Abstract
Objective:
Assess the budgetary impact of adding erlotinib for maintenance therapy (MTx) in advanced non-small cell lung cancer (NSCLC) from a US health plan perspective.
Methods:
A budget impact model was developed to analyze the costs (drug, administration, adverse events) associated with adding erlotinib MTx to a hypothetical 500,000 member US health plan. Treatment durations and dosing were derived from randomized controlled trials, FDA labeling, and National Comprehensive Cancer Network guidelines. Treatment patterns and assumptions were based on market research data, the SEER registry, and published literature. Cost data were obtained from Centers for Medicare and Medicaid Services payment rates and a drug pricing database. Sensitivity analyses were conducted to assess uncertainty.
Results:
Overall health plan expenditures increased by $0.010 per member per month (PMPM). The main driver of additional cost was the erlotinib drug cost (∼$66,000) with the administration ($464) and side-effect ($47) costs being relatively modest. One-way sensitivity analyses showed that the results were most sensitive to the proportion of members receiving MTx; however, the PMPM did not exceed $0.013.
Conclusions:
The overall budget impact to a health plan of expanding the use of erlotinib from the 2nd/3rd-line advanced NSCLC setting to include the maintenance setting was relatively small. This was primarily due to the proportion of patients who would receive erlotinib MTx, the low cost of side-effects and minimal cost of drug administration. Additional research may be warranted to estimate the relative clinical and economic impacts of erlotinib MTx versus alternative MTx treatments.
Introduction
Lung cancer is the most common cause of cancer death in the United States, with more men and women dying from lung cancer than breast, colon and prostate cancers combinedCitation1. It is estimated that in 2010, 116,750 men and 105,770 women were diagnosed with lung cancer and 86,220 men and 71,080 women died from lung cancer (accounting for roughly 28% of all cancer deaths)Citation1. The large majority, 84%Citation2, of these lung cancers are non-small cell lung cancer (NSCLC) and patients are most commonly diagnosed with advanced or metastatic disease for which there is no curative treatment.
Erlotinib is a human epidermal growth factor receptor type 1/epidermal growth factor receptor (HER1/EGFR) tyrosine kinase inhibitor. Erlotinib was approved for 2nd or 3rd-line therapy in patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) in the US in 2004. More recently, erlotinib was approved (April 2010) for patients with locally advanced or metastatic NSCLC whose disease has not progressed after four cycles of platinum-based 1st-line chemotherapy by the FDA – also called maintenance therapy (MTx)Citation3. Approval for erlotinib MTx was based on a phase III trial, the SATURN trial, which compared erlotinib (150 mg/day) to placeboCitation4. In this trial, erlotinib met its primary endpoint, prolonging progression free survival over placebo (HR 0.71, 95% CI 0.62 to 0.82, p < 0.0001)Citation4. In addition, overall survival was significantly longer with erlotinib – extending median survival from 11 months to 12 months (HR 0.81, 95% CI 0.70–0.95, p = 0.0088)Citation4.
As a result of the high incidence of advanced NSCLC and the potential high cost of treatment, health plans may be concerned about the potential impact of adding erlotinib MTx to their formulary. We thus developed a budget impact model to analyze the incremental health care expenditures of adding erlotinib to the health plan formulary. The model considers patients from a hypothetical payer population of 500,000 members with advanced or metastatic (stage IIIB/IV) NSCLC whose disease has not progressed after four cycles of platinum-based 1st-line therapy and were eligible for erlotinib treatment.
Methods
Model structure
This deterministic cohort model was developed in Microsoft Excel (Microsoft, Redmond, WA, USA). Two scenarios were considered in this model. In the first scenario, erlotinib was not available for MTx, and thus only the current standard of care treatment algorithm was considered. In the second scenario, erlotinib was an option for MTx in addition to the current standard of care treatment algorithm. Comparing the costs (drug, administration and adverse effects) of treating patients in these two scenarios provides an estimate of the economic impact on a health plan’s budget of adding erlotinib in the MTx setting to the formulary in the 2010 plan year.
