Abstract
Objective:
To compare the health care costs of patients with metastatic colorectal cancer (mCRC) who received second-line treatment with Avastin (bevacizumab) versus Erbitux (cetuximab), from the third-party payer’s perspective.
Methods:
Patients with mCRC were selected from the PharMetrics claims database if they received second-line therapy containing either bevacizumab (second-line bevacizumab cohort) or cetuximab (second-line cetuximab cohort). Six-month costs following second-line therapy start date and average monthly healthcare costs while on second-line therapy (in 2009 US$) were calculated and compared between the two groups.
Results:
A total of 2188 patients with mCRC who met the eligibility criteria were included in the analysis, including 1808 patients receiving bevacizumab and 380 patients receiving cetuximab in second-line treatment. Demographic and baseline characteristics were similar between the two groups. Patients’ mean age was 61 years and 56% were males. In second-line treatment, bevacizumab was commonly used with oxaliplatin (43.5%) and irinotecan-based regimens (40.4%), whereas cetuximab was commonly used with irinotecan-based regimens (68.2%). Bevacizumab patients had significantly lower total all-cause healthcare costs than cetuximab patients (adjusted difference: –$10,231, p = 0.020), and lower medical costs (–$10,796, p = 0.012) during the 6 months following second-line therapy initiation. Approximately half of the difference in total all-cause healthcare costs was attributable to the lower chemotherapy and targeted therapy costs (–$5635, p = 0.032) of bevacizumab patients than those of cetuximab patients. While on second-line therapy, bevacizumab patients also had lower average monthly all-cause healthcare costs than cetuximab patients.
Limitations:
Second-line treatment in the current study was defined based on changes in mCRC medications, not based on disease progression due to the limited clinical information available in claims.
Conclusion:
The use of bevacizumab in second-line therapy was associated with significantly lower healthcare costs in mCRC patients, compared to the use of cetuximab.
Introduction
Colorectal cancer (CRC) is the third most common cancer and the second leading cause of cancer-related death in the United States (US); in 2009, an estimated 147,000 new cases were diagnosed and 50,000 people died from this diseaseCitation1. If detected early when the disease is still localized, the 5-year survival rate is approximately 90%. However, only 39% of cases are detected at that early stage, and for the approximately 25% of new cases that are diagnosed after CRC has metastasized, the 5-year survival rate is less than 10%. Approximately 75,000 patients are treated for metastatic CRC (mCRC) every year in the USCitation2.
The development of new chemotherapy and targeted agents has increased the therapeutic options and has improved outcomes for mCRC patients. Phase III trials of combination chemotherapy regimens which include 5-FU, leucovorin, and oxaliplatin or irinotecan have demonstrated significant survival benefits in mCRC patientsCitation3. Recent studies show that the inclusion of targeted therapies with chemotherapy further improves overall survival in mCRCCitation4–7. Avastin (bevacizumab), an antivascular endothelial growth factor antibody, was approved for use in combination with intravenous 5-FU-based chemotherapy for first-line and second-line treatment of mCRC in 2004 and 2006, respectivelyCitation8. Erbitux (cetuximab), an anti-epidermal growth factor receptor (EGFR) antibody, received FDA approval in 2004 as a single agent for treating EGFR-expressing mCRC patients after the failure of both irinotecan- and oxaliplatin-based regimens or in patients who are intolerant to irinotecan-based regimensCitation9. Cetuximab could also be used in combination with irinotecan in EGFR-expressing mCRC patients who are refractory to irinotecan-based chemotherapy. Vectibix (panitumumab), another anti-EGFR antibody, was approved in 2006 for the treatment of mCRC patients after failure of fluoropyrimidine, oxaliplatin, and irinotecan chemotherapy regimensCitation10. In July 2009, product labels of cetuximab and panitumumab were updated restricting them to patients without KRAS mutations based on retrospective analyses demonstrating the lack of clinical benefit for cetuximab-treated mCRC patients with KRAS mutations in codon 12 or 13Citation9,Citation10. On the contrary, the clinical benefit of bevacizumab is independent of KRAS statusCitation11.
