Abstract
Objective: To compare, in elderly Medicare beneficiaries, chronic obstructive pulmonary disease (COPD)-related healthcare costs for patients initiating treatment with fluticasone propionate/salmeterol 250 μg/50 μg (FSC) with those for patients initiating treatment with ipratropium bromide/albuterol (IPA), ipratropium bromide (IPR), and tiotropium bromide (TIO).
Methods: In this retrospective, observational, cohort study, COPD-related medical costs (inpatient/emergency department, outpatient) and pharmacy costs were assessed in Medicare beneficiaries ≥65 years old who were enrolled in a commercial Medicare health maintenance organization plan and had a diagnosis of COPD (ICD-9-CM codes 491.xx, 492.xx, or 496.xx) within 12 months before initial treatment with FSC, IPA, IPR, or TIO.
Results: In these ≥65-year-old patients (N=14,689), initial maintenance treatment with FSC was associated with total COPD-related cost savings (medical + pharmacy) of $295 versus IPA, $1,235 versus IPR, and $110 versus TIO (p<0.05, each comparison) over a 1-year follow-up period.
Conclusions: Initiation of maintenance therapy with FSC was associated with significant reduction in total costs (medical + pharmacy) relative to costs associated with the short-acting anticholinergic bronchodilators IPR and IPA and the long-acting anticholinergic bronchodilator TIO in an elderly Medicare-eligible population. These data considered in the context of the substantial efficacy and effectiveness data suggest that early introduction of maintenance treatment with FSC has both clinical and economic benefits. Limitations inherent in handling of administrative data include lack of objective clinical measures such as spirometry and smoking status. Furthermore, accuracy of diagnosis codes cannot be verified.
Introduction
Chronic obstructive pulmonary disease (COPD) is manifested by progressive deterioration in lung function that impairs patients’ general health and quality of life and eventually leads to respiratory failure and premature death. In the US, COPD affects as many as 24 million individualsCitation1 and is the fourth leading cause of death after coronary artery disease, cancer, and strokeCitation1,2. The economic burden of COPD is substantialCitation3,4. In 2004, the annual costs of COPD in the US were $37.2 billion including $20.9 billion in direct medical costs and $16.3 billion in indirect costsCitation5. Most of the medical costs of COPD are attributed to hospitalizations and emergency department (ED) visits for acute exacerbationsCitation6, which occur twice yearly, on average, and are associated with significant risk of persistent disability and deathCitation7–12. COPD-related hospitalizations and ED visits numbered 673,000 and 1.5 million, respectively, in the US in 2000, and their incidence does not appear to be waning with timeCitation6,13. COPD is particularly common in the veterans population, in whom it commonly coexists with cardiovascular diseases including coronary artery disease, congestive heart failure, and atrial fibrillationCitation14.
Control of COPD-related medical costs depends on reducing the incidence of acute exacerbations and the associated risk of hospitalization and ED visitsCitation15. Bronchodilators, namely β2-agonists and anticholinergics, can reduce the frequency and severity of acute exacerbations and are considered central to the management of COPDCitation6. For patients with severe disease and repeated exacerbations, addition of inhaled corticosteroids to bronchodilator therapy is recommended on the basis of evidence suggesting that long-acting β2-agonist bronchodilators combined with inhaled corticosteroids confer greater protection against exacerbations than bronchodilator monotherapyCitation6. Long-acting β2-agonist/inhaled corticosteroid combinations have been demonstrated to be effective versus placebo, long-acting β2-agonist monotherapy, and inhaled corticosteroid monotherapy in reducing the incidence of acute exacerbations of COPDCitation16–19. In the INSPIRE study, a randomized, double-blind, parallel-group, 2-year trial, the long-acting β2-agonist/inhaled corticosteroid combination fluticasone propionate/salmeterol (FSC) 500 μg/50 μg twice daily appeared to be more effective than the long-acting bronchodilator tiotropium 18 μg once daily at reducing hospitalizations and significantly reduced mortality in patients with COPD (n=1,323), but the treatments did not differ with respect to exacerbation rates. The incidence of exacerbations requiring hospitalizations was 16% for FSC and 13% for tiotropium (p=0.085)Citation20. In a recent study of 42,090 patients with a diagnosis of COPD in the Veterans Affairs healthcare system, the combination of inhaled corticosteroid + long-acting β-agonists + tiotropium was associated with a 40% reduction in the risk of death compared with that in patients in a historic control prior to the introduction of tiotropiumCitation21. This benefit of tiotropium was observed when it was administered with inhaled corticosteroids and β-agonists, but not with other medications.
