Abstract
Objective:
This study evaluated the rate of uncontrolled chemotherapy-induced nausea and vomiting (CINV) after initiating antiemetic prophylaxis with palonosetron versus other 5-HT3 receptor antagonists (RAs) in patients diagnosed with hematologic malignancies (lymphoma and leukemia) and receiving highly emetogenic chemotherapy (HEC) or moderately emetogenic chemotherapy (MEC) in a hospital outpatient setting.
Methods:
Patients aged ≥ 18 years and diagnosed with hematologic malignancies initiating HEC or MEC and antiemetic prophylaxis with palonosetron (Group 1) and other 5-HT3 RAs (Group 2) for the first time in a hospital outpatient setting between 4/1/2007 and 3/31/2009 were identified from the Premier Perspective Database. Within each cycle, CINV events were identified (in the hospital outpatient, inpatient, and emergency room settings) through ICD-9 codes for nausea, vomiting, and/or volume depletion (from each CT administration day 1 until the end of the CT cycle), or use of rescue medications (day 2 until the end of the CT cycle). Negative binomial distribution generalized linear multivariate regression model estimating the CINV event rate on CT, specific CT cycles, and cancer diagnosis (leukemia/lymphoma)-matched groups in the follow-up period (first of 8 cycles or 6 months) was developed.
Results:
Of 971 identified patients, 211 initiated palonosetron (Group 1). Group 1 patients comprised of more females [50.2 vs. 41.4%; p = 0.0226], Whites [74.4 vs. 70.4%, and Hispanics [7.6 vs. 6.3%; all races p = 0.0105], received more HEC treatments [89.6 vs. 84.2%; all CT types p = 0.0129], and had more lymphoma diagnosed patients [89.6 vs. 76.3%; all cancer types p = 0.0033] at baseline. After controlling for differences in several demographic and clinical variables, the regression model predicted a 20.4% decrease in CINV event rate per CT cycle for Group 1 versus Group 2 patients. Study limitations include potential lack of generalizability, absence of data on certain confounders including alcohol consumption and prior history of motion sickness, potential underestimation of incidence of uncontrolled CINV, and inability to draw conclusions pertaining to cause and effect relationship.
Conclusion:
In this retrospective hospital study, patients with hematologic malignancies treated with HEC or MEC and initiated on antiemetic prophylaxis with palonosetron in the hospital outpatient setting were more likely to experience significantly lower CINV event rates (in the hospital outpatient, inpatient, and emergency room settings) versus patients initiated on other 5-HT3 RAs.
Introduction
Chemotherapy-induced nausea and vomiting (CINV) is among the most common and feared adverse events for patients undergoing chemotherapy (CT)Citation1–3. Chemotherapy treatment-related factors associated with more frequent and higher rates of CINV include the emetogenicity of the chemotherapeutic agents, repeated CT cycles, and patient risk factors (i.e., age, gender, history of prior CT)Citation4,Citation5. Most CT regimens used to treat hematologic malignancies are highly emetogenic chemotherapy (HEC) and moderately emetogenic chemotherapy (MEC), with emetogenicity degrees of 90% for HEC and 30–90% for MEC regimensCitation5,Citation6. The use of older 5-hydroxytryptamine3 receptor antagonists and other antiemetic agents have improved acute emetic control (≤24 hours after CT); however, delayed emesis (>24 hours up to 5 days) remains a significant problem for patients receiving HEC and MECCitation7–9.
Studies have shown that palonosetron, a newer 5-HT3 receptor antagonist, compared with other 5-HT3 RAs has been effective in treating both acute and delayed emesis in patients with hematologic malignancies who received HEC and MECCitation10–14. Results also reported that complete response rates of CINV were higher in this patient population after receiving palonosetronCitation10–14. This difference might be potentially attributed to stronger receptor-binding characteristics and a prolonged plasma half-life (∼40 h) for palonosetron versus the other agentsCitation15,Citation16.
CINV remains a serious complication for patients with hematologic malignancies treated on emetogenic CTCitation1,Citation2,Citation17. Not much real-world data exists; hence, the following real-world analysis assessed the rate of uncontrolled CINV associated with initiation of antiemetic prophylaxis with palonosetron versus other 5-HT3 RAs in patients with hematologic malignancies on HEC/MEC in a hospital outpatient setting.
