Abstract
Objective:
To compare the cost-utility of exenatide once weekly (EQW) and insulin glargine in patients with type 2 diabetes in the United Kingdom (UK).
Research design and methods:
The IMS CORE Diabetes Model was used to project clinical and economic outcomes for patients with type 2 diabetes treated with EQW or insulin glargine. Treatment effects and patient baseline characteristics (mean age: 58 years, mean glycohaemoglobin: 8.3%) were taken from the DURATION-3 study. Unit costs and health state utility values were derived from published sources. As the price of EQW is not yet known, the prices of two currently available glucagon-like peptide-1 products were used as benchmarks. To reflect diabetes progression, patients started on EQW switched to insulin glargine after 5 years. The analysis was conducted from the perspective of the UK National Health Service over a time horizon of 50 years with costs and outcomes discounted at 3.5%. Sensitivity analyses explored the impact of changes in input data and assumptions and investigated the cost utility of EQW in specific body mass index (BMI) subgroups.
Main outcome measures:
Incremental cost-effectiveness ratio (ICER) for EQW compared with insulin glargine.
Results:
At a price equivalent to liraglutide 1.2 mg, EQW was more effective and more costly than insulin glargine, with a base case ICER of £10,597 per quality-adjusted life-year (QALY) gained. EQW was associated with an increased time to development of any diabetes-related complication of 0.21 years, compared with insulin glargine. Three BMI subgroups investigated (<30, 30–35 and >35 kg/m2) reported ICERs for EQW compared with insulin glargine ranging from £9425 to £12,956 per QALY gained.
Conclusions:
At the prices investigated, the cost per QALY gained for EQW when compared with insulin glargine in type 2 diabetes in the UK setting, was within the range normally considered cost effective by NICE. Cost effectiveness in practice will depend on the final price of EQW and the extent to which benefits observed in short-term randomised trials are replicated in long-term use.
Introduction
The World Health Organization estimated the global prevalence of diabetes at 171 million people (2.8% of the population) in 2000; the number of people with diabetes is predicted to increase to 366 million by 2030Citation1,Citation2. In the UK the prevalence of type 2 diabetes has been estimated at 3.9% in 2005Citation3. The incidence of type 2 diabetes increases with age, obesity and lifestyle factors such as diet and low levels of physical activityCitation4–6.
The International Diabetes Federation estimated that 6% of total healthcare expenditure in the UK in 2010 could be attributable to diabetesCitation7. Much of the medical cost of diabetes relates to the management of diabetes-related complicationsCitation8. Improving glycaemic control has been shown to reduce the risk of diabetes-related complications in the UK Prospective Diabetes Study (UKPDS)Citation9, and to have beneficial effects on costsCitation10. However, many patients with type 2 diabetes do not achieve glycaemic control targets: for example, Guilliford et al. reported that the family practice median percentage of patients achieving a glycohaemoglobin (HbA1c) level of ≤7.5% in 2007–2008 was 75%Citation11. Another study reported that only 38% of patients achieved an HbA1c under 7%, and that only 67% had an HbA1c under 8%, suggesting a need for improved treatment optionsCitation12.
Exenatide is a glucagon-like peptide-1 (GLP-1) receptor agonist belonging to the class of incretin mimetics, currently available as a prefilled pen formulation for injection and approved for twice-daily (BID) administrationCitation13. A long-acting release formulation of exenatide requiring once-weekly injection (EQW) has been developed. Recent studies have shown that EQW improved glycaemic control compared with exenatide BID, with a similar reduction in body weightCitation14,Citation15. Additionally, in other published phase 3 clinical trials of EQW in combination with oral antidiabetic therapies, EQW resulted in superior improvement in glycaemic control compared to sitagliptin, pioglitazone and insulin glargineCitation16,Citation17.
