Abstract
Objective:
Evaluation of cost-effectiveness of levodopa/carbidopa intestinal gel (LCIG), compared to standard care (SC) in patients with advanced Parkinson’s disease (aPD) in the UK.
Design:
Markov model to quantify costs and outcomes associated with LCIG versus SC in aPD patients at Hoehn and Yahr (H&Y) stages 3, 4 or 5 experiencing >50% OFF time per day. Time horizon was lifetime, LCIG treatment was assumed to last maximal 5 years after which patients revert to SC. Model comprised 12 aPD health states according to H&Y status and daily time spent in OFF state. Cost analyses are reported from a UK NHS and Personal Social Services perspective. Uncertainties were assessed through one-way sensitivity analyses.
Comparators:
LCIG, providing patients with continuous dopaminergic stimulation to maximise functional ON time during the day and SC, defined as medically determined best available oral medication.
Main outcome measures:
Cost-effectiveness, based on quality adjusted life years gained, presented as an incremental cost-effectiveness ratio.
Results:
Lifetime analysis yields an incremental cost per QALY of £36,024 for LCIG compared to SC (incremental cost £39,644, QALY gain 1.1). Results were sensitive to time on treatment, health state on treatment initiation, and estimates of long term benefit (OWSA results from £32,127 to £66,421 per QALY). Findings must be considered in the context of the study limitations which were mainly due to data availability constraints.
Conclusions:
LCIG is an effective treatment, reducing OFF time and improving quality of life in advanced PD. It provides value for money in levodopa-responsive aPD patients with severe motor fluctuations when no other treatment options are effective or suitable. Given LCIG is an orphan drug, it is reasonable to suggest that it may be considered cost-effective in the UK setting. However, further research is needed to complete current data gaps and increase robustness of the model.
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disease that causes significant disability, substantially impacting patients’ activities of daily life. PD symptoms are characterised by increasingly incapacitating movement difficulties. Symptoms are well controlled in the early stages of disease; however, with disease progression patients spend increased proportions of waking time experiencing a loss of treatment effect, an ‘OFF state’. The presence of the OFF state typically develops after 4–6 years of therapy. It is a particularly debilitating aspect of the disease characterised by patients having trouble controlling movements and experiencing tremor, stiffness, slowness of movement and/or periods of unpredictable immobilityCitation1. In advanced PD, patients’ and carers’ quality of life (QoL) is severely impactedCitation2–5 and caregiver burden is considerableCitation6–9.
The management of advanced PD (aPD) is challenging and there is a considerable unmet need for effective, symptomatic treatments that can reduce the amount of time patients spend in an OFF state. Current treatments include oral strategies and, less commonly, infusion strategies (such as apomorphine or intraduodenal levodopa) and brain surgery such as deep brain stimulation (DBS) techniques. For those patients who have failed on or are unsuitable for apomorphine, or are inappropriate candidates for DBS treatment, treatment choices are limitedCitation10. Levodopa/carbidopa intestinal gel (LCIG, Duodopa ) provides continuous dopaminergic stimulation.
LCIG has recognised orphan drug status in the USCitation11, EuropeCitation12, AustraliaCitation13, and JapanCitation14 and is indicated for the treatment of advanced levodopa-responsive PD patients with severe motor fluctuations and hyper-/dyskinesia when available combinations of medicinal products have not given satisfactory results. The orphan drug legislation aims to support the principle of equity in access to treatment for patients suffering from rare conditions. The EU criteria for an orphan drug is “a medicine intended for the diagnosis, prevention or treatment of a life-threatening or chronically debilitating condition affecting 5 cases or less per 10,000 persons, or if it has a higher prevalence but it is unlikely that marketing the compound would be economically attractive and there is no effective alternative”Citation15. According to the legislation, these patients should be entitled to the same opportunity in receiving treatment as other patients with more frequently occurring disordersCitation16. LCIG is indicated for a very limited patient population as it is a last resort treatment – patients are only treated with LCIG if available combinations of PD medicinal products have not given satisfactory results or if they are contraindicated. Therefore approximately only 1% of the total PD patient population is likely to be suitable for LCIGCitation17,Citation18. It is important to bear in mind that since the number of patients suffering from rare diseases is small, it is difficult to enrol sufficient patients into a standard randomised controlled trialCitation16.
Cost-effectiveness analysis is an important decision-making tool and is increasingly relied upon by payers to inform decisions around treatment availability and appropriate allocation of resources. Until recently, treatments for orphan indications were viewed with less rigour; however, the reimbursement landscape is changing and increasing scrutiny is applied to the cost-effectiveness of these products too. This paper reports an economic analysis for LCIG based on available clinical data and the results of a recently published costing studyCitation19. The objective of the analysis was to update previously developed models assessing the cost-effectiveness of LCIG relative to standard care (SC) in patients with advanced PD (H&Y ≥ 3, OFF > 50%) in the UK setting. The small patient population of this orphan indication resulted in limited availability of efficacy and cost data making an economic assessment particularly difficult. Data gaps were supplemented by regression analyses, extrapolated data, and best estimates. Some of the data used in the analysis is yet to be published, mainly because the trials are still ongoing. This data will be made available as soon as possible and in the meantime interested readers can contact the authors for further detail. In order to address the uncertainties in the data, a number of one-way sensitivity analyses were undertaken to identify their impact on the outcomes of the economic evaluation.