Patients and methods
Data source
In this retrospective, longitudinal, observational study, data were derived from the Premier Perspective Database, a hospital-service database that includes detailed patient-level data, associated with inpatient stays and visits to outpatient facilities for participating database hospitals. The Premier Perspective Database contains data from over 600 hospitals across the US that details information on patient demographics (age, sex, race, marital status), hospital characteristics, principal and secondary diagnoses, payer type, medication utilization (name, strength, quantity dispensed, day of administration), length of stay, and physician specialty. A unique patient identification number allows linkage of visits within the same hospital outpatient facility for a given patient. Drugs are not linked to their specific indication for use within the database. The data are de-identified in accordance with the Health Insurance Portability and Accountability Act (HIPPA). This retrospective analysis did not involve patient intervention and used data with masked data identifiers.
Study patients and follow-up time
The overall study cohort consisted of patients aged 18 years or older with hematologic malignancies initiating single or multi day CT [HEC/MEC] and antiemetic prophylaxis with palonosetron (Group 1) and other 5-HT3 RAs [ondansetron, granisetron, or dolasetron] (Group 2) for the first time (index date) between April 1, 2007 and March 31, 2009 at an outpatient hospital facility (). Study patients were chemotherapy-naïve as those patients receiving any chemotherapy in the 6 months prior to index date were excluded from the study. Cancer diagnosis was defined using appropriate International Classification of Diseases, 9th Revision (ICD-9) codes and was recorded on an outpatient hospital claim 60 days before or after the index date and was classified as a primary cancer site. Primary hematologic malignancies were defined as lymphoma (Hodgkin lymphoma, non-Hodgkin lymphoma), myeloma, and leukemia (acute lymphocytic leukemia, chronic lymphocytic leukemia, acute myeloid leukemia, chronic myeloid leukemia, and other leukemia), classified as ICD-9-Clinical Modification (CM) codes 200 to 208. The primary cancer site was identified using a hierarchy of 18 anatomical cancer groupings based on the likelihood of each cancer grouping being a primary versus secondary tumorCitation18. Patients with more than one cancer site grouping were classified into a single primary cancer site using this hierarchy. Additional inclusion criteria were patients aged 18 years or older with no evidence of nausea and vomiting [identified through appropriate ICD-9-CM codes] or a hospital charge for a CT agent or antiemetic medication in the 6-month pre-index period, and 36 consecutive months of hospital submission data (). Patients were followed through 8 CT cycles or 6 months post-index date, whichever occurred first. Within each CT cycle, the follow-up time was from day 1 (except use of rescue medications that was identified from day 2) to end of cycle. Chemotherapy was categorized as HEC or MEC per National Comprehensive Cancer Network (NCCN) guidelinesCitation6. In case of multiple single-day CT agents, the CT with the highest emetogenic potential per NCCN guidelines was used to classify the emetogenic risk for each CT cycle. The unit of analysis was a patient CT cycle. A cycle length was defined as 7 days or less (i.e., if there was a gap of more than 7 days between any two CT administrations, the second CT administration was considered day 1 of cycle 2).
Figure 1. Overall study population patient flow chart. CT, chemotherapy; CINV, chemotherapy-induced nausea and vomiting; N, total number of patients; HEC, highly emetogenic chemotherapy; MEC, moderately emetogenic chemotherapy; 5-HT3 RAs, 5-hydroxytryptamine3 receptor antagonists.
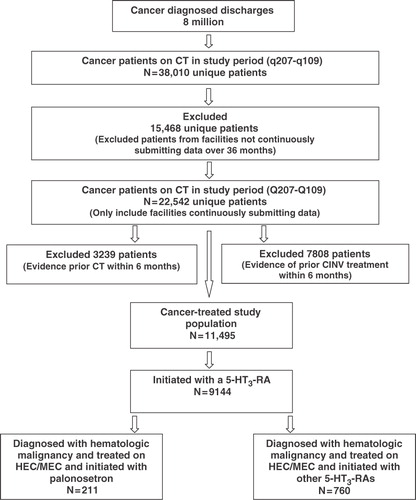
Since the Premier Perspective Database did not contain information on body surface area (BSA) or patient height and weight required to calculate BSA, values of 1.6 m2 for adult women and 1.9 m2 for adult men were assumed for the calculation of appropriate dosing of CT agents to determine the emetogenicity level. The data represented medications (doses) billed which was used as a surrogate for medications (doses) administered, whether scheduled or as needed. An antiemetic medication was considered to be for prophylaxis use rather than for treatment if the medication was given on the day of CT. If a patient received more than one emetogenic risk category CT agent (i.e., a HEC and a MEC), then the patient was considered as prophylaxed if he or she received an antiemetic prophylaxis medication for all the highest emetogenic risk CT administration days within each CT cycle.
