Abstract
Objective:
To estimate the cost effectiveness of solifenacin 5 mg/day compared to oxybutynin immediate-release (IR) 15 mg/day in patients with overactive bladder, from the perspective of the Canadian healthcare (payer) system.
Research design and methods:
A Markov model was adapted to estimate the incremental cost per quality-adjusted life-year (QALY) of solifenacin and oxybutynin IR over a 1-year time horizon, based on efficacy and discontinuation data from the Canadian VECTOR (VEsicare in Comparison To Oxybutynin for oveRactive bladder patients) study. In the model, patients who discontinued treatment were offered tolterodine extended release 4 mg/day as second-line. Model robustness was tested using various sensitivity analyses. Utility values were derived from published literature; incontinence pads were included in a secondary analysis.
Results:
In the base-case analysis, total costs over 1 year were CAN$695 and CAN$550 in the solifenacin and oxybutynin IR groups, respectively. When including incontinence pad costs, there was an incremental saving of CAN$1,831 per patient with solifenacin. Solifenacin was associated with an incremental QALY gain of 0.01 over 1 year. In the base-case analysis without incontinence pads, the incremental cost-utility ratio for solifenacin was CAN$14,092. Probabilistic analyses showed no overlap in the 95% confidence intervals for total costs or QALYs with or without incontinence pads. Solifenacin was cost effective in >90% of cases, based on a willingness-to-pay threshold of CAN$50,000 per additional QALY, irrespective of whether pad costs were included in the model. The most influential variables were the discontinuation rates and the cost of incontinence pads. Limitations of the analysis relate mainly to the fact that data in the VECTOR study were collected using a direct questioning approach, which might have increased the reporting of dry mouth.
Conclusions:
Solifenacin 5 mg/day was a cost-effective treatment compared with oxybutynin IR 15 mg/day.
Conclusions:
NCT00431041 (of the VECTOR study, upon which the analysis in this paper was based).
Introduction
Overactive bladder (OAB) is a chronic condition that is defined by the International Continence Society as urinary urgency, with or without urgency incontinence, usually with increased daytime frequency and nocturiaCitation1. Urgency is a sudden compelling desire to pass urine which is difficult to deferCitation1.These symptoms can have a significant impact on a person’s quality of life (QoL), interfering with daily routines and causing embarrassment and lowered self-esteem. Moreover, OAB is also associated with an increased risk and prevalence of comorbidities, including falls and fractures, urinary tract and skin infections, and depressionCitation2,Citation3.
OAB is a common condition. In a population-based survey of over 19,000 adults in Canada and Europe (the EPIC study), the overall prevalence of OAB was 11.8%, (12.8% women, 10.8% men)Citation4. The Canadian Urinary Bladder Survey of 1,000 adults reported OAB in 13.9% of respondents (14.7% women, 13.1% men)Citation5, while the NOBLE study of over 5,000 adults in the United States reported an overall prevalence of 16.5% (16.9% women, 16.0% men)Citation6. In all these surveys, the prevalence of OAB symptoms increased with age.
The economic burden of OAB is considerable. The NOBLE study estimated the overall cost of OAB in the United States to be US$12.02 billion per year (US$9.17 billion in the community and US$2.85 billion in nursing homes), based on costs in year 2000Citation7. The cost included treatment of associated consequences such as falls and fractures, urinary tract and skin infections, and depression, as well as indirect costs such as lost productivity.
Initial treatment options for OAB include bladder training, fluid management, pelvic floor muscle exercises, and the use of antimuscarinic drugs. The efficacy and safety profiles of different antimuscarinic drugs depend on their interactions with muscarinic receptors, of which five distinct subtypes (M1–M5) have been identified. Interactions with M3 and M2 receptors in the bladder are mostly responsible for the control of OAB symptomsCitation8, but similar interactions in other tissues can cause unwanted anticholinergic-type adverse events such as dry mouth, constipation and blurred vision; this complicates the determination of the optimum balance between efficacy and tolerability for patients.
