed in the control group versus that in the palivizumab trial (15% versus 9.7%, respectively), again produces more favourable cost-effectiveness outcomes for palivizumab. The ICER without discounting is €2,683/QALY, which becomes €4,458/QALY after discounting, both of which are substantially more favourable than the base-case results. Another scenario analysis was performed, excluding mortality from the model, which increases the ICER to €99,103/QALY and €137,239/QALY for respectively preterm/BPD children and CHD children.
Sensitivity analyses
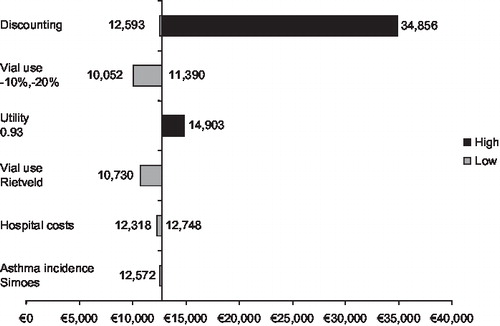
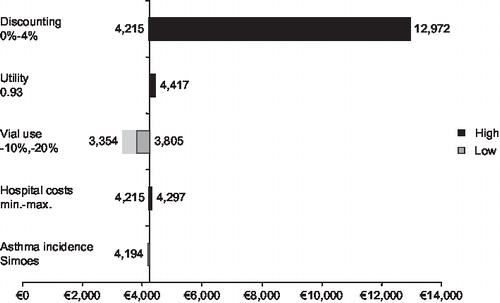
and illustrate the results of sensitivity analyses and shows that the model outcomes for both the preterm/BPD and CHD populations are sensitive to the rate of discounting applied. This is because many of the costs included in the model, such as the costs of prophylaxis and RSV-related hospitalisation, are short-term costs that are not affected by discounting over the long-term. Discounting does, however, have an impact on the health benefits gained from use of palivizumab that persist beyond the single RSV season. This explains the higher costs per QALY when discounting is applied: the ICER for the preterm/BPD population without discounting is €12,593/QALY while a discount rate of 4% yields an ICER of €34,856/QALY.
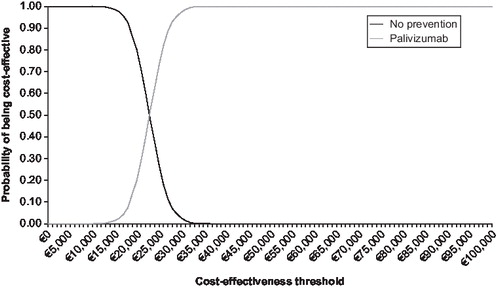
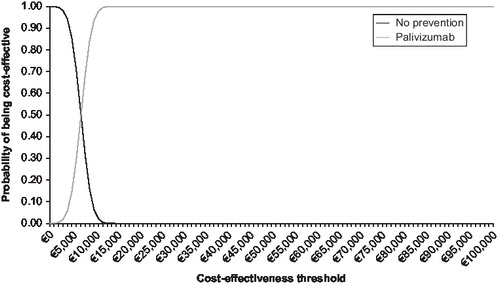
and show the cost-effectiveness acceptability curve from the societal perspective, based on a probabilistic sensitivity analysis with effectiveness discounted for respectively the preterm/BPD population and the CHD population. This illustrates that there is a 97% chance that the ICER associated with use of palivizumab in the preterm/BPD population is less than €30,000/QALY. The probabilistic sensitivity analysis with effectiveness discounted shows that there is a 90% chance that the ICER associated with use of palivizumab in the CHD population is less than €10,000/QALY ().
Discussion
Although two studies have attempted to quantify the financial impact of RSV infection in the NetherlandsCitation18,19, this is the first study to date to examine the cost effectiveness of palivizumab in high-risk children in this healthcare setting.