Methods
Model structure
A simple Markov model was developed to quantify the costs and outcomes associated with the use of LCIG and standard care (SC) for the treatment of aPD. The model was based on a previous simulation model used in a reimbursement submission to the Dental and Pharmaceutical Benefits Board in Sweden (Tandvårds- och läkemedelsförmånsverket, TVL)Citation20. The perspective of the analysis was that of the UK National Health Service (NHS) including Personal Social Services (PSS). SC was defined as medically determined best available oral treatment. The comparator is appropriate according to the licensed indication of LCIG which is for use when other treatments do not give satisfactory results or are not indicated; oral strategies are in this case the only option available for aPD patients. The model cohort comprised aPD patients with H&Y status 3, 4 or 5 experiencing more than 50% of waking time in the OFF state at treatment initiation. These characteristics are representative of patients initiating treatment with LCIG. A lifetime time horizon was applied; however, LCIG treatment was truncated at 5 years with the assumption that after 5 years patients revert back to standard care with orals.
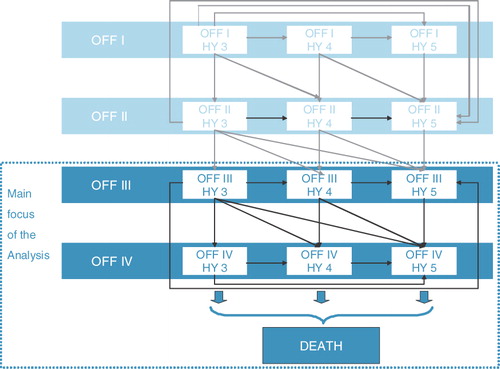
A schematic overview of model structure and health states is given in . The model comprised 12 aPD health states plus death. Cycle length was 6 months. Each health state was associated with costs derived from the UK costing study and utility weights. Costs and outcomes were both discounted at 3.5% annually. PD health states were defined by H&Y staging (stages 3, 4 or 5) and amount of OFF time experienced per waking hours (0–25% time in OFF = OFF I, 26–50% = OFF II, 51–75% = OFF III, 76–100% = OFF IV). Transition probabilities between health states were defined on the basis of progression rates reported for SC (), adjusted by expected treatment effects in the LCIG armCitation21–23. During the first cycle of the model, patients may experience improvement due to their treatment. In subsequent cycles, patients can move to a worse H&Y state and/or any adjacent worse OFF category, but cannot experience improvement. This is an established approach to measurement of progression in PD patientsCitation21–23. The extent of improvement is detailed in the clinical data section below.
Table 1. Transition probabilities (per 6 month cycle): natural history data.
Input data
Clinical data
Patients experience initial benefit from LCIG demonstrated through improvement in H&Y score and the amount of time spent in the OFF stateCitation24,Citation25. The modelled improvement in H&Y scores was based on benefit after 6 weeks of LCIG treatment using evidence from a pooled analysis of two LCIG clinical studies (n = 30)Citation26. The transitions reported thereCitation26 were used to inform the distribution of patients across H&Y states at the completion of cycle 1 ().
Table 2. Cohort health state distribution inputs: health state distribution prior to treatment initiation and at the completion of cycle 1.
There are a limited number of publications which explicitly assess the impact of the current LCIG formulation on OFF time. Initial improvement in OFF state, reported as reduction of time in OFF state, was informed by a trial from Eggert and colleagues who studied LCIG’s impact on motor and non-motor symptomsCitation25. In the base case, the clinical focus is on the sub-group of patients experiencing more than 50% OFF time on treatment initiation – the scenario in line with expected clinical implementation of LCIG. For these patients a mean reduction in OFF time from 60% (baseline) to 15% (at 6 months) was reported (n = 8). All LCIG patients were therefore distributed in OFF I post initial treatment while SC patients were distributed across OFF II and OFF III () based on the baseline distribution of patients in the trialCitation25.
Following initial benefit patients can only remain in the same state or deteriorate. For deterioration to a worse state it is assumed that LCIG patients do so at a slower rate than those patients on SC. For OFF state transitions, based on calculations from limited data as outlines below, a relative OFF progression rate of 0.5 for LCIG, i.e. 50% fewer LCIG patients worsening during each 6 month cycle compared to SC, is assumed. The estimate reflects continued positive effects of treatment observed 4 to 7 years after treatment initiation reported from the long-term study of LCIG use in aPD patientsCitation27,Citation28. Specifically, it is based on indirect data suggesting sustained benefit on measurements of hyperkinesias in aPD patients treated with LCIGCitation27,Citation28. The impact of this assumption on the model outcomes is assessed in sensitivity analyses. No additional impact on H&Y progression over the initial treatment effect for either treatment arm is assumed.