Early discontinuation of treatment with antimuscarinic drugs is a well-recognized problemCitation9–12. A retrospective pharmacy claims analysis in the United States reported that 46.9% of patients with OAB discontinued antimuscarinic therapy after 3 months, and that less than 20% of patients remained on therapy after 12 monthsCitation9. Studies carried out in the UK and the Netherlands reported a high discontinuation rate with all the antimuscarinics studied, but noted differences among individual antimuscarinicsCitation10–12. In a Canadian study, solifenacin 5 mg/day was associated with significantly fewer withdrawals due to dry mouth compared with oxybutynin immediate release (IR) 15 mg/day (3 vs. 19%, respectively; p = 0.003)Citation13.
In addition to considerations of efficacy, tolerability and discontinuation rates, the choice of an antimuscarinic will often be influenced by economic considerations. Unfortunately, clinical trials carried out over a relatively short duration are of limited value in determining long-term economic outcomes directly. Therefore, it is usually necessary to use economic models designed to project costs over a longer period, by combining clinical, observational and economic data from various sourcesCitation14. The objective of the present analysis was to evaluate the cost effectiveness of solifenacin 5 mg/day compared to oxybutynin IR 15 mg/day as first-line treatment of OAB, from the perspective of the Canadian healthcare (payer) system. The analysis was based on data from the Canadian VECTOR study (VEsicare in Comparison To Oxybutynin for oveRactive bladder patients), which was a randomized, double-blind study in 132 patients with OAB who received solifenacin 5 mg once-daily or oxybutynin IR 15 mg/day (5 mg three-times daily) for 8 weeksCitation13. For the pharmaco-economic analysis, the 8-week data were projected over a 1-year time horizon, using a validated Markov modelCitation14 which was modified to reflect Canadian clinical practice and reimbursement policies.
Patients and methods
Data on efficacy, tolerability and discontinuation rates from the VECTOR studyCitation13 were used as the basis for this economic analysis. The VECTOR study was a prospective, randomized, double-blind, double-dummy, multicenter, two-arm comparative parallel group study including 132 subjects with ≥1 urgency episode/24 h (with or without urgency urinary incontinence) and ≥8 micturitions/24 h for ≥3 months. After a 2-week washout period, patients were randomized to receive either solifenacin 5 mg once-daily (n = 68) or oxybutynin IR 15 mg/day (5 mg three-times daily) (n = 64) for 8 weeks. The primary objective was to evaluate the tolerability of solifenacin and oxybutynin IR, as assessed by the patient-reported incidence and severity of dry mouth. Adverse events were determined by the following direct questioning by the physician at baseline and at each follow up visit: ‘Do you think you are having any side-effects from the medications? What are they?’ If dry mouth was reported, its severity was graded as mild, moderate, or severe according to predefined criteria. The Informed Consent Form made clear to patients that dry mouth was a primary outcome of the study.
Markov model
A dynamic decision analytic (Markov) model, using Microsoft Excel, was used to estimate the incremental cost per quality-adjusted life-year (QALY) of solifenacin 5 mg/day compared with oxybutynin IR 15 mg/day. The estimated incremental cost per QALY was projected over a 1-year time horizon, with cycle lengths of 4 weeks, consistent with the availability of data from the VECTOR study. The Markov model was adapted from a previously published modelCitation14. To ensure the model reflected actual clinical practice and common reimbursement policies across Canada, patients who discontinued treatment with oxybutynin IR or solifenacin were offered second-line therapy with tolterodine extended release (ER) 4 mg/day.
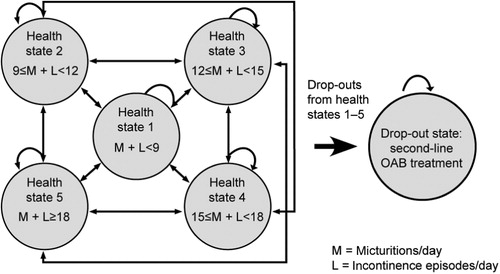
The model followed a cohort of patients through five discrete health states with varying degrees of OAB severity, and one drop-out state (). Health states 1–5 were based on a composite measure of micturitions and incontinence episodes per day, in which health state 1 was considered ‘mild’ and health state 5 ‘severe’. Health state 6 (the drop-out state) acted as the absorbing state, but also captured the cohort who received second-line treatment with tolterodine ER. The initial health state probability values (i.e., distribution amongst the health states at baseline) for solifenacin and oxybutynin IR were extracted from the VECTOR study results. Two independent data researchers (CP and CV) extracted the data separately and compared results to ensure accuracy. Given the VECTOR trial ran for 8 weeks, it was assumed that no further efficacy gains would occur for the subsequent 44 weeks in the model. Thus, patients still on treatment at the end of modeled year are in the same health state as was calculated at week 8 with the exception of patients who have transitioned into the ‘drop-out’ health state. Yearly drop-out rates, or discontinuation rates, were derived from a proprietary provincial claims database [Brogan Inc, Ottawa, Ontario, Canada; www.broganinc.com] and assumed to be linear over time.