Healthcare decision makers will need economic data on EQW in comparison with other therapies for type 2 diabetes to assess its optimum place in therapy. Currently, NICE recommends exenatide BID as third-line therapy when control of blood glucose remains or becomes inadequate (HbA1c ≥7.5%) and the person has either a body mass index (BMI) ≥35.0 kg/m2 and specific psychological or medical problems associated with high body weight, or a BMI <35.0 kg/m2 and therapy with insulin would have significant occupational implications or weight loss would benefit other significant obesity-related comorbiditiesCitation18. Insulin is an alternative third-line therapy. Long-acting preparations such as insulin glargine are recommended by NICE for use in patients requiring support for insulin injection, with significant hypoglycaemia or who would otherwise require two daily insulin injections and oral antidiabetic drugsCitation18.
The modelling study described here is a cost-utility analysis comparing EQW with insulin glargine in type 2 diabetes in the UK setting. A computer simulation model was used with data from the DURATION-3 clinical trial, which compared EQW with insulin glargine over 26 weeks. Clinical results from the trial have been presented elsewhereCitation16.
Patients and methods
The IMS Centre for Outcomes Research (CORE) Diabetes Model has been previously publishedCitation19. Briefly, it is a Markov-based model developed to assess the long-term clinical and economic outcomes of interventions in diabetes using a series of sub-models to simulate the progression of diabetes-related complications. Five input databases permit definition of patient cohort, clinical, treatment, patient management and economic characteristics. The model can perform either type 1 or type 2 diabetes analyses, and has been validated against results from clinical and epidemiological studiesCitation20.
Cohort characteristics were taken from the DURATION-3 trial subjects ()Citation16,Citation21. As the CORE model is based on a single cohort of patients the baseline characteristics were averaged across the whole trial cohort, rather than by arm as reported in the study manuscriptCitation16. Since the full medical history data were not collected in this study, data on diabetic complication prevalence at baseline were taken from the proposed modelling cohort for type 2 diabetes mellitus described by NICE in clinical guideline 87Citation22.
Table 1. Cohort characteristics, base case. Data from the DURATION-3 trial report unless otherwise statedCitation21.
Treatment effects were taken from the DURATION-3 trial results and are summarised in . EQW and insulin glargine were shown to decrease mean HbA1c levels from baseline by 1.47% and 1.31%, respectively (treatment difference −0.16%, p = 0.017). Mean body weight decreased in the EQW arm (−2.6 kg), but increased (+1.4 kg) in the insulin glargine arm (treatment difference −4.0 kg, p < 0.0001).
Table 2. Treatment effect data, base case, from the DURATION-3 trialCitation16,Citation17.
All patients who commenced the simulation on EQW were assumed to switch to insulin glargine after 5 years of treatment. Patients with type 2 diabetes typically experience worsening glycaemic control as the disease progressesCitation23,Citation24 and need intensification of therapy. This assumption attempts to replicate clinical practice and is consistent with the modelling in NICE clinical guideline 87 on type 2 diabetes which also used a treatment duration of 5 years for a GLP-1 agonistCitation22.
Health-state utilities for diabetes complications were derived from the UKPDS, supplemented with data from other published sources as necessary (). Treatment-associated utilities for excess weight were derived from Bagust and Beale 2005Citation25, and for nausea from Matza et al. 2007Citation26. Nausea disutility was calculated by multiplying the published disutility of 0.04 by the percentage of patients in each treatment group with nausea as an adverse event in the DURATION-3 trial (12.9% for EQW, 1.3% for insulin glargine)Citation16, applied for the first 6 months of treatment as the incidence of nausea appears to decrease over timeCitation27. In the DURATION-1 clinical trial, for example, the proportion of patients experiencing nausea during the first 30 weeks of the trial was 26.4% for EQW and 34.5% for exenatide BIDCitation14. This proportion fell to 7.0% and 7.7% (for patients previously on exenatide BID switching to EQW), respectively, in the following open-ended assessment period (week 30 to week 52)Citation28.
Table 3. Health state utilities and disutilities.