The therapeutic benefits of combination therapy with long-acting β2-agonists and inhaled corticosteroids appear to be associated with reductions in use of healthcare services and medical costs relative to monotherapy with short- or long-acting bronchodilators or inhaled corticosteroids. In retrospective analyses, combination therapy with long-acting β2-agonists and inhaled corticosteroids was associated with a lower risk of rehospitalization or death within a year of a first COPD-related hospitalization than treatment with long-acting β2-agonists only, inhaled corticosteroids only, or short-acting β-agonists onlyCitation22. Furthermore, in observational studies using medical and pharmacy claims, combination therapy with the long-acting β2-agonist salmeterol and an inhaled corticosteroid was associated with a significantly lower risk of COPD-related hospitalizations and reduced medical costs compared with the use of the short-acting anticholinergic bronchodilator ipratropium in various populations of patients with COPD including those not selected with respect to disease severityCitation23–26.
The current study was conducted to further explore the economic ramifications of the use of combination long-acting β2-agonist/inhaled corticosteroid pharmacotherapy for the maintenance treatment of COPD by comparing, in elderly Medicare beneficiaries, COPD-related costs of healthcare services between FSC 250 μg/ 50 μg twice daily and each of the following common anticholinergic bronchodilator therapies: ipratropium bromide/albuterol (IPA), ipratropium bromide (IPR), and tiotropium bromide (TIO). To the authors’ knowledge, this study is the first economic assessment of FSC and anticholinergics to include TIO (a long-acting bronchodilator approved in 2004 in the US) for COPD treatment. In addition, it is one of the few to assess COPD-related treatment costs between FSC and anticholinergics in the elderly Medicare-eligible population. As COPD-associated morbidity appears to increase with ageCitation27, the elderly population is a particularly important target of efforts to reduce disease burden and to control COPD-related costs.
Methods
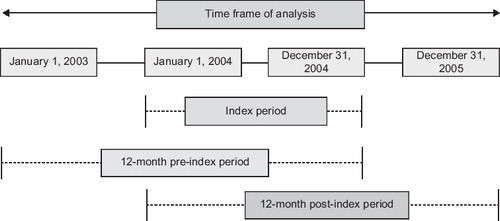
In this retrospective, observational cohort study, medical claims data were used to compare COPD-related healthcare costs associated with exacerbations among elderly Medicare beneficiaries initiating treatment with FSC (fluticasone propionate/salmeterol 250 μg/50 μg) with costs among those initiating treatment with IPA, IPR, or TIO. depicts the study design and timings.
Data source
Data were obtained from the Integrated Healthcare Information Services (IHCIS), an administrative claims database that includes patient-level data on enrollment, facility, professional, and pharmacy services from approximately 33 million patients covered by more than 30 managed-care health plans across the US. The health plans submit administrative data for IHCIS and collaborate with IHCIS to maintain and ensure the validity of the database. Data available for each medical claim (including facility claims and professional service claims) included dates of service and International Classification of Diseases 9th Revision, Clinical Modification (ICD-9-CM) diagnosis codes and ICD-9-CM or Current Procedural Terminology, Version 4 (CPT-4) procedure codes. Data available for each pharmacy service claim included the drug dispensed (in National Drug Code) and the dispensing date. This study met the criteria for exempt status from institutional review board oversight because study investigators only had access to de-identified data, and conduct conformed to the Health Insurance Portability and Accountability Act (HIPAA).