Estimation of the eligible population
The number of erlotinib-eligible patients in a health care system was based on a stratification of 500,000 health plan enrollees by age group and applying age specific incidence estimates of stage IIIB/IV NSCLC for each group. The age distribution of the health plan enrollees was based on US Census Bureau dataCitation5. Age and gender stratified incidence estimates of advanced NSCLC, defined by the American Joint Committee on Cancer (AJCC) stage IIIB/IV, were applied to the plan population based on the SEER 17 RegistryCitation2. Among all NSCLC patients, 54% were assumed to receive initial treatment with chemotherapy (based on an analysis SEER, United Healthcare claims data, and Medicare claim data) ().
Table 1. Number of plan members with stage IIIB/IV NSCLC.
Treatment assumptions
The proportion of patients eligible for and receiving erlotinib MTx was based on the SATURN trial and market research data, respectively. Subsequent treatment patterns in the 2nd and 3rd line were based on patient eligibility for further treatments and whether patients were alive and likely to accept any additional drug therapy based on market research data and a patterns of care study using the SEER Medicare dataCitation6. After the MTx phase, only those treatment options that were: (1) FDA approved in the particular indication and (2) recommended by the National Cancer Care Network (NCCN) guidelines for NSCLC were includedCitation7. Thus, patients in the 2nd-line setting were assumed to receive pemetrexed, docetaxel, or erlotinib. Patients in the 3rd-line setting were assumed to receive erlotinib if eligible. Patients who received erlotinib in either the MTx or 2nd-line settings were not eligible to receive erlotinib in subsequent lines of therapy. Pemetrexed was only considered to be an option for patients with nonsquamous cell NSCLC ().
In the scenario where erlotinib MTx was not available, we assumed that 58% of eligible patients would receive erlotinib as 2nd-line treatment in squamous NSCLC, 42% in nonsquamous NSCLC and 100% in 3rd-line treatment based on market research data and a patterns of care study using SEER-Medicare dataCitation6. In the scenario where erlotinib maintenance therapy was given, the use of pemetrexed and docetaxel increased in 2nd-line therapy due to our assumption that patients would not be given erlotinib in 2nd line if they had received it as maintenance therapy.
Adverse events
We included treatment for grade 3 or 4 adverse events with an incidence rate of 5% or greater as listed in the package inserts for each of the therapies. Adverse events requiring hospitalizations and supportive care for hematologic adverse events were included in the model regardless of the magnitude of incidence, if reported in the pivotal trial publications of the respective therapies. presents the incidence and costs of treating the adverse events.
Table 2. Incidence and costs of treatment of adverse events.
For erlotinib therapy the two most commonly associated grade 3 or 4 adverse events were rash and diarrhea. It was assumed that patients experiencing rash would require one outpatient visit and clindamycin gel for the remainder of the treatmentCitation8. All patients experiencing diarrhea were assumed to require one outpatient visit and treatment with loperamide. Costs of hospitalizations due to grade 3/4 diarrhea were assumed to include one inpatient and one follow-up physician visit as well as the cost of the inpatient stay based on the associated diagnosis related group (DRG 640)Citation9.
For pemetrexed and docetaxel, the most common serious adverse events were severe anemia, neutropenia and febrile neutropenia. Febrile neutropenia was assumed to require hospitalization (DRG 814) and include one inpatient physician visit and one follow-up consultation. Severe anemia patients were assumed to have one outpatient visit, with a proportion of those patients receiving treatment with erythropoietin and/or a red blood cell (RBC) transfusion. Proportions of patients receiving erythropoietin or RBC transfusions were obtained from the pivotal trial publication of pemetrexed compared to docetaxel in the 2nd-line treatment of NSCLC patientsCitation10. Patients with afebrile neutropenia were assumed to have one outpatient physician visit, with a proportion of them receiving one course of filgrastim (G-CSF) as prophylaxis for neutropenia. The proportion of patients receiving G-CSF was based on the pivotal trial publication of pemetrexed compared to docetaxel in the 2nd-line treatment of NSCLC patientsCitation10.
Costs
Drug costs for erlotinib were based on the dosing and duration of use as reported in the pivotal trial for erlotinib in MTx (the SATURN trial)Citation4 (). This trial included information on dose reductions in response to adverse events, which was utilized in the model. Dosing for other treatment options in this model was obtained from the respective full prescribing information. The average durations of treatment for pemetrexed and docetaxel were based on the mean number of cycles from the phase III trial comparing pemetrexed to docetaxel as 2nd-line monotherapy for NSCLCCitation10 ().