Acquisition costs for insulin glargine, injection needles and the materials needed for self-monitoring of blood glucose were obtained from the Monthly Index of Medical Specialities (MIMS) for August 2009Citation2Citation9. The price of EQW was not yet known, therefore the prices listed in MIMS August 2009 for the two other GLP-1 products currently available in the UK were used as benchmarks. These products were exenatide 10 µg BID and liraglutide at doses of 1.2 mg and 1.8 mg daily. The mid-priced product, liraglutide 1.2 mg (£955.49 per year), was chosen as the price for the base case, with the prices for the lowest-priced product (exenatide BID, £830.82 per year) and the highest-priced product (liraglutide 1.8 mg, £1433.24 per year) used in sensitivity analyses. This assumption was made for the purposes of this modelling study, and does not indicate the potential UK price for EQW. UK-specific unit costs for diabetes management and complications were derived from published sources, inflated where necessary to 2009 values (). All costs are given in British pounds sterling.
Table 4. Unit costs for diabetes management and complications.
The analysis was performed using a non-parametric bootstrapping approach in which the progression of diabetes was simulated in a cohort of 1000 patients, repeated 1000 times to calculate the mean and standard deviation (SD) for costs, life expectancy and quality-adjusted life expectancy. Results from the 1000 iterations were used to generate scatter plot diagrams and cost-acceptability curves comparing the two treatment regimens.
The analysis was conducted from the perspective of the UK National Health Service (NHS) over a time horizon of 50 years (covering the remaining lifetime of the patients in the cohort) with costs and outcomes discounted at 3.5% per yearCitation30.
Sensitivity analyses were conducted to assess the impact of varying assumptions on model outcomes, including: time horizons of 5 and 10 years; discount rates of 0% or 6%; EQW price set equal to that of exenatide BID (yearly drug price of £830.82) or liraglutide 1.8 mg daily (yearly drug price of £1433.24) (see above for rationale); costs of complications and management varied by ±20% from base case values; and a switch to insulin glargine after 10 years instead of 5 years.
The effect of treatment-related utilities was assessed in sensitivity analyses. Variation 1 included injection site reaction disutility from Boye et al. 2010Citation31 (−0.011), applied for patient lifetimes for affected patients. The percentage of patients affected was assumed to be the percentage of patients in each treatment group with injection site reaction as an adverse event in the DURATION-3 trial (12.9% for EQW, 1.8% for insulin glargine)Citation16. Variation 2 included injection site reaction disutility as in Variation 1, together with dose flexibility utility from Boye et al. 2010Citation31 (0.006) applied to both treatments, and dose frequency utility for once-weekly administration from Boye et al. 2010Citation31 (0.023) applied for as long as patients remained on EQW treatment. Variation 3 considered a utility change related to body weight derived from Matza et al. 2007Citation26, rather than from Bagust and Beale 2005Citation25 as in the base case. Variation 4 considered only health-state utilities related to complications (no treatment-related utilities or disutilities applied).
The impact of treatment effect was assessed in sensitivity analyses in which the treatment benefits of EQW over insulin glargine on individual parameters were set to zero, to determine which aspects of the profile contributed most to economic benefits. Treatment effects (changes from baseline) for HbA1c, systolic blood pressure, weight, lipids and hypoglycaemia rates were successively removed in the EQW arm. Further subgroup analyses investigated the cost utility results in three different BMI sub-groups, BMI <30, 30–35 and >35 kg/m2.
Finally, to assess the effect of uncertainty around the treatment effect values derived from DURATION-3, a probabilistic sensitivity analysis was performed where probability distributions were applied to key treatment effect parameters and samples were drawn with replacement from these distributions, for each bootstrap iteration, to generate an empirical distribution of the cost-effectiveness ratioCitation32.
Results
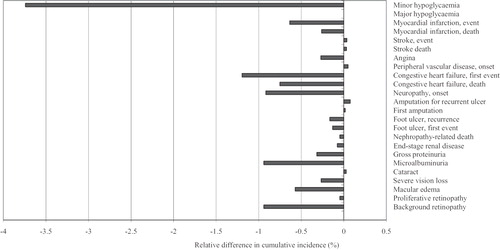
In the base case analysis, life expectancy and quality-adjusted life expectancy were higher with EQW treatment (11.925 years and 8.032 quality-adjusted life-year (QALY)) versus insulin glargine treatment (11.808 years and 7.849 QALYs) (). Treatment with EQW was associated with an increased time to any complication of 2.5 months relative to insulin glargine, and for most diabetes-related complications the onset was delayed by 2–3 months. EQW reduced the cumulative incidence of most diabetes-related complications relative to insulin glargine ().