Sample
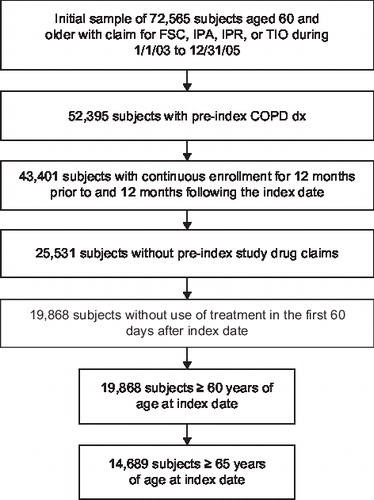
The sample comprised eligible Medicare beneficiaries ≥65 years old who were enrolled in a commercial Medicare health maintenance organization (HMO) plan (Medicare Advantage); had a medical claim of COPD (defined by ICD-9-CM codes 491.xx, 492.xx, or 496.xx) within 12 months before initial treatment with FSC (fluticasone propionate/salmeterol 250 μg/50 μg), IPA, IPR, or TIO; and had a first pharmacy claim (the index event) for one of the study medications between January 1, 2003, and December 31, 2005. This study was limited to COPD patients aged 65 years or older since this criterion coincides with Medicare eligibility due to age entitlement. The first observation of a pharmacy claim for one of the study drugs was defined as the index date. Patients were excluded if they received any of the study medications during the 12 months before the index treatment. A ‘treatment-free’ 60-day window after the index medication was examined for presence of study medications other than the index treatment. If other study medications in addition to the index treatment had been taken during that window, the patient was excluded. This window was primarily to ensure patients were on assigned index treatment at least 60 days or one refill at a minimum. Patients were also excluded if they did not have continuous enrollment in the health plan for at least 24 months during the study period.
Measures
COPD-related medical and pharmacy costs were computed for the 12-month post-index period for all patients having data for this period. COPD-related medical costs included any service (including inpatient, outpatient, office visit, ED visit) assigned a primary diagnosis code of COPD (491.xx, 492.xx, or 496.xx) or any COPD-related medical procedure (i.e., spirometry, oxygen therapy, pulmonary rehabilitation) that was billed. COPD-related costs were calculated for the following categories: pharmacy costs (i.e., the sum of all costs associated with COPD-related pharmacy claims including those for oral corticosteroids, short-acting β-agonists, leukotriene modifiers, theophylline and xanthine derivatives, and antibiotics frequently used for COPD [penicillins, cephalosporins, macrolides, sulfa/trimethoprim, quinolones, tetracyclines, lincomycins]); outpatient costs (i.e., all costs incurred for COPD-related non-ED/inpatient claims); and ED/inpatient costs (i.e., all costs incurred for COPD-related inpatient and ED claims). Medical costs were defined as sum of inpatient/ED and outpatient cost categories. Similarly, total costs were the sum of medical and pharmacy costs.
Cost data were standardized to facilitate comparisons across services and time periods. All costs were adjusted for inflation and standardized to 2007 dollars using the consumer price index (CPI)Citation28. Facility inpatient costs were calculated based on primary diagnosis categories, length of stay, and presence of intensive care unit/surgery. Facility outpatient costs were calculated based on requested (submitted) charges. Professional service costs were calculated using a standardized payment scheduled based on a resource-based relative value scale. Pharmacy costs were calculated using wholesale acquisition costs (WAC).
Data analyses
Demographics, baseline clinical characteristics, and healthcare utilization data were summarized with descriptive statistics. Differences between the FSC cohort and each of the cohorts receiving an anticholinergic were tested with Student's t-tests or chi-square tests, depending on their distribution.
Multivariate generalized linear models with gamma distributions and log-link functions were used to estimate COPD-related total, medical, inpatient/ED, outpatient, and pharmacy costs. Covariates included age, sex, COPD sub-type (defined by ICD-9-CM diagnosis codes for conditions chronic bronchitis (491.xx), emphysema (492.xx) and COPD (496.xx), lower respiratory tract infection, upper respiratory tract infection, total count of healthcare events (i.e., prescriptions, ED visits, hospitalizations) during the 12 months before the index event, and the total months of prescriptions for oral antibiotics, oral corticosteroids, short-acting β-agonists, xanthines, all antibiotics, and oral non-COPD antibiotics in the 12-month pre-index period. Cost differences were calculated relative to costs in the FSC cohort. The bootstrap method with 1,000 replications was used to compute 95% confidence intervals (CIs) of cost differences between the FSC cohort and each of the other cohorts, and between-cohort differences were tested with Student's t-test.