Phase III trials have shown that the addition of bevacizumab to chemotherapy increases median overall survival of mCRC patients in both first-line therapy (hazard ratio [HR] = 0.66; p < 0.001) and second-line therapy (HR = 0.75; p = 0.001)Citation4,Citation7. The use of cetuximab is associated with improved overall survival compared to the best supportive care alone (HR = 0.77; p = 0.005) in later-line treatment of mCRCCitation6. Adding irinotecan to cetuximab further increases the objective response rate in mCRC patients (22.9 vs. 10.8%; p = 0.007)Citation5,Citation6. To date, the relative efficacies of bevacizumab and cetuximab as second-line therapies are still unknown due to the lack of head-to-head trials directly comparing these two targeted therapies.
Despite the lack of head-to-head trials comparing bevacizumab and cetuximab, both of them are widely used in the treatment of mCRC. The availability of multiple targeted agents as second-line treatment in mCRC thus raises important questions over the costs associated with these treatments. Currently, there is a paucity of published analyses examining the economic outcomes associated with the use of targeted therapies in mCRC. Based on the SEER-Medicare data, real-world estimates of CRC-related healthcare costs per year of survival are $8853 for all CRC, and $30,794 for stage IV CRC (in 2006 US$)Citation12. However, the study did not specifically focus on patients treated with targeted agents. Past economic studies on targeted therapies for mCRC have been model-based with efficacy and safety assumptions derived from randomized controlled trials, publicly available unit costs, and on-label dosing regimensCitation13–15. Tappeden and colleagues have showed that adding bevacizumab to irinotecan, 5-FU and leucovorin costs approximately £62,857 per quality-adjusted life-year (QALY) gained and adding bevacizumab to 5-FU and folinic acid costs approximately £88,658 per QALY gained in the UK populationCitation16. Starling and colleagues estimated an ICER of £57,608 per QALY gained for cetuximab plus irinotecan versus the best supportive careCitation14. The estimates from the above cost-effectiveness modeling studies may not reflect the real-world costs due to the variations in the actual clinical practice patterns and patient compliance.
In the real world, the drug’s treatment patterns and outcomes may be very different from those in the clinical trials. Given the lack of real-world data describing the economic outcomes associated with targeted therapies in mCRC, the objectives of this study were to estimate the healthcare costs associated with second-line therapy with bevacizumab and cetuximab in mCRC patients from the perspective of a commercial payer using a claims database. Evaluating the real-world economic outcomes for alternative therapies can provide helpful information for both payers and physicians to assess alternative therapy choices.
Materials and methods
Data source
This study is based on administrative claims data from the PharMetrics database (2002–2009). The database contains fully adjudicated medical and pharmacy claims for more than 61 million unique patients from approximately 100 health plans across the US. The database contains integrated medical claims data (e.g., ICD-9 diagnosis, Current Procedural Terminology [CPT] or Healthcare Common Procedure Coding System [HCPCS] codes, and date and place of services), pharmacy claims data (e.g., national drug codes [NDC] and date of dispensing), and de-identified patient information.
Study design
This was a retrospective cohort study of patients with mCRC treated with bevacizumab or cetuximab in a second-line therapy. Patients receiving panitumumab in a second-line treatment were not included in the study due to a small sample size. Patients with mCRC were identified on the basis of claims with a diagnosis of CRC (ICD-9-CM 153.x, 154.0, 154.1, 154.8, 230.3, and 230.4) and a subsequent diagnosis for secondary neoplasm (ICD-9-CM 196.x, 197.x, and 198.xx). The date of secondary neoplasm diagnosis was defined as the mCRC diagnosis date.