Table 3. Medication use and treatment costs for pemetrexed, docetaxel and erlotinib.
Erlotinib is a self-administered oral tablet; hence, no administration costs were assumed for this medication. One outpatient physician office visit was assumed for the first prescription with refill prescriptions for the remaining prescriptions not requiring additional physician office visits. The cost of an outpatient physician visit (office or other outpatient visit) was based on the 2010 Centers for Medicare and Medicaid Services (CMS) payment rates for the CPT (Current Procedural Terminology) code 99215Citation11.
The cost of treatment administration for pemetrexed and docetaxel was based on the average number of infusions per patient and the cost of administering the infusion. The cost per infusion includes one physician office visit for each infusion plus the cost of the chemotherapy infusion per hour. The cost of infusion was based on 2010 CMS payment rates for CPT 96413 (chemotherapy infusion first hour). The total administration cost per patient was a product of the number of infusions and the cost per infusion.
The model attached costs of hospitalization based on the hospitalization rates for pemetrexed and docetaxel as listed in the pivotal trial publication of pemetrexed. Costs for hospitalization are based on the 2010 National CMS payment rates. Drug costs are based on wholesale acquisition costs provided by First DataBank’s National Drug Data FileCitation12.
Analysis
In addition to aggregate costs to the health plan, an estimate of costs per member per month (PMPM) was computed. The PMPM cost was calculated by dividing the total cost to the plan by the number of members in the plan, then dividing by 12 months. After analyses were performed using base-case (default) estimates for the parameters, sensitivity analyses were performed on key model parameters to assess the robustness of the model results. Parameters were varied across confidence intervals, when available from clinical studies, or by ±25%, when data were unavailable. In addition, a scenario analysis was performed to evaluate the budget impact of adding erlotinib to standard care when pemetrexed MTx is available. In this scenario, it was assumed that MTx treatment prior to erlotinib availability consisted of pemetrexed MTx being used in all patients with nonsquamous histology. With the introduction of erlotinib, a market share shift from 100% to 80% was assumed for pemetrexed in nonsquamous patients corresponding with the assumption of a 20% market share for erlotinib in the nonsquamous MTx setting based on market research data.
Results
In a hypothetical health plan of 500,000 enrollees, it was estimated that 133 patients would be diagnosed with locally advanced or metastatic NSCLC per year. Accounting for 1st-line chemotherapy use and MTx eligibility, an estimated 11 stage IIIB/IV NSCLC patients would receive erlotinib MTx in the first year (). The total expected cost of treating stage IIIB/IV NSCLC patients who do not progress after four cycles of chemotherapy without bevacizumab was estimated to be $255,800 when erlotinib MTx was not a treatment option. With erlotinib MTx as a treatment option, the treatment cost was $318,100 – an increase of $69,300). When expressed as a PMPM cost, the addition of erlotinib MTx to the formulary resulted in an increased cost of $0.010 PMPM. The budget impact of erlotinib is detailed in .
Table 4. Treatment costs ($) and budget impact by treatment setting.
The average per person drug cost of treatment with erlotinib in the MTx ($18,900) and 2nd/3rd-line ($19,100) settings was nearly equivalent, accounting for wastage and treatment discontinuation. The drug costs of pemetrexed and docetaxel as 2nd-line treatments were estimated to be $22,200 and $12,300 per person on average, respectively. Additionally, the use of pemetrexed and docetaxel increased in the 2nd line because of the assumed loss of erlotinib as a 2nd-line treatment option for patients receiving erlotinib MTx. Specifically, the use of pemetrexed and docetaxel in nonsquamous patients in the 2nd line rose from 29% to 50% and the use of docetaxel increased from 42% to 100% in patients with squamous NSCLC. Conversely, since erlotinib was not available subsequent to patients receiving erlotinib MTx, its use decreased from 42% to 0% in 2nd line and 100% to 0% in the 3rd line. The increased cost associated with the increased utilization of docetaxel and pemetrexed in the 2nd line (∼$7,900) was offset by the decreased cost and utilization of erlotinib in the 2nd and 3rd line (∼$9,000).