Table 5. Life expectancy, cost and cost-effectiveness results, base case.
Figure 1. Relative change in cumulative incidence of diabetes-related complications with EQW compared with insulin glargine, base case.
At a yearly cost equal to liraglutide 1.2 mg daily, treatment with EQW was associated with higher expected drug acquisition costs than insulin glargine, partially offset by reductions in the expected costs associated with complications such as cardiovascular and renal disease (). Management costs were higher due to increased life expectancy. Lifetime direct costs were higher with EQW, and the incremental cost-effectiveness ratio (ICER) for EQW compared with insulin glargine was £10,597 per QALY gained ().
Table 6. Lifetime costs by component, base case.
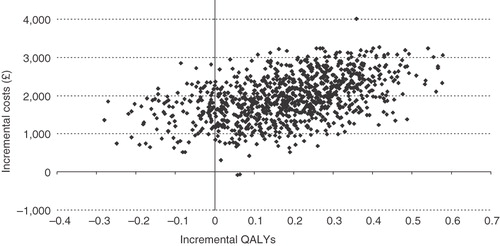
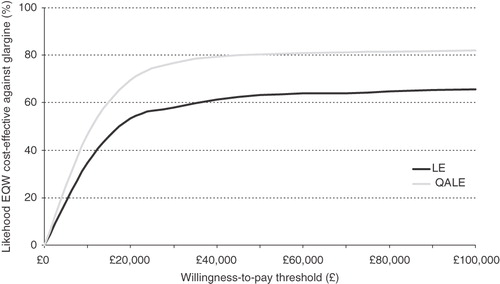
A scatter plot of the ICER results from 1000 iterations of the model is shown in . Most of the results (88.4%) fell within the upper right quadrant of the plot, indicating higher costs and greater quality-adjusted life expectancy for EQW compared with insulin glargine. The same data are represented in the cost-effectiveness acceptability curve (), indicating the likelihood of cost effectiveness over a range of willingness-to-pay (WTP) thresholds. EQW was cost effective compared with insulin glargine in 79.9% of iterations at a WTP threshold of £30,000 per QALY gained. At a WTP threshold of £20,000 per QALY gained, EQW was cost effective in 74.6% of iterations.
The results of the sensitivity analyses are summarised in . The ICER was robust to changes in the cost of complications. Shortening the time horizon to 5 or 10 years worsened the ICER. In the probabilistic sensitivity analysis, the ICER decreased slightly to £10,389 per QALY gained. Including the utility benefits of dose flexibility and reduced dosing frequency (utility variation 2) decreased the ICER to £6971 per QALY gained.
Table 7. Summary of sensitivity analyses for EQW compared with insulin glargine.
EQW showed better cost effectiveness over insulin glargine in patients in higher BMI categories, compared to non-obese patients. In patients with BMI >35 mg/m2, the ICER was £9425 per QALY gained and in patients with BMI 30–35 kg/m2 the ICER was £9430 per QALY gained, compared with £12,956 per QALY gained in non-obese patients (BMI <30 kg/m2).
As the price of EQW is not yet known, sensitivity analysis explored the effect of a range of prices. Setting the price of EQW as equal to that of exenatide BID reduced the ICER to £7622 per QALY gained, while setting it as equal to that of liraglutide 1.8 mg increased the ICER to £21,996 per QALY gained.
Removing the beneficial relative treatment effects of EQW by setting each of them as equal to the effect of insulin glargine worsened the ICER compared with the base case, as would be expected, with the largest effect seen for weight change followed by HbA1c. Removing the effect of this weight treatment difference altogether produced an ICER of £18,364 per QALY.
Discussion
The DURATION-3 study reported improvement in risk factors for EQW over insulin glargine, including greater reductions in HbA1c, decreased body weight and a lower incidence of hypoglycaemiaCitation16. The present study applied the clinical results from the DURATION-3 trial to an established economic model to investigate the likely impact on complications and outcomes and hence the cost effectiveness of EQW compared with insulin glargine. The overall reduction in complications in the EQW arm led to a reduction in associated costs, which partially offset the higher drug acquisition cost of EQW assumed in the base case. Consequently, this modelling study projected that EQW would be cost effective compared with insulin glargine in the UK setting over the tested range of prices. Therefore, EQW would likely be a cost-effective alternative third-line treatment of type 2 diabetes, given the current third-line positioning of insulin glargine by NICE. The base case ICER was £10,597 per QALY gained, well below the cost-effectiveness threshold range of £20,000–30,000 per QALY generally considered cost effective by NICECitation33.