This model provides a more favourable cost-effectiveness outcome for palivizumab than previously published studies in this area. Previous studies have tended to focus on sub-populations of high-risk children in a limited geographical areaCitation37 or have expressed results in terms of cost per hospitalisation or infection avoidedCitation38–40. This is not a generally accepted cost-effectiveness outcome and no thresholds are available for this measure. The model presented here is the first to consider cost per QALY in the Netherlands for premature infants and children with BPD or CHD, based on the results of relatively large international randomised controlled trials.
The modelled approach described above reflects a broader range of opportunity costs by including long-term downstream consequences of RSV such as asthma. The model also reconsiders mortality from a new analytical perspective. Mortality rates must be included in order to calculate the cost per life-year gained associated with use of palivizumab. While the US study by Yount etal employs a similar modelling approach to that described here, it does not include the indirect costs resulting from lost lifetime productivity following the death of a childCitation41. Inclusion of these indirect costs leads to a substantial improvement in the cost effectiveness of palivizumab, which becomes the dominant strategy in children with CHD.
Limitation
A limitation of the model is that costs per hospitalisation were based on a single study carried out in the southwest region of the NetherlandsCitation18. Regional and seasonal variations play a major role in connection with RSV, both with respect to disease severity and approaches to disease management. There is no guarantee that the results from the southwest region are representative of the Netherlands as a whole. As well, the use of data from only regional hospitals is likely to lead to an underestimation of the costs associated with RSV hospitalisation, because the large hospitals from the cities are not included. A further methodological concern is that the regression model developed by Rietveld etal was based mainly on children who had not been born prematurely and did not have BPD. Hospitalisation costs in the Netherlands are therefore likely to be greater for the high-risk groups of children for whom palivizumab is indicated than those previously reported.
Despite these limitations, the modelling approach allows us to demonstrate the possible long-term health-economic outcomes associated with palivizumab without the need for a naturalistic prospective study, which may require a 5- to 10-year follow-up period. The use of a decision-tree model allows us to extrapolate clinical outcomes beyond the duration of the existing clinical trials. Scenario and sensitivity analyses were performed to ensure robustness of the model. These analyses show that the outcomes of the model are robust to variance in the underlying assumptions and input data: palivizumab remains cost effective in all analyses.
All analyses were performed with and without discounting. Although discounting results in higher ICERs, the cost effectiveness of palivizumab remains acceptable in all scenarios, even after discounting.
The results presented here for the Dutch at-risk children are in line with those reported recently from the perspective of the UK National Health Service and the health insurance fund perspective in Austria using a similar model structureCitation24,42. In the UK, in preterm/BPD babies, the use of palivizumab resulted in an ICER of £16,720/QALY. From the health insurance fund perspective in Austria, the incremental cost-effectiveness ratio without discounting was estimated to be €14,439/QALY for the preterm population and €21,672 for the BPD population.
In the UK, in babies with CHD, the use of palivizumab resulted in an ICER of £6,664/QALY, whereas in Austria, the incremental cost-effectiveness ratio was estimated to be €9,754/QALY for the CHD indication. This confirms that the cost-effectiveness outcomes for palivizumab are not dependent on country-specific input data, and suggests that the results for the Netherlands, Austria and the UK may guide decision makers in other European countries.
Conclusion
The use of palivizumab represents a cost effective means of prophylaxis against RSV infection in high-risk children in the Netherlands is a cost-effective strategy, providing both short- and long-term health-economic benefits.
Transparency
Declaration of funding
This analysis was funded by Abbott GmbH & Co. KG, Ludwigshafen, Germany.
Declaration of financial/other relationships
W.W. has disclosed that he is an employee of Abbott, the manufacturer of palivizumab. M.N. has disclosed that he has served as consultant to Abbott and was paid by Abbott to conduct the analyses presented in this article.
Some peer reviewers receive honoraria from CMRO for their review work. The peer reviewers of this paper have disclosed that they have no relevant financial relationships.