Figure 1. Schematic of Markov model. Diagram adapted from Speakman et al.Citation15.
Utility values were derived from the published literatureCitation14 in order to indicate the preference (or value) patients had for a health state (). The utility value for the drop-out state was calculated as an average of utilities for health states 1–5, to try and capture the potential benefits of second-line treatment.
Table 1. Utility values used in the Markov model. Adapted from Kobelt et al.Citation14*.
The primary outcome of this analysis was the incremental cost-utility ratio (ICUR) at 1 year with solifenacin 5 mg/day and oxybutynin IR 15 mg/day. The QALY for each treatment option was calculated by multiplying each health state’s utility value by the time spent in each health state. The ICUR was determined by dividing the difference in total costs of solifenacin from oxybutynin IR by the difference in QALYs between the two treatments:
Costings
Unit costs are summarized in . The analysis was conducted mainly from the Canadian public payer perspective. The base-case analysis did not include the cost of incontinence pads, as these items are not covered by the Canadian public health system. However, given the economic burden of additional out-of-pocket expenses related to incontinence pads, a secondary analysis was also carried out which included the cost of adult incontinence pads. Drug costs were derived from the Ontario Drug Benefit Formulary, unless otherwise notedCitation19, based on a daily dose of 5 mg solifenacin and 15 mg oxybutynin IR, adjusted for adherence rates from the VECTOR study (i.e., the percentage of patients remaining on each drug after 8 weeks) and then by real-world annual adherence rates (solifenacin 34%, oxybutynin IR 16%, tolterodine ER 34%). It was assumed that all patients in the drop-out state started second-line treatment with tolterodine ER 4 mg, adjusted for real-world adherence. This assumption was validated based on a review of publicly funded claims for tolterodine in Ontario, which demonstrated a high market share, and based on the listed status of tolterodine in Ontario, which suggest it would only be used after failure to or intolerance to oxybutynin, i.e. that its use would be reserved as a second-line therapy. The utilization of incontinence pads by health state was derived from two placebo-controlled, double-blind trials with solifenacinCitation17,Citation18, with supplementary unpublished data on pad use from these clinical trials. The cost of a typical medium-sized adult pad was derived from a pharmacy wholesaler. Given that the model was contained within a 1-year time horizon, discounting of costs and outcomes was not performed. With the exception of dry mouth, adverse events rates in the VECTOR study were similar between solifenacin and oxybutynin IR; thus, the costs of adverse events other than dry mouth were excluded from the analysis. In the VECTOR study, dry mouth was reported by 24/68 patients in the solifenacin group and by 53/64 patients in the oxybutynin IR group (p < 0.0001)Citation13. The relatively high incidence of dry mouth in both groups may be the result of the direct questioning methodology used and patients being informed that dry mouth was the primary study objective.
Table 2. Unit costs used in each analysis.
Sensitivity analyses
One-way and multivariate probabilistic sensitivity analyses using Monte Carlo simulations of 10,000 iterations were performed to test the robustness of the model to variations in input parameters (). In the one-way analyses, the unit cost for incontinence pads was adjusted to reflect the cost of large rather than medium sized pads, and was also adjusted to zero to reflect that incontinence pads are not funded by the Canadian healthcare system. The unit cost of tolterodine was adjusted to zero to determine the impact of not offering tolterodine as a second-line alternative. In addition, the utility values for all health states were adjusted to evaluate the impact of overall patient preferences and to determine if a higher preference for the drop-out state would alter the results. For the Monte Carlo analysis, health-state transition probabilities and utilities were modeled using a beta distribution. Costs of tolterodine were modeled using a normal distribution; this method was chosen (rather than a gamma distribution) because there were no concerns about having a skewed range of potential prices and discontinuation rates.