Results
Sample
The number of patients ≥65 years old was 14,689 (3,188 in the FSC cohort; 6,385 in the IPA cohort; 2,713 in the IPR cohort; 2,403 in the TIO cohort). shows the derivation of the study sample. summarizes demographics and baseline clinical characteristics. Males comprised approximately half of the patients in each cohort (45.9–56.0%). Age distributions differed slightly (p<0.05) between the FSC cohort and the IPA and IPR cohorts such that the latter cohorts included more patients 75–79 years old and slightly fewer patients ages 65–69 years than the FSC cohort. In each cohort, COPD not otherwise specified was the most common COPD diagnosis (90.8–92.9%) followed by chronic bronchitis and emphysema. Asthma was more common in the FSC cohort (27.8%) than the other cohorts (14.0–19.2%) (p<0.05). During the pre-index period, patients in the FSC cohort used slightly fewer prescriptions than patients in the IPR and TIO cohorts and had fewer ED visits and hospitalizations than patients in the IPA cohort and the IPR cohort (p<0.05, each comparison).
Table 1. Demographics and baseline characteristics.
Costs
Sample ≥65 years old (n=14,689).
Treatment with FSC was associated with significantly lower annual mean COPD-related total costs, medical costs, inpatient/ED costs, and outpatient costs than treatment with IPA, IPR, or TIO ( for unadjusted costs). Total unadjusted COPD-related costs in 2007 dollars were $3,210 for FSC compared with $3,518 for IPA, $4,289 for IPR, and $3,328 for TIO (p<0.05 FSC versus each comparator). The corresponding values for medical costs were $2,369 for FSC, $3,021 for IPA, $3,713 for IPR, and $2,648 for TIO (p<0.05 FSC versus each comparator).
Table 2. Mean (SD) unadjusted COPD-related costs in 2007 dollars in patients ≥65 years old (n=14,689).
Pharmacy costs were significantly higher with FSC ($841) than with IPA ($497), IPR ($743), or TIO ($680) (p<0.05) (). Treatment with FSC was associated with total COPD-related cost savings of $295 versus IPA, $1,235 versus IPR, and $110 versus TIO (p<0.05, each comparison) ().
Table 3. Mean (95% CI) adjusted cost differences: COPD-related costs with FSC versus IPA, IPR, and TIO in 2007 dollars in patients ≥65 years old (n=14,689).
Healthcare utilization
In the post-index period, the incidence of healthcare utilization (as numbers of patients with an event) was significantly (p<0.05) lower with FSC compared with (1) IPA and TIO for oral COPD antibiotic use, (2) IPA and IPR for COPD emergency-department visit, COPD hospitalization, and COPD-related office visit; (3) IPR and TIO for COPD-related prescription use; and (4) IPA, IPR, and TIO for COPD oral corticosteroid use ().
Table 4. Post-index utilization.
Discussion
In a sample from a large observational medical claims database (N=14,689 patients ≥65 years old), treatment with FSC was associated with significantly lower annual mean COPD-related total costs (medical + pharmacy), medical costs (inpatient/ED + outpatient), inpatient/ED costs, and outpatient costs than treatment with IPA, IPR, or TIO. While pharmacy costs were significantly higher with FSC than with IPA, IPR, or TIO, the reduction in medical costs with FSC more than offset the pharmacy costs to yield a significant reduction in COPD-related total costs. Initial maintenance treatment with FSC was associated with total COPD-related cost savings (medical + pharmacy) of $295 versus IPA, $1,235 versus IPR, and $110 versus TIO. The reduction in total costs is attributed to significant reductions in medical (inpatient/ED, outpatient) costs with FSC versus the anticholinergic bronchodilators. In previous observational studies, FSC was associated with a significantly lower risk of COPD-related hospitalizations and reduced medical costs compared with IPR in various populations of patients with COPDCitation24–26.