Acknowledgement:
The authors have disclosed that they had no outside editorial assistance in preparing this manuscript.
References
- Levy BT, Graber MA. Respiratory syncytial virus infection in infants and young children. J Fam Pract 1997;45:473-81.
- Ottolini M, Hemming V. Prevention and treatment recommendations for respiratory syncytial virus infection. Drugs 1997;54: 867-84.
- Simoes E. Respiratory syncytial virus infection. Lancet 1999;354: 847-52.
- Hall C. Respiratory syncytial virus. In: Feigin R, Cherry J, eds. Infectious Diseases. Philadelphia: W.B. Saunders, 1998.
- Canfield S, Simoes E. Prevention of respiratory syncytial virus (RSV) infection: RSV immune globulin intravenous and palivizumab. Pediatr Ann 1999;28:507-14.
- Smyth RL, Openshaw PJ. Bronchiolitis. Lancet 2006;368:312-22.
- Rothbarth PH, Kimpen JL, Roord JJ, Neijens HJ. Respiratory syncytial virus infections and preventive options. Ned Tijdschr Geneeskd 2000;144:15-19.
- Brandenburg AH, Jeannet PY, Steensel-Moll HA, Local variability in respiratory syncytial virus disease severity. Arch Dis Child 1997;77:410-14.
- Malhotra A, Krilov L. Influenza and respiratory syncytial virus. Update on infection, management and prevention. Pediatr Clin North Am 2000;47:353-72.
- Welliver R, Duffy L. The relationship of RSV-specific immunoglobulin E antibody responses in infancy, recurrent wheezing and pulmonary functions at age 7-8 years. Pediatr Pulmonol 1993;15:19-27.
- Sigurs N, Bjarnason R, Sigurbergsson F, Asthma and immunoglobulin E antibodies after respiratory syncytial virus bronchiolitis: a prospective cohort study with matched controls. Pediatrics 1995;95:500-5.
- Sampalis JS. Morbidity and mortality after RSV-associated hospitalisations among premature Canadian infants. J Pediatr 2003;143(5 Suppl):S150-6.
- Greenough A, Cox S, Alexander J, Health care utilisation of infants with chronic lung disease, related to hospitalisation for RSV infection. Arch Dis Child 2001;85:463-8.
- Bont L, Steijn M, van Aalderen WM, Kimpen JL. Impact of wheezing after respiratory syncytial virus infection on health-related quality of life. Pediatr Infect Dis J 2004;23:414-17.
- Greenough A, Alexander J, Burgess S, Health care utilisation of prematurely born, preschool children related to hospitalisation for RSV infection. Arch Dis Child 2004;89:673-8.
- Wang EE, Law BJ, Stephens D. Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC) prospective study of risk factors and outcomes in patients hospitalized with respiratory syncytial viral lower respiratory tract infection. J Pediatr 1995;126:212-19.
- Rodriguez WJ, Parrott RH. Ribavirin aerosol treatment of serious respiratory syncytial virus infection in infants. Infect Dis Clin North Am 1987;1:425-39.
- Rietveld E, De Jonge HC, Polder JJ, Anticipated costs of hospitalisation for respiratory syncytial virus infection in young children at risk. Pediatr Infect Dis J 2004;23:523-9.
- Miedema CJ, Kors AW, Ten WE, Medical consumption and socioeconomic effects of infection with respiratory syncytial virus in the Netherlands. Pediatr Infect Dis J 2001;20:160-3.
- The European Medicines Agency. Available at: http://www.emea.eu.int/index/indexh1.htm.
- The IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalisation from respiratory syncytial virus infection in high-risk infants. Pediatrics 1998;102:531-7.
- Feltes TF, Cabalka AK, Meissner HC, Palivizumab prophylaxis reduces hospitalisation due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr 2003;143:532-40.
- Weinstein MC Fineberg HV. Clinical Decision Analysis. Philadelphia: WB Saunders, 1980;228-65.