Table 3. Sensitivity analyses performed.
Transition probabilities were not altered in the sensitivity analyses. This was because there was a high degree of certainty around real-world discontinuation used to model drop-out rates in weeks 8–52 post-VECTOR. However, a scenario analysis was performed to determine the impact of lowering the oxybutynin IR drop-out rates by 10–30%. The upper testing limit of 30% was chosen because drop-outs in the VECTOR study were approximately 30% greater with oxybutynin IR compared to solifenacin.
Results
Efficacy analysis
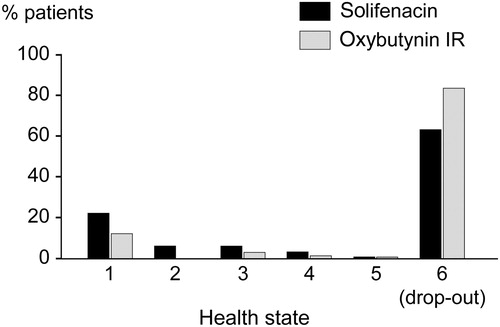
illustrates the distribution of the patients among the five health states at 12 months (the distribution at baseline was previously reported in ). The drop-out rate was estimated to be 62% for solifenacin and 83% for oxybutynin IR after 1 year, reflecting drop-out rates observed through analyses of a provincial claims database.
Cost-effectiveness analysis
In the base-case analysis which excluded incontinence pads, the mean total costs over a 1-year treatment period were CAN$695 in the solifenacin treatment arm and CAN$550 in the oxybutynin IR treatment arm. Probabilistic analysis gave mean total costs of CAN$566 (95% CI: 566–567) and CAN$347 (95% CI: 346–348) in the solifenacin and oxybutynin IR treatment arms, respectively, with no overlap in the CIs.
In the analysis which included the cost of incontinence pads, total costs over a 1-year treatment period were CAN$2,556 for patients receiving solifenacin and CAN$4,387 for those receiving oxybutynin IR, resulting in incremental cost savings of CAN$1,831 per patient on solifenacin. Incontinence pads were the main cost contributor, representing 73% and 87% of costs in the solifenacin and oxybutynin treatment arms, respectively. Probabilistic analyses showed mean total treatment costs of CAN$3,079 (95% CI: 2,901–3,258) with solifenacin and CAN$5,530 (95% CI: 5,162–5,897) with oxybutynin IR, with no overlap in the CIs. This resulted in incremental cost savings of CAN$2,451 per patient on solifenacin.
summarizes total costs, QALYs and the ICURs with and without incontinence pads, based on utility data from the literature. Treatment with solifenacin 5 mg/day resulted in total QALYs of 0.696 versus total QALYs of 0.686 for oxybutynin IR, an incremental QALY gain of 0.01 over the 1-year time period. In the base-case analysis without incontinence pads, solifenacin had an ICUR of CAN$14,092 per QALY. In the analysis which included incontinence pads, solifenacin was dominant compared to oxybutynin IR, as defined by achieving lower costs while having similar or greater benefits (in terms of QALYs). Probabilistic analysis demonstrated mean QALYs of 0.681 (95% CI: 0.679–0.683) for solifenacin, and 0.671 (95% CI: 0.669–0.673) for oxybutynin IR, irrespective of whether incontinence pads were included in the analysis or not. In none of the cases was there an overlap in the CIs.
Table 4. Summary of costs, QALYs, and ICUR results at 1 year with solifenacin 5 mg/day and oxybutynin IR 15 mg/day, with and without the cost of incontinence pads. Costs are in CAN$.