An algorithm was developed to identify second-line treatment from claims data. Following the mCRC diagnosis date, the start of first-line therapy was identified as the initiation (i.e., first-line start date) of any of the following chemotherapeutic or targeted agents: 5-FU/leucovorin, oxaliplatin, irinotecan, capecitabine, bevacizumab, cetuximab, and panitumumab. Chemotherapeutic and targeted agents were identified using NDC and HCPCS codes. Any changes to the initial first-line agents occurring within 4 weeks of the first-line start date were considered part of first-line therapyCitation17,Citation18. If a different chemotherapeutic or targeted agent was used after 4 weeks, the new agent defined the initiation of second-line therapy. In order to be considered as initiating a second-line therapy, a patient must initiate a new agent that is not used in first-line treatment, while he/she may continue or discontinue the use of the first-line agents. Treatment modifications occurring within 4 weeks of the initiation of second-line therapy were considered as part of second-line therapy. The date on which second-line therapy was initiated was considered as the index date.
Patients were included in the study if they initiated at least two lines of therapy following the mCRC diagnosis date, and bevacizumab or cetuximab were part of the second-line therapy. Patients had to be aged 18 years or older as of the index date and have continuous eligibility (i.e., enrolled in an insurance plan) between the CRC diagnosis date and the index date. In addition, patients were required to have a minimum 6 months of eligibility before the index date and 6 months after the index date. The earliest index date observed in the study was in 2004 when bevacizumab and cetuximab became available on the market. Patients who were administered both bevacizumab and cetuximab for second-line treatment were excluded. Patients were categorized into two mutually exclusive cohorts depending on whether their second-line therapy contained bevacizumab or cetuximab.
Outcome measures and statistical analyses
Demographic characteristics were assessed as of the index date. Sites of primary CRC were based on the first claim with a CRC diagnosis, and sites of metastases were based on the first claim of a secondary tumor diagnosis following the initial CRC diagnosis. In addition, baseline comorbidities and healthcare utilization were assessed based on claims during the 6-month period prior to the index date. A summary measure for comorbid conditions, the Charlson Comorbidity Index (CCI) was also examinedCitation19. For healthcare utilization, claims for various types of services (e.g., inpatient, outpatient, and pharmacy) were measured. The types of healthcare services were classified based on the PharMetrics-provided standard algorithm that utilized the place-of-service, record type, CPT code, HCPCS code, and the revenue center code. Briefly, pharmacy services included all prescription drug claims using NDC codes. Inpatient services included an aggregated set of medical claims occurred during hospitalization with a room and board number, and all other medical services (e.g., emergency room service, laboratory/pathology, radiology, physician office visits, etc.) were classified as outpatient services. Continuous variables were compared between second-line bevacizumab and second-line cetuximab patients using Wilcoxon rank-sum tests and categorical variables were compared using chi-square tests.
Treatment patterns of study patients during first-line and second-line therapies were assessed. Patients were identified as receiving regimens containing oxaliplatin, capecitabine, irinotecan, 5-FU, leucovorin, bevacizumab, or cetuximab, and these regimen categories were not mutually exclusive as combinations of these drugs may have been administered. Panitumumab was not observed in the first-line and second-line therapies of study patients. The duration of first-line therapy was calculated as the time between the first and last claim of the applicable treatments used in first-line while patients were still on first-line treatment. The duration of second-line therapy was calculated as the time between the first and last claim of the applicable treatments while patients were still on second-line treatment and before the end of continuous eligibility. The usage rate of chemotherapy and targeted agents in first-line and second-line treatment were compared between study cohorts using chi-square tests. The durations of first-line and second-line therapy were compared between study cohorts using Wilcoxon rank-sum tests and log-rank tests (considering potential censoring), respectively.
Healthcare costs (all-cause) in each cohort were calculated using two different metrics. First, costs were calculated over a fixed 6-month period beginning on the index date, i.e. second-line therapy start date. Second, costs were calculated as a monthly cost while on second-line therapy to account for the variability in second-line duration (i.e., shorter or longer than 6 months). Costs incurred during second-line therapy were divided by the number of months on second-line therapy. Presenting monthly costs while on therapy can minimize the differences caused by the different second-line therapy durations between bevacizumab and cetuximab patients.