These results are in accordance with a previous cost-effectiveness analysis of exenatide BID versus insulin glargine in the UK setting, which projected a value of £22,420 per QALY gainedCitation34. In that study, the key treatment effects modelled were a reduction of HbA1c of −0.99% versus −1.07%, a BMI change of −0.80 kg/m2 versus +0.50 kg/m2 and a systolic blood pressure reduction of −4.15 mmHg versus −0.57 mmHg for exenatide BID and insulin glargine, respectively. The lower ICER for EQW versus insulin glargine in the present study can therefore be explained by the increased clinical benefits, particularly with regards effect on HbA1c, observed for EQW over insulin glargine in the DURATION-3 studyCitation16, compared with those observed for exenatide BID over insulin glargineCitation35.
In the present modelling study, the improved quality-adjusted life expectancy projected for EQW compared with insulin glargine was driven mainly by delays and reductions in diabetes-related complications, which in turn were driven by the greater improvements in body weight, HbA1c, blood pressure, and serum lipids in the EQW group compared with the insulin glargine group. EQW was associated with an increased time to development of any diabetes-related complication of 2.5 months compared with insulin glargine. The cumulative incidences of most complications were reduced with EQW compared with insulin glargine. The exceptions were cataract, amputations, peripheral vascular disease, stroke and stroke death (), which showed a small increase in cumulative incidence in the EQW group due to the survival paradox. Patients treated with EQW have a longer modelled life expectancy than patients treated with insulin glargine (about 2.5 months when undiscounted), and thus have a longer period of time during which they are at risk for developing these late complications of type 2 diabetes.
As type 2 diabetes is a progressive disease, glycaemic control typically worsens over timeCitation23,Citation24. Treatment guidelines in the UKCitation18 and USCitation36 address this by recommending stepwise intensification of therapy from monotherapy with oral antidiabetic drugs through dual and triple therapy and eventually to insulin therapy. It would thus be unreasonable to assume that patients in the EQW cohort would remain on the same treatment for the lifetime horizon of the model, therefore the base case analysis reflects clinical practice by assuming that patients would switch to insulin glargine after 5 years of EQW treatment. This is consistent with the approach taken by NICE in the clinical guideline 87Citation1Citation8, and is the approach used in the modelling undertaken for the recently completed NICE evaluation of liraglutideCitation37, a human GLP-1 analogue also approved for the treatment of type 2 diabetesCitation38. We tested the effect of this assumption in sensitivity analysis by setting the duration of treatment to 10 years instead of 5. The ICER was higher with a 10-year treatment duration, at £16,798 per QALY gained.
Attributes of injectable treatments such as injection site reactions, dose frequency and dose flexibility (e.g., ability to take the dose at various times of day) have been shown to influence utility scores, with dose frequency having the largest effectCitation31. When these attributes were taken into account in the current study, together with nausea and weight change (utility variation 2 in the sensitivity analysis), the ICER estimate for EQW over insulin glargine improved from £10,597 (base case) to £6971 per QALY gained. The reduced dosing frequency with EQW (once-weekly injection, compared with once daily for insulin glargine) offset the higher rate of nausea and injection site reactions. This finding suggests that patient convenience attributes such as reduced dosing frequency can further improve the cost effectiveness of the intervention. In other indications, once-weekly treatment regimens have been associated with better adherence than once-daily regimens; this may be a further benefit for EQWCitation39.
Shortening the time horizon markedly worsened the ICER. This illustrates the importance of conducting economic evaluations in chronic conditions such as type 2 diabetes over a lifetime horizon, as shorter time horizons do not fully capture the effect of long-term complications developing at the late stages of disease.