Identification of CINV events
The primary study outcome for the overall study population was rate of uncontrolled CINV events in the study follow-up period at a CT (HEC/MEC) cycle level in patients with hematologic malignancies treated with antiemetic prophylaxis formerly defined as Group 1 and Group 2. CINV events in all analyses were defined as one of the following: any hospital visit [inpatient, hospital outpatient, or emergency room (ER)] associated with a primary or secondary ICD-9 code of 787.0 (nausea and vomiting), 787.01 (nausea with vomiting), 787.02 (nausea alone), 787.03 (vomiting alone), 276.5 (volume depletion), 276.50 (volume depletion, unspecified), 276.51 (dehydration), and/or 276.52 (hypovolemia) from each CT administration day 1 (index date) until the end of the CT cycle, or use of a rescue medication a day after each CT administration (day 2 until the end of the CT cycle). The CINV events per CT cycle were summed to get the total number of CINV events across all cycles in the follow-up period for the two study groups. The list of rescue medications identified through appropriate J-codes included 5-HT3 RAs, NK1 RAs, antihistamines, benzodiazepine, dexamethasone, butyrophenones, cannabinoids, phenothiazines, and other steroids (non-dexamethasone).
Statistical analysis
Descriptive statistics were calculated for continuous and categorical variables at baseline for the overall study population. For continuous variables, means, standard deviations (SDs), and medians were generated. For categorical variables, percentages were reported. Patient demographic and clinical characteristics between groups were analyzed using t-test for means and chi-square test for frequencies. An a priori level of significance of 0.05 was set for all of the analyses. Study patient demographics including age, gender, geographic region, payer type, and race were tabulated for each group. Comorbidity burden was assessed using the Charlson Comorbidity Index (CCI) score, based on a review of medical claims occurring during the 6 months prior to index dateCitation19. The CCI score considers patient age and assigns a weight ranging from 0 to 6 corresponding to each comorbid condition, identified by ICD-9-CM codes found in medical claims. Weights are summed for a score between 0 and 29, with higher scores indicating greater comorbidity burden in the patient. For this analysis, all cancer codes and weights were removed from the CCI score to eliminate double counting.
Propensity score matching was used to neutralize the distribution of CT emetogenicity between patients in Groups 1 and 2. In addition to CT emetogenicity, propensity matching was also performed on specific CT cycle, cancer diagnosis (leukemia/lymphoma), and with at least one CINV event per cycle in the follow-up period. Only leukemia and lymphoma patients were included for propensity matching since the number of patients with myeloma was identified to be too low for matching. A generalized, linear multivariate regression model using a negative binomial distribution was developed on the study population after adjusting for several available demographic and clinical variables to estimate the adjusted uncontrolled CINV event rate per CT cycle between the two groups matched on CT emetogenicity, cycle, cancer diagnosis, and with at least one CINV event per cycle in the follow-up period. The main dependent variable was any CINV event in the follow-up period. The independent variables in the model included study group, age, CCI score, gender, ethnicity, primary payer, cycle length (in days), and number of CT days within a cycle.
Having tested for under- or overdispersion of the dependent variable (i.e., conditional variance is greater than the mean) and finding that the dependent variable does not have an excessive number of zeroes, negative binomial regression was used as an appropriate model for the study. The regression models the log of the expected count as a linear function of the predictor/independent variables. The interpretation is as follows: for a one unit change in the predictor variable, the difference in the logs of expected counts of the dependent variable is expected to change by the respective regression coefficient, given the other independent variables in the model are held constant. Multicollinearity diagnostics were performed via assessment of variance inflation factors for all model covariates. No significant collinearity existed between final reported model covariates. All analyses were conducted using SAS software, Version 9.1 (SAS Institute, Cary, NC, USA).