Total cost components included medical costs (including inpatient and outpatient) and pharmacy costs. In addition, chemotherapy drug costs, targeted agents costs, and costs of infusion procedures were calculated as a subset of total cost. The majority of the expenses for chemotherapeutic and targeted agents were extracted from the outpatient section of medical claims, since they are administered by healthcare professionals. The expenses of oral drug capecitabine and occasionally some infusion drugs were extracted from pharmacy claims.
The differences between costs incurred by the second-line bevacizumab and second-line cetuximab cohorts were analyzed on an unadjusted and adjusted basis. Unadjusted differences were analyzed using Wilcoxon rank-sum tests. Adjusted differences between the two groups were calculated as the differences between the estimated means of the two groups using multivariate generalized linear models or two-part models, adjusting for potential confounding factors. If no more than 10% of patients had zero costs for a specific cost measure, generalized linear models with log-link and gamma distributions were used to generate the estimated mean for each group. If more than 10% of patients had zero costs, two-part models were used to estimate the mean of each group, where the first part was a logistic regression to estimate the probability of observing zero value and the second part was a generalized linear model with log link and gamma distribution. The factors adjusted in the regression models were patient demographics, primary tumor locations, secondary metastases sites, baseline comorbidities and healthcare utilization, first-line treatment, first-line duration, and the gap between first-line therapy end date and second-line therapy start date. All statistical analyses were performed using SAS 9.2 (SAS Institute, Inc., Cary, NC, USA). Costs represent actual dollar amount paid by the third-party payer and were inflation-adjusted to 2009 US$.
Results
Patient disposition and baseline characteristics
A total of 2188 patients satisfied the selection criteria and were included in the analysis. Approximately 83% were assigned to the second-line bevacizumab cohort (n = 1808) and 17% to the second-line cetuximab cohort (n = 380; ). Demographic characteristics were similar between both cohorts (). For both cohorts, more than half of them were male (55.6% of bevacizumab patients vs. 59.5% of cetuximab patients; p = 0.171), and the average age was 61.3 (SD 11.3) years for the bevacizumab cohort and 61.3 (12.9) for the cetuximab cohort (p ≈ 1.000). Approximately half of the sample were members of a preferred provider organization health plan, and most patients (>80.0%) were enrolled in a commercial plan. The primary site of the initial CRC diagnosis was the colon (70.9% of bevacizumab patients vs. 64.7% of cetuximab patients; p = 0.017). The most common site of metastasis was the respiratory and digestive systems (64.2 vs. 71.3% for bevacizumab and cetuximab, respectively; p = 0.013).
Table 1a. Patient demographic and disease characteristics.
Baseline comorbidities were comparable between the second-line bevacizumab and second-line cetuximab cohorts with no statistically significant differences observed in the parameters measured (). With respect to healthcare utilization during the 6-month baseline period prior to second-line therapy, the second-line bevacizumab patients, compared to second-line cetuximab patients, had a higher proportion of patients with inpatient visits (50.8 vs. 40.5%; p < 0.001) and with laboratory/pathology visits (97.7 vs. 95.8%; p = 0.037). Other healthcare utilization categories, including outpatient and pharmacy services, were not statistically different between the two groups at baseline.
Table 1b. Baseline comorbidities and healthcare utilization during the 6-month period prior to second-line therapy initiation.
Treatment patterns
Treatment regimens received by mCRC patients in first-line and second-line therapy are summarized in and . Among patients who used bevacizumab for second-line therapy, 28.5% received bevacizumab treatment in first-line and 2.2% received cetuximab in first-line. Among patients who received cetuximab for second-line therapy, 55.0% and 2.9% were treated with bevacizumab and cetuximab in first-line, respectively. The most common first-line chemotherapy regimen was oxaliplatin-based for both cohorts (62.7% of second-line bevacizumab patients vs. 52.9% of second-line cetuximab patients; p < 0.001). The median duration of first-line therapy was longer in the second-line cetuximab cohort than in the second-line bevacizumab cohort (4.2 vs. 3.3 months; p < 0.001).