Drug administration costs and side-effect treatment costs for erlotinib MTx were $464 and $47, respectively. Differences between the administration costs and side-effects costs for pemetrexed and docetaxel were relatively minimal between the two scenarios, with the largest difference occurring as a result of treating docetaxel side-effects ($1,003 per 500,000 person health plan). Given the relatively minimal cost impacts due to market share shifts in the 2nd/3rd line as well as those related to drug administration and side-effects, the increase in overall costs was primarily due to the additional drug costs of erlotinib MTx (∼$66,000) per 500,000 person health plan).
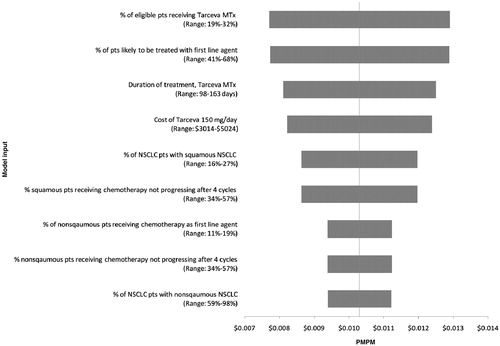
Sensitivity analyses demonstrated that the results were relatively insensitive to the percent of eligible patients who receive erlotinib MTx (range: $0.008–0.013), the percent of patients likely to be treated with a 1st-line agent (range: $0.008–0.013), and the duration of erlotinib MTx (range: $0.008–0.013). However, none of the PMPM added costs increased by more than $0.003 (equivalent to ∼25% increase). The budget impact was also insensitive to the percent of patients with nonsquamous NSCLC or changes in the incidence of grade 3 and 4 erlotinib-related adverse events (). In the scenario analysis in which pemetrexed was available for MTx therapy the PMPM was reduced to $0.002.
Discussion
The objective of this analysis was to estimate the budget impact of adding erlotinib MTx to a health plan formulary. In a hypothetical health plan of 500,000 enrollees, the estimated difference in costs when adding erlotinib as MTx was $62,000 (PMPM: $0.010) during the first year. This increase was primarily driven by the addition of erlotinib drug costs in the MTx setting and the subsequent increased use of 2nd/3rd-line agents that have higher costs than erlotinib as patients receiving erlotinib in the MTx setting were no longer eligible for erlotinib in the 2nd/3rd-line setting. Overall, a PMPM increase of $0.010 may be considered a small impact on overall health plan expenditures, and was driven by the relatively low number of patients who remain eligible for and receive erlotinib MTx after 1st-line chemotherapy and the relatively modest side-effect profile for erlotinib relative to alternative therapies.
The study results are similar to those of the study by Ramsey et al., in which the budgetary impact of erlotinib in the 2nd- and 3rd-line settings were considered. The estimated PMPM increase was $0.01, which is equal to the current study PMPM increase of $0.01013. The study results were similar due to: (1) similar treatment settings, (2) similar treatment durations, and (3) the relatively few number of patients receiving erlotinib in both studies (11 in this current study compared to 16 in Ramsey et al.). These results were similar despite differences in SEER registries used to estimate the population eligible and treatment costs.
A recent economic evaluation by Klein and colleagues compared pemetrexed MTx to no MTx, and included a comparison to erlotinib MTx as a secondary outcome. The incremental per patient cost for erlotinib versus observation was $7,500, roughly comparable to the present study estimate of $5,700Citation1Citation4. However, the authors assumed equal treatment durations, side-effect costs, and 2nd-line treatment costs for all treatment strategies in their analysis, making interpretation of the results somewhat challenging.