Results
Demographic and clinical characteristics: overall study population
summarizes attrition as inclusion and exclusion criteria were applied. Out of 38,010 cancer diagnosed patients on CT within the study time period, we excluded 15,468 patients who did not continuously submit data over 36 months, 3239 patients who had evidence of CT use in the 6 months prior to index date, 7808 patients with evidence for CINV treatments in the 6 months prior to index date, and 2351 patients who did not initiate with a 5-HT3 RA. Of the remaining 9144 patients, a total of 971 patients with hematologic malignancies receiving HEC/MEC were analyzed (). The baseline demographic and clinical characteristics of this sub analysis are summarized in . The mean age of all patients was 61.9 (SD: 16.2), and 50.7% were female and 71.2% were White, with 49.1% of all patients aged 65 years or above. Emetogenicity distribution was 85.4% HEC and 14.6% MEC for CT cycle 1; p = 0.0129. Lymphoma (79.2%), leukemia (14.4%), and myeloma (6.4%) represented the primary cancer sites for these patients; p = 0.0033. Most patients had a payer status of traditional Medicare (43.8%) followed by non-capitated managed-care coverage (22.8%). The average CCI score was 0.2 (SD: 0.5) ().
Table 1. Baseline demographic and clinical characteristics of patient with hematologic malignancy receiving HEC/MEC regimens.
Of the 971 patients, 211 patients (21.7%) were administered palonosetron (Group 1) and 760 patients (78.3%) received other 5-HT3 RAs (Group 2) for the entire study follow-up period (). The mean age for Group 1 and Group 2 was statistically comparable [60.1 (SD: 18.4) and 62.4 (SD: 15.4) years, respectively; p = 0.0722]. Group 1 had a higher proportion of females (50.2 vs. 41.4%; p = 0.0226), Whites (74.4 vs. 70.4%), and Hispanics (7.6 vs. 6.3%), while more patients in Group 2 were African-Americans (11.6 vs. 10.4); all races p = 0.0105. Group 1 patients had a higher percentage of HEC administrations (89.6 vs. 84.2%); all CT p = 0.0129 and comprised of more patients with lymphoma (89.6 vs. 76.3%); all cancer diagnoses p = 0.0033. The two groups had similar CCI scores at baseline ().
Unadjusted CINV event rate
For the overall study population, prior to propensity matched regression analysis, Group 1 patients had 748 CINV events over 882 CT cycles (4.2 cycles per patient) in the study follow-up period, while Group 2 patients had 3036 events over 2849 cycles (3.7 cycles per patient) during the same follow-up period. The unadjusted average CINV event rate per CT cycle was statistically similar between Group 1 and Group 2 patients (0.85 vs. 1.07, respectively; p = 0.1012) (). Among Group 1 patients, out of 748 CINV events, 65 events (8.7%) were classified through ICD-9-CM diagnosis codes for nausea, vomiting, nausea with vomiting, or volume depletion, while 732 events (97.9%) were identified through use of CINV-related rescue medications. Forty-nine events (6.6%) were classified through both diagnosis codes and use of rescue medications. Among Group 2 patients, out of 3036 CINV events, 250 events (8.2%) were classified through ICD-9-CM diagnosis codes for nausea, vomiting, nausea with vomiting, or volume depletion, while 3028 events (99.7%) were identified through use of CINV-related rescue medications. A total of 242 events (8.0%) were classified through both diagnosis codes and use of rescue medications.
Table 2. Unadjusted CINV event rate.
Among HEC cycles, prior to propensity matched regression analysis, Group 1 patients had 665 CINV events over 824 cycles in the follow-up period, while Group 2 patients had 2414 events over 2526 cycles during the same follow-up period. The unadjusted average CINV event rate per CT cycle was statistically similar between Group 1 and Group 2 patients (0.81 vs. 0.96, respectively; p = 0.2505) (). Among MEC cycles, prior to propensity matched regression analysis, Group 1 patients had 83 CINV events over 58 cycles in the follow-up period, while Group 2 patients had 622 events over 323 cycles during the same follow-up period. The unadjusted average CINV event rate per CT cycle was statistically similar between Group 1 and Group 2 patients (1.43 vs. 1.93, respectively; p = 0.4807) ().