The cost-effectiveness of FSC versus that of IPR and IPA has previously been assessedCitation25. The current study is one of the first to include TIO and to examine outcomes in a real-world setting since TIO was approved in 2004 for treatment of COPD. FSC was cost-effective in this study against TIO, the primary comparison of interest, as well as against IPR and IPA. At baseline, the FSC and TIO groups were fairly similar with respect to demographic and clinical characteristics while patients in the IPR and IPA groups were generally sicker and older than patients in the FSC group. These baseline differences were controlled for in the multivariate model. In this study, risk adjustors were used to control for differing baseline severity that may have existed between study groups in the absence of randomization. In order to address selection bias and control for severity, proxy measures of severity such as ED visits, hospitalizations, and prescription medications used in the pre-index baseline period were used. In addition, antibiotics and oral corticosteroids (OCS) were used as markers for moderate exacerbations in COPD within the multivariate model. The fact that unadjusted IP/ED costs for IPA were significantly higher than those for FSC but were numerically (but not statistically significantly) lower in a multivariate model controlling for disease severity and other covariates is a testament to the success of statistical methods used to control for differences in baseline severity. COPD patients with comorbid asthma were included because asthma and COPD commonly co-existCitation29. Because of lack of reversibility information in claims data (which lack spirometry information), asthma cannot conclusively be ruled out based on excluding ICD-9-CM diagnosis code. Asthma and COPD are commonly comorbid and are often treated with similar methodsCitation30. The two diseases can be differentiated only with systematic clinical assessment. Administrative data lack these prospective methods, a limitation inherent in all retrospective cohort studies.
The improved clinical and economic outcomes with FSC relative to anticholinergic bronchodilator therapy are consistent with the results of short-term, controlled clinical trials. In two randomized, double-blind, parallel-group, 8-week studies in patients with COPD, FSC (fluticasone propionate/salmeterol 250 μg/50 μg BID) significantly improved lung function measured by spirometry and symptoms and reduced supplemental albuterol use compared with IPA (ipratropium/albuterol 36 μg/206 μg QID)Citation27,31. The short duration of these studies precluded valid assessment of the impact of therapy on exacerbations. The drivers of cost reductions with FSC relative to TIO, a long-acting anticholinergic bronchodilator, have not been elucidated. The INSPIRE study, a head-to-head comparison of FSC (fluticasone propionate/salmeterol 500 μg/50 μg BID) and TIO, showed no difference in the primary outcome of exacerbationsCitation20. However, hospitalizations were numerically lower with FSC relative to TIO in the INSPIRE study. Possibly, FSC-associated reduction in the need for hospitalization may be a cost driver.
The results of the current study corroborate and extend those of previous medical and pharmacy claims analyses involving patient populations 40 years and older with COPD treated with FSC or IPRCitation24,25. In a retrospective, observational study involving Texas Medicaid beneficiaries ages 40–64 years (n=6,793), combination therapy with FSC compared with IPR was associated with a 27% lower risk of COPD-related hospitalization or ED visit and with similar COPD-related total costs (medical + pharmacy costs)Citation24. Similarly, in a study that, like the current one, involved the IHCIS database, initial maintenance therapy with FSC compared with IPR was associated with a 56% lower risk of COPD-related hospitalization or ED visit and with similar COPD-related total costs (and lower all-cause total costs) in patients ≥40 years old with COPDCitation25. Whereas COPD-related total costs were similar with FSC compared with IPR in the latter studiesCitation24,25, they were lower with FSC than IPR, IPA, or TIO in the current study. This difference might be explained by the older patient population in the current study: as COPD morbidity appears to increase with ageCitation27, the elderly population might have yielded a more sensitive test of the economic impact of therapy. This possibility is consistent with the results of a retrospective claims analysis involving 1,051 adults ≥65 years old with COPDCitation26. FSC compared with IPR was associated with a 45% reduction in the risk of COPD-related hospitalization or ED visit, lower COPD-related medical costs, and higher COPD-related pharmacy costs. In that studyCitation25 as in the current study, the reduction in medical costs appeared to more than offset the increase in pharmacy costs such that total costs (medical + pharmacy) were lower with FSC; however, total costs were not reported in the latter studyCitation26. Considered in aggregate, the data from previously published claims analyses and the current study suggest that FSC is associated with significantly lower risk of hospitalization and ED visits at total medical costs lower than those of IPR in older patient populations and similar to those of IPR in younger patient populations. The current study extends these observations to IPA and TIO.