Exploratory sensitivity analyses were performed on BMI subgroups, although the DURATION-3 patient population was not stratified according to BMI, nor was the trial powered to investigate the differences in treatment effects among subgroups. In all three BMI subgroups investigated, a favourable ICER for EQW compared with insulin glargine was found. Value for money was improved in patients with BMI above 30 kg/m2 at baseline (ICER of £9430 and £9425 per QALY gained for the BMI 30–35 kg/m2 and >35 kg/m2 subgroups) compared with patients who had BMI <30 kg/m2 at baseline (ICER = £12,956). The differences in ICER among the three BMI subgroups in the present study may be due to differences in age (mean age 56.13 years in the BMI >35 kg/m2 group, 57.77 years in the BMI 30–35 kg/m2 group and 59.26 years in the BMI <30 kg/m2 group) and shorter duration of diabetes in the non-obese subgroup. The between-treatment difference in HbA1c in the BMI subgroups was: −0.25% in the >35 kg/m2 group, +0.01% in the 30–35 kg/m2 group, and –0.23% in the <30 kg/m2 group.
A limitation of the present study is that it uses data on relatively short-term changes (26 weeks) in surrogate endpoints such as HbA1c, blood pressure, weight, and blood lipids to project future effects on hard clinical outcomes such as death and the development of complications. However, this limitation is inherent when evaluating new treatments for diabetes, since long-term clinical outcome data require many years to collect. For healthcare providers who need to make timely decisions on access to novel therapies, mathematical modelling offers the best available option for estimating potential future effects. The IMS CORE Diabetes Model has been validated against clinical and epidemiological dataCitation20.
Another limitation of the study is that EQW is not currently licensed in Europe and therefore the price of EQW in this analysis was assumed to be equal to the acquisition cost of liraglutide 1.2 mg. Nevertheless, the price sensitivity analyses conducted were robust and only moderately changed the cost-utility results.
Future research could aim to compare effectiveness of daily diabetic treatment versus once-weekly treatment to assess whether convenient dosing improves clinical and economic outcomes. Additional data exploring the longer-term effectiveness of GLP-1 agonists would reduce the need for modelled extrapolation of results. However, if confirmed, this modelled analysis suggests that EQW should be considered as an option for patients with type 2 diabetes who would otherwise require insulin, as it offers improved glycaemic and body mass control at acceptable cost effectiveness.
Conclusions
The DURATION-3 study showed that EQW treatment resulted in a greater reduction in mean HbA1c and fewer hypoglycaemic events compared with insulin glargine. Patients receiving EQW also experienced a significant mean weight loss, compared with weight gain in the insulin glargine group. The cost-utility analysis reported here found that, hypothesising price parity with liraglutide 1.2 mg, the clinical benefits resulted in an ICER for EQW compared with insulin glargine of £10,597 per QALY gained, well within the range normally considered cost effective by NICE. EQW had favourable cost effectiveness in all three BMI subgroups analysed in this study, with better, and almost equivalent, cost effectiveness in the two higher BMI sub-groups (30–35 kg/m2 and >35 kg/m2) compared with the lowest BMI subgroup (<30 kg/m2).
Transparency
Declaration of funding
This study was funded by Eli Lilly & Co.
Declaration of financial/other relationships:
L.T., B.W., D.B. and K.S.B. are employees of Eli Lilly & Co. Ltd. A.B., J.L.P. and A.L. are employees of IMS.
Acknowledgements
Medical writing assistance was provided by Carole Nadin.