Multivariate regression model results: adjusted CINV event rate
After adjusting for baseline differences in available demographic (age, gender, and ethnicity) and clinical variables (CCI score, cycle length (in days), and number of CT days within a cycle), the regression model predicted a 20.4% decrease in the CINV event rate per CT cycle (converted parameter estimate of palonosetron: 4.56 vs. 5.73 for the intercept) in patients with hematologic malignancies receiving HEC/MEC in a hospital outpatient setting and were administered palonosetron (Group 1) compared to patients given other 5-HT3 RAs (Group 2). Female patients were shown to have a higher (2.9%) (statistically non-significant) CINV event rate versus male patients (5.90; p = 0.7895). Cycle length (a decrease of 0.2%) (5.72; p = 0.04) was also found to be significantly associated with CINV event rate (). Among payer types, commercially insured patients were found to have the largest decrease (39.4%) in the event rate while Medicaid patients showed a 17.9% decrease as compared to Medicare patients (3.47; p = 0.0028).
Table 3. Multivariate regression model: adjusted CINV event rate.
Discussion
This retrospective, longitudinal, hospital outpatient study is the first analysis to report CINV event rates (both acute and delayed nausea/emesis) at a CT cycle level in patients with hematologic malignancies receiving HEC/MEC regimens. In this study, the end of cycle 1 was the day before the start of the next CT administration (if the gap was >7 days). As observed in this study and other clinical studies, the follow-up time for identifying CINV events within a cycle was not limited to the first 5 days following CT administrationCitation20. Additionally, it has been reported that patients with cancer receiving HEC/MEC can experience not only CINV symptoms at cycle 1, but also during subsequent CT cyclesCitation1,Citation2. In this study, patients with hematologic malignancies receiving single or multiday HEC/MEC experienced a lower (20.4%) adjusted CINV event rate per CT cycle after palonosetron initiation compared to other 5-HT3 RAs that was statistically significant.
Approximately 30% of patients with hematologic malignancies on HEC/MEC continue to suffer from CINV even after the administration of 5-HT3 RAsCitation21,Citation22. Additionally, few comparative 5-HT3 RAs’ studies have assessed CINV event rates in patients with various types of leukemia and lymphomaCitation17,Citation23–29. In one study comparing the antiemetic efficacy of granisetron, tropisetron, and ondansetron in patients with malignant lymphoma on HEC, nausea and/or emesis control failure during the CT period and during the observation period was 24% and 51%, respectivelyCitation27. To date, few studies have also assessed the CINV event rates in patients with hematologic malignancies treated with HEC/MEC after palonosetron administration compared with other 5-HT3 RAsCitation10–14. A phase II MEC trial and a phase III HEC trial evaluating palonosetron with dolasetron and ondansetron in patients with non-Hodgkin’s lymphoma and Hodgkin’s lymphoma reported that palonosetron was associated with significantly higher rates of complete response rates in the acute and delayed phases of the trial as compared to the other 5-HT3 RAsCitation10,Citation11. Rzepecki and associates found palonosetron plus dexamethasone to be more effective than ondansetron plus dexamethasone in the prevention of acute and delayed nausea and vomiting after HEC before hematopoietic stem cell transplantation in patients with lymphoma, relapsed germ cell tumors, and acute myeloid leukemiaCitation12. Another study comparing palonosetron with ondansetron showed that palonosetron was associated with significantly less severe nausea on days 1 to 5 and less impact of CINV on daily activities on days 6 and 7 in patients with acute myelogenous leukemia treated with MECCitation13. Musso and associates reported that during the CT period in patients with hematologic malignancies (non-Hodgkin’s lymphoma, Hodgkin’s lymphoma, acute myeloid leukemia, and solid tumors) and in the 5 days following, CINV symptoms were absent in 80% of patients in the palonosetron group versus 60% of the ondansetron group (p < 0.05)Citation14. Study results show similar favorable reduction in CINV events over a period of 8 HEC/MEC cycles in patients diagnosed with leukemia/lymphoma who received palonosetron prophylaxis over the other 5-HT3 RAs.