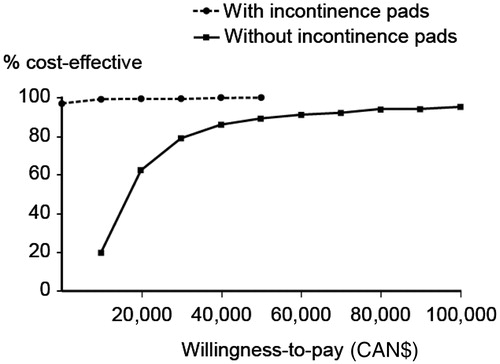
shows the cost-effectiveness acceptability curves of solifenacin versus oxybutynin IR, with and without the cost of incontinence pads, based on the probabilistic sensitivity analyses (Monte Carlo simulations). For the base-case analysis without incontinence pads, solifenacin was cost effective compared to oxybutynin IR in approximately 90% of cases, based on the traditional willingness-to-pay threshold for cost effectiveness in the Canadian healthcare system of CAN$50,000 per additional QALY gainedCitation20. When the cost of incontinence pads was included in the analysis, solifenacin was cost effective compared with oxybutynin IR in over 95% of cases, even if the willingness-to-pay threshold was placed at zero.
Additional sensitivity analyses
A summary of the results of one-way deterministic sensitivity analyses are presented in . The model was robust to changes in key assumptions, indicating a high degree of certainty of the base-case results. When the cost of adult incontinence pads was added to the base-case analysis, solifenacin was dominant compared to oxybutynin IR. The only sensitivity analysis which had an impact on the results was when the utility for the drop-out state was varied to be equal to that of the second health state (≥9, <12); this is an unlikely scenario given that less than 10% of patients were in this health state at the start of the study.
Table 5. Summary of results of sensitivity analyses, in which the cost of incontinence pads was excluded from the base case (costs in CAN$).
One-way sensitivity analyses showed no changes to the ICUR values calculated in the base-case analysis unless the oxybutynin drop-out rate was reduced by approximately 30% (when the ICUR was CAN$166,508 per QALY gained).
Discussion
Oxybutynin IR has been traditionally used as first-line treatment for OAB, partially because of its low cost compared to other antimuscarinics. However, it is often associated with a high incidence of dry mouthCitation13,Citation21,Citation22, which might contribute to early discontinuation of therapyCitation13. In recent years, solifenacin has become a well-established treatment for OAB in many countries, and its efficacy has been consistently demonstrated in numerous randomized controlled trials, using a wide range of objective and subjective measuresCitation17,Citation18,Citation23–25. The Canadian VECTOR study reported a significantly lower incidence and severity of dry mouth with solifenacin 5 mg/day compared with oxybutynin IR 15 mg/day, resulting in fewer withdrawals in the solifenacin groupCitation13.
The economic analysis described here estimated the cost effectiveness of solifenacin 5 mg/day compared with oxybutynin IR 15 mg/day in patients with OAB, from the perspective of the Canadian healthcare system. It used a Markov model that was primarily driven by assumptions on drop-out rates, costs, and health outcomes attributable to drop-outs. These assumptions were tested with sensitivity analyses which used a wide range of parameters and input values. In the analysis which excluded the cost of incontinence pads, acceptability curves showed that solifenacin was a cost-effective treatment option in approximately 90% of cases, based on a standard threshold of <CAN$50,000 per QALY gained. Indeed, solifenacin remained cost-effective in about 70% of cases even if the willingness-to-pay threshold was reduced to CAN$20,000. The costs of incontinence pads are not covered by the Canadian public healthcare system, but nevertheless remain a significant expense for patients. In the analysis which included the cost of incontinence pads, first-line treatment with solifenacin 5 mg/day over the 1-year time period resulted in incremental cost savings of CAN$1,831 per patient and an incremental QALY gain of 0.01 compared with oxybutynin IR 15 mg/day. Solifenacin remained cost effective even in the analysis that assumed the health-state values for drop-outs were (optimistically) equivalent to health state 2. Probabilistic analyses showed that there was no overlap in the 95% CIs for mean total costs and QALYs in each treatment arm, irrespective of whether or not incontinence pads were included in the analysis.