References
- Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047-53
- World Health Organization. Diabetes - country and regional data. 2010. Available at: http://www.who.int/diabetes/facts/world_figures/en/
- Gonzalez EL, Johansson S, Wallander MA, et al. Trends in the prevalence and incidence of diabetes in the UK: 1996–2005. J Epidemiol Community Health 2009;63:332-6
- Brunner EJ, Mosdol A, Witte DR, et al. Dietary patterns and 15-year risks of major coronary events, diabetes, and mortality. Am J Clin Nutr 2008;87:1414-21
- Lyssenko V, Jonsson A, Almgren P, et al. Clinical risk factors, DNA variants, and the development of type 2 diabetes. N Engl J Med 2008;359:2220-32
- Perry IJ, Wannamethee SG, Walker MK, et al. Prospective study of risk factors for development of non-insulin dependent diabetes in middle aged British men. BMJ 1995;310:560-4
- IDF Diabetes Atlas. Economic impact of diabetes. 2010 [Accessed 2010 Mar 23]. Available at: http://www.diabetesatlas.org/content/economic-impacts-diabetes
- American Diabetes Association. Economic costs of diabetes in the U.S. in 2007. Diabetes Care 2008;31:596-615
- UKPDS. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:837-53
- Wagner EH, Sandhu N, Newton KM, et al. Effect of improved glycemic control on health care costs and utilization. JAMA 2001;285:182-9
- Gulliford MC, Dodhia H, Sivaprasad S, et al. Family practices' achievement of diabetes quality of care targets and risk of screen-detected diabetic retinopathy. PLoS One 2010;5:e10424
- Leese GP, Boyle P, Feng Z, et al. Screening uptake in a well-established diabetic retinopathy screening program: the role of geographical access and deprivation. Diabetes Care 2008;31:2131-5
- Exenatide (Byetta) Summary of Product Characteristics. 2010 May 4 [Accessed 2010 July 20]. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000698/WC500051845.pdf
- Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008;372:1240-50
- Blevins T, Pullman J, Malloy J, et al. DURATION-5: exenatide once weekly resulted in significantly greater improvement in glycemic control with less nausea than exenatide twice daily in patients with type 2 diabetes. American Diabetes Association Annual Meeting 2010 Abstract 0008-LB. 2010
- Diamant M, Van GL, Stranks S, et al. Once weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION-3): an open-label randomised trial. Lancet 2010;375:2234-43
- Bergenstal RM, Wysham C, Macconell L, et al. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet 2010;376:431-9
- National Institute for Clinical Excellence. type 2 diabetes: newer agents. May 2009
- Palmer AJ, Roze S, Valentine WJ, et al. The CORE diabetes model: projecting long-term clinical outcomes, costs and cost-effectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin 2004;20(Suppl 1):S5-26
- Palmer AJ, Roze S, Valentine WJ, et al. Validation of the CORE diabetes model against epidemiological and clinical studies. Curr Med Res Opin 2004;20(Suppl 1):S27-40
- Eli Lilly and company. GWBR Clinical Study Report, 2009
- National Institute for Clinical Excellence. NICE short clinical guideline 87 – type 2 diabetes: newer agents for blood glucose control in type 2 diabetes. May 2009. Available at http://www.nice.org.uk/cg87
- UK Prospective Diabetes Study 16. Overview of 6 years' therapy of type II diabetes: a progressive disease. UK Prospective Diabetes Study Group. Diabetes 1995;44:1249-58
- Turner R, Cull C, Holman R. United Kingdom Prospective Diabetes Study 17: a 9-year update of a randomized, controlled trial on the effect of improved metabolic control on complications in non-insulin-dependent diabetes mellitus. Ann Intern Med 1996;124(1 Pt 2):136-45
- Bagust A, Beale S. Modelling EuroQol health-related utility values for diabetic complications from CODE-2 data. Health Econ 2005;14:217-30
- Matza LS, Boye KS, Yurgin N, et al. Utilities and disutilities for type 2 diabetes treatment-related attributes. Qual Life Res 2007;16:1251-65
- DiPiro JT, Talbert RL, Yee GC, et al., eds. Pharmacotherapy. New York: McGraw-Hill, 2008
- Buse JB, Drucker DJ, Taylor KL, et al. DURATION-1: exenatide once weekly produces sustained glycemic control and weight loss over 52 weeks. Diabetes Care 2010;33:1255-61
- Haymarket Medical Media. Monthly Index of Medical Specialties, August 2009. Available at: www.mims.co.uk