Antiemetic therapy aims to prevent CINV during the overall emetic risk period after CTCitation6. Not providing the most efficacious antiemetic prophylaxis in the first cycle has been shown to increase CINV symptoms in the subsequent cycles in cancer diagnosed patientsCitation1,Citation2,Citation30,Citation31. In this study, palonosetron compared to other 5-HT3 RAs showed lower CINV event rates per CT cycle. The unique pharmacological characteristics of palonosetron, i.e. prolonged plasma half-life and high binding affinity may be a significant factor in reducing CINV event rates and supports the early use of appropriate antiemetic prophylaxisCitation32. Although hematologic malignancies do not constitute the majority of cancers diagnosed yearly, the recognition of predictable risk factors for CINV is essentialCitation33. Several studies have identified the female gender as a risk factor that increases CINV event rates in patients with hematologic malignanciesCitation34,Citation35. In the overall study population, females with hematologic malignancies were also more likely to have higher CINV event rates than males, though statistically non-significant. Additionally in this study’s population, Hispanics and Asians had higher CINV event rates versus Whites, again though statistically non-significant. Few studies have ascertained the association between racial differences and the incidence of CINV events, especially for African-Americans, Hispanics, and Asians with hematologic malignancies. However, epidemiologic data has shown that Whites had a higher incidence of lymphoma and leukemia, followed by African-Americans and AsiansCitation36. However, African-Americans have been shown to have twice the risk of being diagnosed with myeloma than WhitesCitation37. Another factor found to be associated with an increased CINV event rate in this study included number of days on CT [(11.1% increase) 6.37; p = 0.1483)]. These variables were also found to be predictive of an increased CINV event rate in other studies examining patients with hematologic malignancies on HEC/MEC regimensCitation1,Citation2.
This study has certain limitations worth noting. First, although multivariate analyses were used to adjust for differences in demographic and clinical characteristics, no data were available on certain important variables including use of alcohol that may have altered the authors’ estimates. Also, although the database used in the authors’ analysis contained a nationally representative sample, caution should be exercised in generalizing the results to individuals in other populations or geographic regions, as potentially confounding differences in treatment patterns may have been unobserved or missed. The dataset used (Premier Perspective Database) restricted resource use to hospitals within the Premier Perspective Database system, and thus, could have led to an underestimation of the incidence of CINV events. Additionally, there might be the potential of overestimation of CINV events in this study as antiemetic prophylaxis was considered as medications provided on day 1 of CT, antiemetic medications provided from day 2 to end of cycle were considered as rescue medications. The study patients consisted of patients initiating CT and antiemetic prophylaxis in a hospital outpatient setting; however, CINV event rates in the follow-up period were assessed in the entire hospital setting (inpatient, emergency room, and hospital outpatient). Another limitation was that as the study focused on patients initiating HEC/MEC and a 5-HT3 RA antiemetic prophylaxis with or without other antiemetic medications regularly prescribed including corticosteroids and NK1 receptor antagonist (aprepitant), the actual distribution of these ancillary antiemetics between the study groups could not be accurately ascertained that could have potentially impacted the study outcome (CINV event rate). Also, the adjusted CINV rate per CT cycle difference seen between the study groups was found to be statistically significant, however, whether this translates into a clinically meaningful difference needs to be ascertained. Finally, the study’s findings pertain to observations from real-world practice rather than from a clinical trial. Although the retrospective nature of the study limits the ability to draw conclusions regarding cause and effect, the authors’ findings draw attention to important relationships whose causal and intermediary factors warrant further exploration. There may be some selection bias as a result of the observational nature of the study.
Conclusions
It is well-known that the majority of patients with hematologic malignancies receiving HEC and MEC experience a CINV event and hence it is important to use the most appropriate prophylactic antiemetic regimens with the highest therapeutic index. In this retrospective hospital outpatient study, patients with hematologic malignancies (leukemia and/or lymphoma) receiving HEC/MEC and initiated on palonosetron were more likely to experience a significantly lower rate of CINV events than with other 5-HT3 RAs. Further studies on clinical as well as economic impact of early initiation of palonosetron versus other 5-HT3 RAs in the prevention of CINV among patients with cancer on CT treatment are warranted.
Transparency
Declaration of funding
This study was funded by Eisai Inc.