Table 2a. First-line treatment patterns.
Table 2b. Second-line treatment patterns.
More than 90% of study patients started second-line therapy with bevacizumab or cetuximab between 2005 and 2008, and less than 2% of patients initiated second-line therapy with bevacizumab or cetuximab in 2004. Bevacizumab was approved for treating mCRC as a first-line treatment in February 2004 and as a second-line treatment in June 2006. Interestingly, there were 32.7% of bevacizumab patients (data not shown) who started second-line treatment with bevacizumab before June 2006, which indicated off-label use. For second-line therapy, bevacizumab was most often used in combination with oxaliplatin- and irinotecan-based regimens (43.5% and 40.4% of second-line bevacizumab patients, respectively), and more than 85% of the time 5-FU was also used in the oxaliplatin- and irinotecan-based regimens with bevacizumab. Cetuximab was most commonly used in combination with irinotecan-based regimens (68.2% of second-line cetuximab patients), and in less than 30% of instances 5-FU was used in combination with irinotecan and cetuximab. The median duration of second-line chemotherapy was longer in the second-line bevacizumab cohort than in the second-line cetuximab cohort (4.7 vs. 4.0 months; p < 0.001). The median follow-up time for second-line bevacizumab and cetuximab patients was 14.9 and 13.1 months, respectively (p = 0.003).
Healthcare costs
shows costs measured during the 6-month period following the initiation of second-line therapy. The average total healthcare cost during this period was $73,712 (SD $53,378) and $81,781 (SD $63,830) for bevacizumab and cetuximab patients, respectively. On unadjusted basis, total healthcare costs in the second-line bevacizumab cohort were $8070 less than in the second-line cetuximab cohort (p = 0.080). Outpatient medical costs comprised the major portion of total healthcare costs in both cohorts (approximately 87% and 85% of total healthcare costs in the second-line bevacizumab and second-line cetuximab cohorts, respectively). Physician office visits, lab, radiology, emergency room visits, and ancillary services composed ∼25% of the outpatient costs for both cohorts, with the rest coming from other outpatient services (data not shown).
Figure 2. Mean 6-month all-cause healthcare costs following second-line therapy initiation and adjusted difference between second-line bevacizumab and second-line cetuximab*,†.
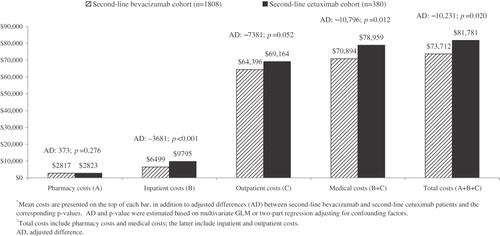
Using regression models that take into account potentially confounding factors including demographics, disease characteristics, comorbidities, baseline utilizations, and first-line treatments, the adjusted difference for the 6-month period showed that second-line bevacizumab patients incurred $10,231 less in total healthcare costs, which was statistically significant (p = 0.020). The primary driver for this adjusted difference in total healthcare costs was medical costs (–$10,796 comparing second-line bevacizumab with second-line cetuximab patients; p = 0.012). Both inpatient cost (–$3681; p < 0.001) and outpatient cost (–$7381; p = 0.052) were lower in second-line bevacizumab patients than in second-line cetuximab patients. The difference in pharmacy costs was small and not statistically significant (cost for second-line bevacizumab patients was $373 greater; p = 0.276).
In both cohorts, the chemotherapy and targeted therapy costs constituted a large share of the total healthcare costs (approximately 57% for both groups; ). Second-line bevacizumab patients had a significantly lower cost of targeted therapy compared with second-line cetuximab patients (adjusted difference: –$10,260; p < 0.001), which more than offset the higher chemotherapy cost ($3824; p < 0.001). The net difference for chemotherapy and targeted therapy costs showed that the second-line bevacizumab cohort incurred $5635 less in costs (p = 0.032). No significant differences between the two cohorts were observed for infusion costs.