Several other economic evaluations of solifenacin have used a similar modeling approach. Hakkaart et al.Citation26 used a Markov model technique to compare solifenacin 5 mg/day and 10 mg/day versus placebo over a 12-month period in patients with OAB, based on pooled results from four 12-week, randomized, double-blind, parallel-group, phase III clinical trials which had similar protocols; the studies generated data from 1,890 patients. Compared with placebo, the incremental cost per QALY was £17,602 (GBP) for solifenacin 5 mg/day and £24,464 for solifenacin 10 mg/day, based on 2004 costs. These values were both within UK national guidelines for considering a treatment to be cost-effective. Speakman et al.Citation15 used a 1-year Markov model to compare the cost utility of flexible dosing with solifenacin (5 mg and 10 mg) versus tolterodine (IR 2 mg bd and ER 4 mg). The study used weighted costs derived from 2008 UK prescription data, and patient-level data obtained from a 12-week, randomized, double-blind study in 1,186 patients with OAB. Solifenacin was found to be a dominant strategy, associated with lower costs and superior effectiveness over the 1-year period. The estimated cost per patient was £509 for patients treated with solifenacin and £526 for tolterodine, whereas treatment with solifenacin was associated with a small incremental gain of 0.004 QALYs over tolterodine.
There are some limitations to the present analysis. Adverse events reported in VECTOR were collected using a direct questioning approach, and the Informed Consent Form made clear to patients that dry mouth was a primary outcome of the study. Furthermore, the patient sample size in VECTOR may be considered relatively small, impacting on the precision of the VECTOR estimates. These factors might have given higher rates of dry mouth in both treatment groups compared with routine practice. However, the scenario analysis showed that drop-out rates due to dry mouth for oxybutynin IR would need to be reduced by nearly 30% before the results of the analysis were altered. Secondly, the costs of adverse events other than dry mouth were not included in the analysis. This was not expected to induce bias, and furthermore the relative consequences of dry mouth were captured in the drop-out-adjusted transition probabilities for each treatment. The VECTOR study did not demonstrate significant differences in adverse outcomes which might result from ineffectively treated OAB patients, e.g. urinary tract infections, or fractures from falls. Thus, they were excluded from this analysis, which may have underestimated total costs and overestimated the preferences attributed to the drop-out state.
Conclusion
This cost-utility analysis was based on the results of the VECTOR study from the perspective of the Canadian healthcare (payer) system. When excluding the cost of incontinence pads, solifenacin 5 mg/day was associated with improved patient outcomes (QALYs) compared with oxybutynin IR 15 mg/day, and was the cost-effective treatment strategy in approximately 90% of cases at a standard threshold of <CAN$50,000 per QALY gained. If the cost of incontinence pads was included, solifenacin was the dominant treatment strategy, providing an incremental cost saving of CAN$1,831 per patient over a 1-year period. Solifenacin 5 mg/day generally remained the most cost-effective strategy even when considering a wide range of variations in key model parameters.
Transparency
Declaration of funding
This was supported by a research grant from Astellas Pharma Canada, Inc.
Declaration of financial/other relationships
The authors declare the following for relevant research, consultancy and/or advisory work: S.H. has disclosed that he has been involved in research, consultancy and/or advisory work for Astellas, Pfizer, Allergan, Coloplast, Johnson & Johnson, and American Medical Systems.
C.V. and C.P. have disclosed that they are employees of PIVINA Consulting Inc., a company that received funding from Astellas to conduct this study.
Acknowledgments
Medical writing and editorial assistance were provided by S. Sharpe PhD of SharpeCom Ltd, funded by Astellas Pharma Canada, Inc.
Some of the results of this study were presented as an abstract and poster at the 34th Annual meeting of the International Urogynaecological Association (IUGA), Como, Italy, June 16–20, 2009 (poster 569).