Declaration of financial/other relationships
C.C. is an employee of Premier, Inc., which received funding to conduct the study. J.G. is an employee of Premier, Inc., which received funding to conduct the study. S.B. is an employee of the Health Outcomes Department at Eisai, Inc. D.B. is an employee of the Health Outcomes Department at Eisai, Inc.
Acknowledgments
Editorial support for the preparation of this manuscript was provided by Michelle A. Adams of Write All, Inc.
Data from this article were previously presented as: Craver C, Gayle J, Balu S, Buchner D. Palonosetron versus other 5-HT3 RAs in preventing chemotherapy induced nausea and vomiting in hematologic malignancies treated in a hospital outpatient setting. ASH (American Society of Hematology) Annual Meeting, December 4–7, 2010; Orlando, FL, USA.
References
- Cohen L, de Moor CA, Eisenberg P, et al. Chemotherapy-induced nausea and vomiting: incidence and impact on patient quality of life at community oncology settings. Support Care Cancer 2007;15:497-503
- Morrow GR, Roscoe JA, Hickok JT, et al. Initial control of chemotherapy-induced nausea and vomiting in patient quality of life. Oncology 1998;12(Suppl 4):32-7
- Sussman N. Reactions of patients to the diagnosis and treatment of cancer. Anticancer Drugs 1995;6(Suppl 1):4-8
- Hesketh PJ. Defining the emetogenicity of cancer chemotherapy regimens: relevance to clinical practice. Oncologist 1999;4:191-6
- Grunberg SM, Osoba D, Hesketh PJ, et al. Evaluation of new antiemetic agents and definition of antineoplastic agent emetogenicity – an update. Support Care Cancer 2005;13:80-4
- National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in OncologyTM: Antiemesis. Version 2.2010. Available at: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp [Accessed March 1, 2011]
- Einhorn LH, Rapoport B, Koeller J, et al. Antiemetic therapy for multiple-day chemotherapy and high-dose chemotherapy with stem cell transplant: review and consensus statement. Support Care Cancer 2005;13:112-16
- Ruhlmann C, Herrstedt J. Palonosetron hydrochloride for the prevention of chemotherapy-induced nausea and vomiting. Expert Rev Anticancer Ther 2010;10:137-48
- Grunberg SM, Deuson RR, Mavros P, et al. Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer 2004;100: 2261-8
- Eisenberg P, Figueroa-Vadillo J, Zamora R, et al. Improved prevention of moderately emetogenic chemotherapy-induced nausea and vomiting with palonosetron, a pharmacologically novel 5-HT3 receptor antagonist: results of a phase III, single dose trial versus dolasetron. Cancer 2003;98:2473-82
- Aapro MS, Grunberg GM, Manikhas GM, et al. A phase III, double-blind, randomized trial of palonosetron compared with ondansetron in preventing chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy. Annal Oncol 2006;17:1441-9
- Rzepecki P, Pielichowski W, Oborska S, et al. Palonosetron in prevention of nausea and vomiting after highly emetogenic chemotherapy before haematopoietic stem cell transplantation – single center experience. Transplant Proc 2009;41:3247-3249 doi:10.1016/j.transproceed.2009.07.071
- Mattiuzzi GN, Cortes JE, Blamble DA, et al. Daily palonosetron is superior to ondansetron in the prevention of delayed chemotherapy-induced nausea and vomiting in patients with acute myelogenous leukemia. Cancer 2010;116:5659-66
- Musso M, Scalone R, Bonanno V, et al. Palonosetron (Aloxi) and dexamethasone for the prevention of acute and delayed nausea and vomiting in patients receiving multiple-day chemotherapy. Support Care Cancer 2009;17:205-9
- Siddiqui MA, Scott LJ. Palonosetron. Drugs 2004;64:1125-32
- Aloxi injection (palonosetron hydrochloride) [prescribing information]. Woodcliff Lake, NJ: Eisai Inc, 2009
- López-Jiménez J, Martín-Ballesteros E, Sureda A, et al. Chemotherapy-induced nausea and vomiting in acute leukemia and stem cell transplant patients: results of a multicenter, observational study. Haematologica 2006;91:84-91