Table 3. Treatment-related 6-month healthcare costs following second-line therapy initiation.
presents the average monthly healthcare costs for mCRC patients during second-line treatment. Similar trends were observed as in the 6-month cost analyses. Second-line bevacizumab was associated with significantly lower monthly total costs compared to second-line cetuximab (adjusted difference: –$6219; p < 0.001), mainly due to differences in medical costs (–$6367; p < 0.001). Among the medical costs, monthly outpatient costs were significantly lower in second-line bevacizumab patients (adjusted difference: –$6319; p < 0.001) while inpatient costs were not significantly different between the two groups (–$64; p = 0.395). The significances of the differences in average monthly inpatient and outpatient costs while on second-line therapy between the two groups differed from those of 6-month post-index period costs, in which 6-month inpatient costs were significantly lower and outpatient costs were not significantly different in the second-line bevacizumab cohort than in the second-line cetuximab cohort. This may be attributed to the possibility that the 6-month costs capture costs beyond second-line therapy while monthly costs account for costs observed in the second-line only. Both analyses confirmed lower total costs and medical costs associated with bevacizumab second-line therapy.
Figure 3. Mean monthly all-cause health care costs while on second-line therapy and adjusted difference between second-line bevacizumab and second-line cetuximab*,†.
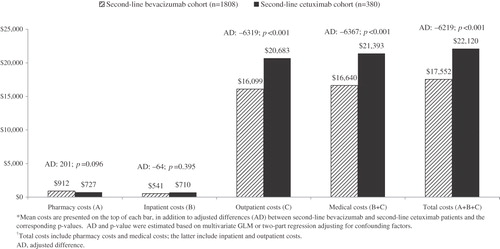
Monthly costs for chemotherapy and targeted therapy () exhibited similar trends as in the 6-month period. Bevacizumab treatment was associated with significantly lower monthly chemotherapy plus targeted therapy costs while on second-line than cetuximab (adjusted difference: –$5193; p < 0.001), mainly due to cost saving from targeted agents (–$6104; p < 0.001). No significant differences were observed in monthly chemotherapy costs while on second-line therapy. Compared to second-line cetuximab patients, second-line bevacizumab patients had lower monthly infusion costs (–$450; p < 0.001).
Table 4. Treatment-related monthly healthcare costs while on second-line therapy.
Discussion
In the current study, we have examined the costs associated with second-line treatment with bevacizumab and cetuximab in mCRC patients. Patients receiving second-line treatment with either bevacizumab or cetuximab were identified from a large insurance claims database and the costs of targeted agents, chemotherapy, as well as medical costs and total costs incurred by study patients were qualified and compared between the two groups. To our knowledge this study provides the first real-world evaluation of healthcare costs associated with bevacizumab and cetuximab when used as second-line therapy in mCRC patients. The results demonstrate that patients on second-line therapy with bevacizumab incur significantly lower healthcare costs than patients on cetuximab.
Both unadjusted and adjusted total healthcare costs and medical costs were significantly lower for second-line bevacizumab patients than second-line cetuximab patients. The adjusted cost difference in total healthcare costs during the 6-month period following second-line therapy initiation was –$10,231 (p = 0.020) comparing bevacizumab to cetuximab patients (). In interpreting this cost difference, it should be noted that the observed duration of second-line treatment was longer for bevacizumab patients than for cetuximab patients (median 4.7 vs. 4.0 months; ). It is also worth noting that second-line bevacizumab patients had higher use of expensive chemotherapeutic agent oxaliplatin and less use of targeted agent as monotherapy than second-line cetuximab patients. Even though bevacizumab patients incurred costs for second-line treatment for a longer period and had higher costs for chemotherapeutic agents, second-line bevacizumab patients still realized a net cost savings versus second-line cetuximab patients over the 6-month observation period.