There are several limitations to this model worth noting. Other treatment options in the MTx setting in the base case-analysis were not considered. In practice, patients may receive chemotherapy beyond four cycles or switch to another anti-cancer agent used to treat NSCLC patients. Pemetrexed is currently the only other agent approved in NSCLC for MTx. In the scenario analysis exploring this issue, the PMPM was reduced from $0.010 to $0.002. This reduction was related to the cost savings associated with shifting a portion of nonsquamous patients from receiving pemetrexed to erlotinib as erlotinib is a less expensive treatment optionCitation14. Next, the model conservatively assumes that health plans incur the cost for the full treatment duration, including wastage, whether or not the patient ultimately fills all their prescriptions. Recurrent NSCLC was not considered in this model; however, the recurrent NSCLC patient population is exceedingly small and is unlikely to make a substantial impact on our resultsCitation15. The findings of the present analysis may not be generalizable to payers with different population distributions. In particular, Medicare has a higher proportion of erlotinib MTx eligible patients because the majority of advanced NSCLC diagnoses occur in patients 65 and over; therefore the PMPM increase is likely to be higher than the present results indicateCitation2. It is worth noting that erlotinib is an oral medication and as such patients might bear additional out-of-pocket expenses (i.e., co-pays), which were not accounted for in the budget impact to the plan. Lastly, this budget impact model does not consider efficacy beyond its immediate impact on expenditures. An evaluation of the clinical and economic outcomes of erlotinib MTx, with outcomes expressed as years of life and/or quality-adjusted life-years (QALY) gained, would be needed to formally assess cost effectiveness.
In conclusion, the results of this study suggest that the overall budget impact to a health plan of expanding the use of erlotinib from the 2nd/3rd-line advanced NSCLC setting to include the maintenance setting was relatively small. This was primarily due to the proportion of patients who would receive erlotinib MTx, the potential cost shifting from pemetrexed to erlotinib for a proportion of MTx eligible patients, the low cost of treating side-effects, and the minimal cost of drug administration. Additional research may be warranted to better estimate the proportion of eligible patients that receive MTx as well the relative clinical and economic impacts of erlotinib MTx versus alternative MTx treatments.
Transparency
Declaration of funding
This study was funded by Genentech, Inc.
Declaration of financial/other relationships
J.C. has been a consultant for Genentech Inc., Pfizer Inc., and Novartis Inc. W.W. was an intern for Genentech Inc at time of manuscript preparation. C.R. is an employee of Genentech Inc. D.V. has been a consultant for Genentech Inc., Pfizer Inc., Novartis Inc., and Medco.
Acknowledgments
The authors would like to acknowledge the assistance of Devi Ramanan in finalizing the analysis.
References
- American Cancer Society. How many people get non-small cell lung cancer? 2010 08/16 http://www.cancer.org/Cancer/LungCancer-Non-SmallCell/DetailedGuide/non-small-cell-lung-cancer-key-statistics
- SEER. Surveillance, epidemiology, and end results (SEER) program, SEER-17 registry, public use dataset 2004-2007. 2010 12/3
- OSI Pharmaceuticals. Tarceva package insert, 2010
- Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 2010;11:6 521-9
- United States Census Bureau. Population estimates. 2010. http://www.census.gov/
- Ramsey SD, Martins RG, Blough DK, et al. Second-line and third-line chemotherapy for lung cancer: use and cost. Am J Manag Care 2008;14:297-306
- NCCN. National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology: non-small cell lung cancer, v.2. 2010
- Tsimboukis S, Merikas I, Karapanagiotou EM, et al. Erlotinib-induced skin rash in patients with non-small-cell lung cancer: pathogenesis, clinical significance, and management. Clin Lung Cancer 2009;10:106-11
- Centers for Medicare and Medicaid Services. Diagnosis related group codes. 2010. http://www.cms.gov/AcuteInpatientPPS/01_overview.asp#TopOfPage
- Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004;22:1589-97
- Centers for Medicare and Medicaid Services. Physician fee schedule (CY 2010). 2010
- Analysource. Analysource online: The online resource for drug pricing and deal information. 2010. www.analysource.com
- Ramsey SD, Clarke L, Kamath TV, et al. Evaluation of erlotinib in advanced non-small cell lung cancer: impact on the budget of a U.S. health insurance plan. J Manag Care Pharm 2006;12:472-8
- Klein R, Wielage R, Muehlenbein C, et al. Cost-effectiveness of pemetrexed as first-line maintenance therapy for advanced nonsquamous non-small cell lung cancer. J Thorac Oncol 2010;5:1263-72
- Okada M, Nishio W, Sakamoto T, et al. Long-term survival and prognostic factors of five-year survivors with complete resection of non-small cell lung carcinoma. J Thorac Cardiovasc Surg 2003;126:558-62