The results of this study should be interpreted in the context of its limitations. Like other observational studies, this one is subject to inherent biases including confounding bias and selection bias. Confounding bias and selection bias exist due to absence of randomization. In a real-world administrative claims database, patients classified based on operational definitions may be (usually are) different at baseline. They also may differ in disease severity, a fact that makes direct comparisons difficult. This study included proxies for disease severity that are routinely accepted as a risk-adjustment methodology in multivariate comparisons so that confounding arising from extraneous factors can be minimized. Bias was also addressed in part by limiting the sample to patients who did not initiate another treatment within 60 days after initial treatment to ensure that the study captured patients initiating treatment rather than those switching treatments. Patient characteristics such as body mass index (BMI) and smoking history and objective clinical data such as results of lung function measures are not available in the administrative data. Other databases, such as electronic medical records (EMR), capture clinical measures; however, they generally lack administrative data and cost information. The IHCIS database was selected for this study because it is large enough to provide ample sample size and represented commercially insured managed Medicare (Medicare Advantage) beneficiaries in the US. However, it is not representative of the US population or the US Medicare population. Accuracy of diagnosis codes could not be verified. These limitations are characteristic of observational studies. In the event that these biases were operating, they are likely to have been operating similarly across cohorts; therefore, the study outcome would have been biased toward the null hypothesis of no differences among cohorts. Other limitations include potential errors in the coding of claims as well as diagnoses codes and the absence of a means of assessing patient compliance.
This study provides new information on the cost impact of major bronchodilator maintenance therapies for COPD in elderly patients enrolled in a Medicare risk-sharing plan. Initiation of maintenance therapy with FSC was associated with significant reduction in total costs (medical + pharmacy) relative to those associated with the short-acting anticholinergic bronchodilators IPR and IPA and the long-acting anticholinergic bronchodilator TIO in an elderly Medicare-eligible population. Considered in the context of the aforementioned data on reductions in exacerbations and in hospitalizations and ED visits with FSC relative to anticholinergic bronchodilator therapy, these data suggest that early introduction of maintenance treatment with FSC has both clinical and economic benefits.
Acknowledgments
Declaration of interest: GlaxoSmithKline funded the study described in this manuscript. A.A.D. has disclosed that he is employed by GlaxoSmithKline. H.P. and C.M.B. have disclosed that they have received research funding from GlaxoSmithKline and other pharmaceutical companies on similar topics, and C.M.B. serves as a consultant to GlaxoSmithKline, and other pharmaceutical companies in respiratory disease research. L.S-W has disclosed that she has received research funding from GlaxoSmithKline for similar research. The authors acknowledge Jane Saiers, PhD, for assistance with writing the manuscript. GlaxoSmithKline funded Dr. Saiers’ work.
References
- Mannino DM. COPD: epidemiology, prevalence, morbidity and mortality, and disease heterogeneity. Chest 2002;121(5 Suppl): 121-6S.
- Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med 2006;343:269-80.
- Foster TS, Miller JD, Marton JP, Assessment of the economic burden of COPD in the US: a review and synthesis of the literature. COPD 2006;3:211-18.
- Menzin J, Boulanger L, Marton J, The economic burden of chronic obstructive pulmonary disease (COPD) in a US Medicare population. Respir Med 2008;102:1248-56.
- American Lung Association. Research on chronic obstructive pulmonary disease. Available at: http://lungaction.org/lungusa/alert-description.html?alert_id=2956848 . Accessed: 27 January 2009.
- Global Initiative for Chronic Obstructive Lung Disease. Executive Summary. National Institutes of Health: National Heart, Lung, and Blood Institute. Updated 2008. Available at: http://www.goldcopd.com/Guidelineitem.asp?l1=2&l2=1&intId=2003. Accessed 27 January 2009.
- MacIntyre N, Huang YC. Acute exacerbations and respiratory failure in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2008;5:530-5.
- Grasso ME, Weller WE, Shaffer TJ, Capitation, managed care, and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;158:133-8.
- Hilleman DE, Dewan N, Malesker M, Pharmacoeconomic evaluation of COPD. Chest 2000;118:1278-85.