- National Institute for Health and Clinical Excellence. Guide to the methods of technology appraisal. National Institute for Health and Clinical Excellence 2008. Available at: http://www.nice.org.uk/media/B52/A7/TAMethodsGuideUpdatedJune2008.pdf
- Boye KS, Matza LS, Walter KN, et al. Utilities and disutilities for attributes of injectable treatments for type 2 diabetes. Eur J Health Econ 2010; Mar 12 [Epub ahead of print]
- Drummond M, Sculpher M, Torrance G, et al. Methods for the Economic Evaluation of Health Care Programmes, 3rd edn. Oxford: Oxford University Press, 2005
- Appleby J, Devlin N, Parkin D. NICE’s cost effectiveness threshold. BMJ 2007;335:358-9
- Ray JA, Boye KS, Yurgin N, et al. Exenatide versus insulin glargine in patients with type 2 diabetes in the UK: a model of long-term clinical and cost outcomes. Curr Med Res Opin 2007;23:609-22
- Heine RJ, Van Gaal LF, Johns D, et al. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2005;143:559-69
- Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract 2009;15:540-59
- National Institute for Clinical Excellence. Liraglutide for the treatment of type 2 diabetes mellitus. Appraisal consultation document. 2010 June 14 [Accessed 2010 July 21]. Available at: http://www.nice.org.uk/guidance/index.jsp?action=article&o=49173
- Liraglutide (Victoza) Summary of Product Characteristics. 2010 February 22 [Accessed 2010 Jul 21]. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001026/WC500050017.pdf
- Kruk ME, Schwalbe N. The relation between intermittent dosing and adherence: preliminary insights. Clin Ther 2006;28:1989-95
- Clarke P, Gray A, Holman R. Estimating utility values for health states of type 2 diabetic patients using the EQ-5D (UKPDS 62). Med Decis Making 2002;22:340-9
- Tengs TO, Wallace A. One thousand health-related quality-of-life estimates. Med Care 2000;38:583-637
- Wasserfallen JB, Halabi G, Saudan P, et al. Quality of life on chronic dialysis: comparison between haemodialysis and peritoneal dialysis. Nephrol Dial Transplant 2004;19:1594-9
- Sharma S, Oliver-Fernandez A, Bakal J, et al. Utilities associated with diabetic retinopathy: results from a Canadian sample. Br J Ophthalmol 2003;87:259-61
- Lloyd A, Nafees B, Gavriel S, et al. Health utility values associated with diabetic retinopathy. Diabet Med 2008;25:618-24
- Hopkins RB, Tarride JE, Bowen J, et al. Cost-effectiveness of reducing wait times for cataract surgery in Ontario. Can J Ophthalmol 2008;43:213-17
- Redekop WK, Stolk EA, Kok E, et al. Diabetic foot ulcers and amputations: estimates of health utility for use in cost-effectiveness analyses of new treatments. Diabetes Metab 2004;30:549-56
- Davies R, Wittrup-Jensen KU, Peters JR, et al. The impact on health-related quality of life (EQ-5Dindex) in people with type 1 diabetes who experience severe hypoglycemia. Diabetologia 2005;48(Suppl 1):A292
- National Institute for Clinical Excellence. Technology appraisal guidance -- No. 53. Guidance on the use of long-acting insulin analogues for the treatment of diabetes -- insulin glargine. December, 2002
- National Health Service. Prescription Cost Analysis 2008. 2009 April 21. Available at: http://www.ic.nhs.uk/statistics-and-data-collections/primary-care/prescriptions/prescription-cost-analysis-2008
- Melville A, Richardson R, McIntosh A, et al. Complications of diabetes: screening for retinopathy and management of foot ulcers. Qual Health Care 2000;9:137-41
- Personal Social Services Research Unit UK. Unit Costs of Health and Social Care 2008. Canterbury, UK: University of Kent
- National Health Service. NHS reference costs 2007-08. 2009 August 5. Available at: http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_098945
- Clarke P, Gray A, Legood R, et al. The impact of diabetes-related complications on healthcare costs: results from the United Kingdom Prospective Diabetes Study (UKPDS Study No. 65). Diabet Med 2003;20:442-50
- The National Collaborating Centre for Chronic Conditions. Chronic kidney disease: national clinical guideline for early identification and management in adults in primary and secondary care. London: Royal College of Physicians, 2008
- Ghatnekar O, Willis M, Persson U. Cost-effectiveness of treating deep diabetic foot ulcers with Promogran in four European countries. J Wound Care 2002;11:70-4