Total healthcare costs assessed in this study included chemotherapy and targeted agent costs, infusion costs, expenditures associated with comorbidities, supportive care agents, and treatment costs for potential adverse events. The majority of the cost savings observed for second-line bevacizumab patients were attributable to the lower cost of the targeted therapy itself. On average, the monthly cost of bevacizumab was $6793 and cetuximab was $11,277 (in 2009 US$). The real-world cost differences from this study are consistent with the findings of other investigators who employed modeling-based analyses with hypothetical cohorts and safety, efficacy, and economic assumptions based on publicly available information. For example, Wong et al. 2009 estimated that 6 months of therapy with bevacizumab would cost $24,123 (in 2008 US$; per month average $4021), while treatment with cetuximab would cost $52,080 (per month average $8680).Citation20
Limitations
The study is subject to the limitations associated with observational claims database analyses. First, this study may contain selection bias. The current study has required patients to have at least 6 months continuous eligibility following the initiation of second-line treatment, so results might not be applicable to severe mCRC patients whose life expectancy is less than 6 months. Additionally, though this study used multivariate regression to adjust for measurable confounding factors, unobserved confounders such as mCRC disease severity or performance status cannot be accounted for in an administrative claims database. Second, in the absence of clinical information, identification of mCRC patients was based on diagnosis codes. Although this method is widely used in retrospective claim analyses, it bears the risk of misclassificationCitation21,Citation22.
Third, second-line therapy was defined as the occurrence of a new agent not used in first-line treatment. This assumption covered two scenarios: (1) enhancement of first-line therapy with the addition of new agents, and (2) discontinuing at least one of the agent(s) used in first-line, and introducing new agents. Due to the lack of clinical information, it is unclear whether the addition of a new agent is reflective of disease progression. This may contribute to the relatively short duration of first-line therapy observed in the current study since patients may switch to second-line due to tolerability issues instead of disease progression.
Fourth, since the data were collected from commercial health plans, caution should be taken in generalizing the findings from this relatively younger population with employer-based health insurance to the overall population, especially to the elderly population.
Fifth, the majority of the patient population in this study received cetuximab treatment prior to the revision of product labeling limiting its use to mCRC patients without KRAS mutations in July 2009Citation9. The study results might not generalize to the treatment patterns and outcomes in more recent years. However, it can be argued that the costs associated with cetuximab are conservative based on the assumption that cetuximab patients without KRAS mutations might incur greater treatment costs with greater treatment benefit and longer treatment duration. Although results of genetic testing cannot be observed in the claims database, we identified 20 cetuximab patients (∼5%) who received genetic testing used for KRAS detection. The low number of patients who received genetic testing reflects a delay in the adoption of KRAS testing in clinical practice. The total costs of these 20 patients are higher than the average costs of all cetuximab patients (data not shown). Nevertheless, this study of second-line bevacizumab and cetuximab provides relevant and valuable information on the outcomes of the therapies since therapy choices for these patients reflects physicians’ decisions based on the clinical information available to them as in a real-world setting.
Despite these limitations, the use of claims data allows for the study of a large and diverse group of patients using various regimen combinations observed in actual clinical practice. Therefore, this study’s findings may be more reflective of national costs and treatment patterns. Future research should further verify the findings of this study with more recent data in order to reflect the changes in mCRC treatment patterns and KRAS mutational status.
Conclusions
The current study has assessed the healthcare costs associated with second-line bevacizumab and cetuximab treatment in mCRC patients. To our knowledge, this is the first real-world study with a detailed cost comparison between the two drugs. Our findings suggest that bevacizumab was associated with lower total healthcare costs than cetuximab when used in second-line treatment for mCRC, and the majority of the cost differences were due to the lower drug cost of bevacizumab compared to cetuximab. Future research on a KRAS wild type population after the adoption of KRAS testing should be conducted to provide further insight on the comparative costs of targeted agents in mCRC population.