- Nuijten MJ, Wittenberg W, Lebmeier M. Cost effectiveness of palivizumab for respiratory syncytial virus prophylaxis in high-risk children: a UK analysis. Pharmacoeconomics 2007;25:55-71.
- Oostenbrink JB, Bouwmans CAM, Koopmanschap MA, Rutten FFH. Handleiding voor kostenonderzoek; Methoden en standaard kostprijzen voor economische evaluaties in de gezondheidszorg [Manual for costing: methods and standard costs for economic evaluations in health care]. Actualised version 2004. Diemen: College voor Zorgverzekeringen.
- College Tarieven Gezondheidszorg (CTG). Tarieflijst instellingen 2004. Utrecht: CTG.
- Riteco JA, Heij LJM de, Luijn JCF van, Wolff I. Richtlijnen voor farmaco-economisch onderzoek. College voor zorgverzekeringen 1999 : Amstelveen. CURE/cuo.228/2.
- Groothuis JR, Simoes EA, Levin MJ, Prophylactic administration of respiratory syncytial virus immune globulin to high-risk infants and young children. The Respiratory Syncytial Virus Immune Globulin Study Group. N Engl J Med 1993;329:1524-30.
- The PREVENT Study Group. Reduction of RSV hospitalization among premature infants and infants with bronchopulmonary dysplasia using respiratory syncytial virus immune globulin prophylaxis. Pediatrics 1997;99:93-9.
- Simoes EA, Sondheimer HM, Top FHJr, Respiratory syncytial virus immune globulin for prophylaxis against respiratory syncytial virus disease in infants and children with congenital heart disease. The Cardiac Study Group. J Pediatr 1998;133:492-9.
- Cabalka A. Physiological risk factors for respiratory viral infections and immunoprophylaxis for respiratory syncytial virus in young children with congenital heart disease. Pediatr Infect Dis J 2004;23:S41-5.
- Centraal Bureau voor de Statistiek. Voor burg, 2005.
- Wren C, O'Sullivan JJ. Survival with congenital heart disease and need for follow up in adult life. Heart 2001;85:438-43.
- Rietveld E, Steyerberg E, Ploder J, Passive immunisation against respiratory syncytial virus: a cost-effectiveness study. Chapter 32. Thesis: 60-69.
- Hoogendoorn EJI, Feenstra TL, Rutten-van Mölken MPMH. Inventarisatie van het gebruik en de kosten van zorg voor astma en COPD in Nederland. RIVM rapport 2004 ;260604001.
- Simoes EAF, Carbonell-Estrany X, Kimpen J, Palivizumab use decreases risk of recurrent wheezing in preterm children. Eur Respir J 2004;24(Suppl) 48:212s.
- Deshpande SA, Northern V. The clinical and health economic burden of respiratory syncytial virus disease among children under 2years of age in a defined geographical area. Arch Dis Child 2003;88:1065-9.
- Roeckl-Wiedmann I, Liese JG, Grill E, Economic evaluation of possible prevention of RSV-related hospitalizations in premature infants in Germany. Eur J Pediatr 2003;162:237-44.
- Wegner S, Vann JJ, Liu G, Direct cost analyses of palivizumab treatment in a cohort of at-risk children: evidence from the North Carolina Medicaid Program. Pediatrics 2004;114:1612-19.
- Lofland JH, O'Connor JP, Chatterton ML, Palivizumab for respiratory syncytial virus prophylaxis in high-risk infants: a cost-effectiveness analysis. Clin Ther 2000;22:1357-69.
- Yount LE, Mahle WT. Economic analysis of palivizumab in infants with congenital heart disease. Pediatrics 2004;114:1606-11.
- Resch B, Gusenleitner W, Nuijten MJC, Cost-effectiveness of palivizumab against respiratory syncytial viral infection in high-risk children in Austria. Clin Ther 2008;30:749-60.