- Weiner MG, Livshits A, Carozzoni C, et al. Derivation of malignancy status from ICD-9 codes. American Medical Informatics Association (AMIA) 2003 Symposium Proceedings: 1050
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83
- Roila F, Donati D, Tamberi S, et al. Delayed emesis: incidence, pattern, prognostic factors and optimal treatment. Support Care Cancer 2002;10:88-95
- Tremblay P, Kaiser R, Sezer O, et al. Variations in the 5-hydroxytryptamine type 3B receptor gene as predictors of the efficacy of antiemetic treatment in cancer patients. J Clin Oncol 2003;21:2147-55
- Kaiser R, Sezer O, Papies A, et al. Patient-tailored antiemetic treatment with 5-hydroxtryptamine type 3 receptor antagonist according to cytochrome P-450 2D6 genotypes. J Clin Oncol 2002;20:2805-11
- Abali H, Celik I. Tropisetron, ondansetron, and granisetron for control of chemotherapy-induced emesis in Turkish cancer patients: a comparison of efficacy, side-effect profile, and cost. Cancer Invest 2007;25:135-9
- Walsh T, Morris AK, Holle LM, et al. Granisetron vs ondansetron for prevention of nausea and vomiting in hematopoietic stem cell transplant patients: results of a prospective, double-blind, randomized trial. Bone Marrow Transplant 2004;34:963-8
- Tan M, Xu R, Seth R. Granisetron vs dolasetron for acute chemotherapy-induced nausea and vomiting (CINV) in high and moderately high emetogenic chemotherapy: an open-label pilot study. Curr Med Res Opin 2004;20:879-82
- Fox-Geiman MP, Fisher SG, Kiley K, et al. Double-blind comparative trial of oral ondansetron versus oral granisetron versus IV ondansetron in the prevention of nausea and vomiting associated with highly emetogenic preparative regimens prior to stem cell transplantation. Biol Blood Marrow Transplant 2001;7:596-603
- Slaby J, Trneny M, Prochazka B, et al. Antiemetic efficacy of three serotonin antagonists during high-dose chemotherapy and autologous stem cell transplantation in malignant lymphoma. Neoplasma 2000; 47:319-22
- Spitzer TR, Friedman CJ, Bushnell W. Double-blind randomized parallel-group study on the efficacy safety of oral granisetron oral ondansetron in the prophylaxis of nausea vomiting in patients receiving hyperfractionated total body irradiation. Bone Marrow Trans 2000;26:203-10
- Orchard PJ, Rogoscheske J, Burns L, et al. A prospective randomized trial of the anti-emetic efficacy of ondansetron and granisetron during bone marrow transplantation. Biol Blood Marrow Transplant 1999;5:386-93
- Feinberg B, Gilmore J, Haislip S, et al. Likelihood of a subsequent chemotherapy induced nausea and vomiting (CINV) event in patients receiving moderately or highly emetogenic chemotherapy (MEC/HEC). Presented at the 2010 ISPOR European Congress, November 6-9, 2010; Prague, Czech Republic
- Schwartzberg L, Szabo S, Gilmore J, et al. Likelihood of a subsequent chemotherapy-induced nausea and vomiting (CINV) event in patients receiving low, moderate or highly emetogenic chemotherapy (LEC/MEC/HEC). Curr Med Res Opin 2011;27:837-45 doi: 10.1185/03007995.2011.556603
- Rojas C, Thomas AG, Alt J, et al. Palonosetron triggers 5-HT3 receptor internalization and causes prolonged inhibition of receptor function. Eur J Pharmacol 2010;626:193-9 doi:10.1016/j.ejphar.2009.10.002
- American Cancer Society. Cancer Facts & Figures 2010. Atlanta: American Cancer Society, 2010
- Molassiotis A, Saunders MP, Valle J, et al. A prospective observational study of chemotherapy-related nausea and vomiting in routine practice in a UK cancer centre. Support Care Cancer 2008;16:201-8
- Osoba D, Zee B, Pater J, et al. Determinants of postchemotherapy nausea and vomiting in patients with cancer. Quality of Life and Symptom Control Committees of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 1997;15:116-23
- Morton LM, Wang SS, Devesa SS, et al. Lymphoma incidence patterns by WHO subtype in the United States. Blood 2006;107:265-276 doi: 10.1182/blood-2005-06-2508
- Benjamin M, Reddy S, Brawley OW. Myeloma and race: a review of the literature. Cancer Metastasis Rev 2003;22:87-93