References
- Abrams P, Artibani W, Cardozo L, et al. Reviewing the ICS 2002 terminology report: the ongoing debate. Neurourol Urodyn 2006;25:293
- Brown JS, McGhan WF, Chokroverty S. Comorbidities associated with overactive bladder. Am J Manag Care 2000;6(11 Suppl):S574-579
- Brown JS, Vittinghoff E, Wyman JF, et al. Urinary incontinence: does it increase risk for falls and fractures? J Am Geriatr Soc 2000;48:721-725
- Irwin DE, Milsom I, Hunskaar S, et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol 2006;50:1306-1315
- Herschorn S, Gajewski J, Schulz J, et al. A population-based study of urinary symptoms and incontinence: the Canadian Urinary Bladder Survey. BJU Int 2007;101:52-58
- Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol 2003;20:327-336
- Hu T-W, Wagner TH, Bentkover JD, et al. Estimated economic costs of overactive bladder in the United States. Urology 2003;61:1123-1128
- Andersson K-E. Antimuscarinics for treatment of overactive bladder. Lancet Neurol 2004;3:46-53
- Darkow T, Fontes CL, Williamson TE. Costs associated with the management of overactive bladder and related comorbidities. Pharmacotherapy 2005;25:511-519
- Wagg AS, Fahey A, Siddiqui E. Persistence with oral antimuscarinics used for the treatment of overactive bladder in different age groups of patients in general practice. British Geriatrics Society, Autumn Meeting, October 7-9, 2009, Harrogate, UK, Abstr 95
- Siddiqui E, Fahey A, Huang M. Persistence with antimuscarinics in patients with overactive bladder syndrome: analysis of a UK database. 30th Congress of the Société Internationale d'Urologie (SIU), November 1–5, 2009, Shanghai, China, Abstr MP-17.10
- Blok B, van Kerrebroeck P, Buijs S, et al. Persistence with antimuscarinics in a European study of patients with overactive bladder syndrome. Int Urogynecol J 2009;20(Suppl 2):S192-193, Abstr 141
- Herschorn S, Stothers L, Carlson K, et al. Tolerability of 5 mg solifenacin once daily versus 5 mg oxybutynin immediate release 3 times daily: results of the VECTOR trial. J Urol 2010;183:1892-1898
- Kobelt G, Jönsson L, Mattiasson A. Cost-effectiveness of new treatments for overactive bladder: the example of tolterodine, a new muscarinic agent: a Markov model. Neurourol Urodyn 1998;17:599-611
- Speakman M, Khullar V, Mundy A, et al. A cost-utility analysis of once daily solifenacin compared to tolterodine in the treatment of overactive bladder syndrome. Curr Med Res Opin 2008;24:2173-2179
- Johannesson M, O'Conor RM, Kobelt-Nguyen G, et al. Willingness to pay for reduced urinary incontinence symptoms. Br J Urol 1997;80:557-562
- Chapple CR, Rechberger T, Al-Shukri S, et al. Randomized, double-blind placebo and tolterodine-controlled trial of the once-daily antimuscarinic agent solifenacin in patients with symptomatic overactive bladder. BJU Int 2004;93:303-310
- Cardozo L, Lisec M, Millard R, et al. Randomized, double-blind placebo controlled trial of the once daily antimuscarinic agent solifenacin succinate in patients with overactive bladder. J Urol 2004;172:1919-1924
- OPDP. Ontario Drug Benefit Formulary/Comparative Drug Index, 2009. www.health.gov.on.ca (accessed February 2009)
- Laupacis A, Feeny D, Detsky AS, et al. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. CMAJ 1992;146:473-481
- Anderson RU, Mobley D, Blank B, et al. Once daily controlled versus immediate release oxybutynin chloride for urge urinary incontinence. J Urol 1999;161:1809-1812
- Versi E, Appell R, Mobley D, et al. Dry mouth with conventional and controlled-release oxybutynin in urinary incontinence. Obstet Gynecol 2000;95:718-721
- Chapple CR, Martinez-Garcia R, Selvaggi L, et al. A comparison of the efficacy and tolerability of solifenacin succinate and extended release tolterodine at treating overactive bladder syndrome: results of the STAR trial. Eur Urol 2005;48:464-470
- Cardozo L, Heßdörfer E, Milani R, et al. Solifenacin in the treatment of urgency and other symptoms of overactive bladder: results from a randomized, double-blind, placebo-controlled, rising-dose trial. BJU Int 2008;102:1120-1127
- Karram MM, Toglia MR, Serels SR, et al. Treatment with solifenacin increases warning time and improves symptoms of overactive bladder: results from VENUS, a randomized, double-blind, placebo controlled trial. Urology 2009;73:14-18
- Hakkaart L, Verboom P, Phillips R, et al. The cost utility of solifenacin in the treatment of overactive bladder. Int Urol Nephrol 2008;41:293-298