- Mapel DW, Hurley JS, Frost FJ, Health care utilization in chronic obstructive pulmonary disease. A case-control study in a Health Maintenance Organization. Arch Intern Med 2000;160:2653-8.
- Strassels SA, Smith DH, Sullivan SD, The costs of treating COPD in the United States. Chest 2001;119:344-52.
- Halpern MT, Stanford RH, Borker R. The burden of COPD in the USA: results from the Confronting COPD survey. Respir Med 2003;97(Suppl C):S81-9.
- Tsai C-L, Sobrino JA, Camargo CA. National study of emergency department visits for acute exacerbation of chronic obstructive pulmonary disease, 1993-2005. Acad Emerg Med 2008;15:1275-83.
- Mapel DW, Dedrick D, Davis K. Trends and cardiovascular co-morbidities of COPD patients in the Veterans Administration Medical System, 1991-1999. COPD 2005;2:35-41.
- Decramer M, Nici L, Nardini S, Targeting the COPD exacerbation. Respir Med 2008;102(Suppl 1):S3-15.
- Szafranski W, Cukier A, Ramirez A, Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J 2003;21:74-81.
- Calverley PM, Pauwels R, Vestbo J, Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2003;361:449-56.
- Calverley PM, Anderson JA, Celli B, Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007;356:775-89.
- Ferguson GR, Anzueto A, Fei R, Effect of fluticasone propionate/salmeterol (250/50 μg) or salmeterol (50 μg) on COPD exacerbations. Respir Med 2008;102:1099-108.
- Wedzicha JA, Calverley PM, Seemungal TA, The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med 2008;177:19-26.
- Lee TA, Wilke C, Joo M, Outcomes associated with tiotropoium use in patients with chronic obstructive pulmonary disease. Arch Intern Med 2009;169:1403-10.
- Soriano JB, Kiri VA, Pride NB, Inhaled corticosteroids with/without long-acting beta-agonists reduce the risk of rehospitalization and death in COPD patients. Am J Respir Med 2003;2:67-74.
- Rascati KL, Stanford RH, Borker R. A comparison of the risk of hospitalizations due to chronic obstructive pulmonary disease in Medicaid patients with various medication regimens, including ipratropium, inhaled corticosteroids, salmeterol, or their combination. Clin Ther 2005;27:346-54.
- Rascati KL, Akazawa M, Johnsrud M, Comparison of hospitalizations, emergency department visits, and costs in a historical cohort of Texas Medicaid patients with chronic obstructive pulmonary disease, by initial medication regimen. Clin Ther 2007;29:1203-13.
- Akazawa M, Hayflinger DC, Stanford RH, Economic assessment of initial maintenance therapy for chronic obstructive pulmonary disease. Am J Manag Care 2008;14:438-48.
- Blanchette CM, Akazawa M, Dalal A, Risk of hospitalizations/emergency department visits and treatment costs associated with initial maintenance therapy using fluticasone propionate 500 μg/salmeterol 50 μg compared with ipratropium for chronic obstructive pulmonary disease in older adults. Am J Geriatr Pharmacother 2008;6:138-46.
- National Heart, Lung, and Blood Institute. 2007 NHLBI morbidity and mortality chartbook. Bethesda, Maryland: US Department of Health and Human Service, National Institutes of Health. 2007 . Available at: http://www.nhlbi.nih.gov/resources/docs/cht-book.htm . Accessed 30 January 2009.
- US Department of Labor. Bureau of Labor Statistics. Consumer Price Indexes. Available at: http://www.bls.gov\CPI . Accessed 21 September 2009.
- Gibson PG, Simpson JL. The overlap syndrome of asthma and COPD: what are its features and how important is it? Thorax 2009;64:728-35.
- Blanchette CM, Gutierrez B, Ory C, Economic burden in direct costs of concomitant chronic obstructive pulmonary disease and asthma in a Medicare Advantage population. J Manag Care Pharm 2008;14:176-85.
- Make B, Hanania NA, ZuWallack R, The efficacy and safety of inhaled fluticasone propionate/salmeterol and ipratropium/albuterol for the treatment of chronic obstructive pulmonary disease: an eight-week, multicenter, randomized, double-blind, double-dummy, parallel-group study. Clin Ther 2005;27:531-42