Transparency
Declaration of funding
This study was carried out by Analysis Group, Inc. Funding for this study was provided by Genentech Inc. H.Y., A.P.Y., Y.Y., E.Y., and E.Q.W. contributed to the conception and design of this study; H.Y. and A.P.Y. performed data analysis; H.Y., A.P.Y., Y.Y., E.Y., and E.Q.W. provided data interpretation; and H.Y., A.P.Y., Y.Y., E.Y., and E.Q.W. wrote and approved the manuscript.
Declaration of financial/other relationships
A.P.Y., H.Y., and E.Q.W. are employees of Analysis Group Inc., which has received consulting fees from Genentech for research related to this manuscript. Y.Y. and E.Y. are employees of Genentech.
References
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin 2009;59:225-49
- Goldberg RM, Rothenberg ML, Van Cutsem E, et al. The continuum of care: a paradigm for the management of metastatic colorectal cancer. Oncologist 2007;12:38-50
- Funaioli C, Longobardi C, Martoni AA. The impact of chemotherapy on overall survival and quality of life of patients with metastatic colorectal cancer: a review of phase III trials. J Chemother 2008;20:14-27
- Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 2007;25:1539-44
- Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 2004;351:337-45
- Jonker DJ, O'Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med 2007;357:2040-8
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335-42
- Genentech Inc. Avastin® (bevacizumab) [prescribing information]. http://www.gene.com/gene/products/information/pdf/avastin-prescribing.pdf; 2011
- Bristol-Myers Squibb. Erbitux® (cetuximab) [prescribing information]. http://packageinserts.bms.com/pi/pi_erbitux.pdf; 2011
- Amgen Inc. Vectibix® (Panitumumab) [prescribing information]. http://pi.amgen.com/united_states/vectibix/vectibix_pi.pdf; 2011
- Hurwitz HI, Yi J, Ince W, et al. The clinical benefit of bevacizumab in metastatic colorectal cancer is independent of K-ras mutation status: analysis of a phase III study of bevacizumab with chemotherapy in previously untreated metastatic colorectal cancer. Oncologist 2009;14:22-8
- Lang K, Lines LM, Lee DW, et al. Lifetime and treatment-phase costs associated with colorectal cancer: evidence from SEER-Medicare data. Clin Gastroenterol Hepatol 2009;7:198-204
- Shiroiwa T, Fukuda T, Tsutani K. Cost-effectiveness analysis of bevacizumab combined with chemotherapy for the treatment of metastatic colorectal cancer in Japan. Clin Ther 2007;29:2256-67
- Starling N, Tilden D, White J, et al. Cost-effectiveness analysis of cetuximab/irinotecan vs active/best supportive care for the treatment of metastatic colorectal cancer patients who have failed previous chemotherapy treatment. Br J Cancer 2007;96:206-12
- Tappenden P, Jones R, Paisley S, et al. The cost-effectiveness of bevacizumab in the first-line treatment of metastatic colorectal cancer in England and Wales. Eur J Cancer 2007;43:2487-94
- Tappenden P, Jones R, Paisley S, et al. Systematic review and economic evaluation of bevacizumab and cetuximab for the treatment of metastatic colorectal cancer. Health Technol Assess 2007;11:1-128, iii-iv
- Ramsey SD, Martins RG, Blough DK, et al. Second-line and third-line chemotherapy for lung cancer: use and cost. Am J Manag Care 2008;14:297-306
- Saif MW, Shi N, Zelt S. Capecitabine treatment patterns in patients with gastroesophageal cancer in the United States. World J Gastroenterol 2009;15:4415-22
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613-19
- Wong YN, Meropol NJ, Speier W, et al. Cost implications of new treatments for advanced colorectal cancer. Cancer 2009;115:2081-91
- Delea TE, Vera-Llonch M, Edelsberg JS, et al. The incidence and cost of hospitalization for 5-FU toxicity among Medicare beneficiaries with metastatic colorectal cancer. Value Health 2002;5:35-43
- Paramore LC, Thomas SK, Knopf KB, et al. Estimating costs of care for patients with newly diagnosed metastatic colorectal cancer. Clin Colorectal Cancer 2006;6:52-8