The rate and types of device related adverse events (AEs) associated with LCIG that are included in the model are based on data reported for the original administration device from the long-term NPP-002-02 safety studyCitation28 (). SC regimen AEs are not considered within the model, a conservative approach since it is likely that they would result in resource-incurring AEs.
Table 3. LCIG device related complications, from NPP-002-02.
Patients who drop out of LCIG treatment revert to SC rates of efficacy and resource utilisation. Drop-out rates from the NPP-002-02 studyCitation28 were estimated at 15.38% for the first treatment cycle and 1.11% for subsequent cycles. People who dropped out from treatment because they died were excluded from the drop out calculation as their mortality is reflected in the background mortality rate (transition probabilities from each state to death).
Resource use
Mean resources associated with LCIG and SC were estimated based on clinical usage and additional assumptions of resources validated by a PD clinician. Medication usage under standard care and LCIG was based on the NPP-001-02 DIREQT studyCitation29. Unit costs were based on costs from the British National Formulary, March 2009 and were applied to the reported dosages in order to estimate cycle treatment costs. Hospital costs are taken from the UK NHS Reference Costs 2007–2008. At the start of treatment, LCIG patients experience a test phase involving administration via a temporary nasoduodenal tube. During the test the viability of the treatment is assessed. A positive test of the clinical response to LCIG results in surgery to place a permanent tube for chronic administration. Test phase costs were included as a separate component since all patients in the LCIG arm experience this cost. Follow up visits and resource use due to adverse events were estimated and validated by the PD clinician. Note that it is conservatively assumed that LCIG patients will be managed by a multi-professional team resulting in higher unit cost per follow up visit for LCIG patients. Annual medical resource use and costs specific to LCIG and SC are detailed in .
Table 4. Medical resource use and costs related to LCIG and SC, annual.
Costs
Health state costs were estimated from the results of an observational study of PD by the Adelphi Group, based on defined Disease-Specific Programmes (DSP) methodologyCitation30, conducted through neurologists and geriatricians from hospitals across the UK from March to July 2008 (). Resource use from the DSP data comprised hospitalisations, consultations and tests, institutional care, professional caregiver and respite care costs. Mean health state costs were estimated using a simple ordinary least squares regression (Appendix). No cost was applied to death within the model.
Table 5. Health state costs and quality of life utilities.
Utilities
Health state quality of life utilities were estimated from an interim analysis of an ongoing study (DAPHNE: Duodopa in Advanced Parkinson’s: Health outcomes and Net Economic Impact, 2007 [data on file, to be published after study finalisation, Abbott]) as used in the original TLV analysis for Sweden. EuroQol Group 5-Dimension Self-report Questionnaire (EQ-5D) data were reported by H&Y status and time spent in OFF and utility weightings were estimated for each health state employed in the model. Due to the small patient numbers in some health states, data from six health states were excluded and re-calculated based on a series of extrapolation calculations ().
Sensitivity analyses
To assess the impact of uncertainty on core model outputs, a series of structured one-way sensitivity analyses (OWSAs) were conducted. Clinical and cohort OWSAs included scenarios widening/narrowing the inclusion criteria for the initial cohort, changed assumptions for initial treatment effect on OFF time, and an assumption of no additional benefit of LCIG on OFF progression over time. Treatment-related OWSAs included variation in reported drop-out/adverse event rate within plausible ranges and removal of the assumption that LCIG treatment was truncated at 5 years. Health state costs, discount rates and the time horizon of the analysis were also varied to assess their impact on the results.
Results
Base case
In the base-case analysis, per patient costs over the lifetime of the patient were estimated at £201,192 for LCIG compared to £161,548 for standard care. Expected life years gained per patient were 5.30 for LCIG and 4.53 for SC and the expected per patient quality adjusted life years (QALYs) were estimated at 1.88 and 0.78 respectively. The model estimated an incremental cost per life year gained (LYG) for LCIG relative to SC of £51,741 and a cost per QALY of £36,024.
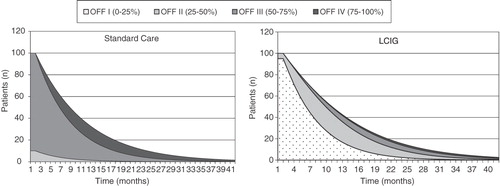
Additional analyses explored the impact of treatment on the time spent in OFF states. The expected distribution of patients over time in terms of the time spent within OFF states differed according to the treatment arm (). LCIG patients spent less time in higher OFF (=worse) states compared to patients maintained on standard care. This is important as the percentage of OFF time significantly impacts QoL and provides a transparent measure of symptom severity. Comparison of the graphs in illustrates the better prognosis for patients managed on LCIG (more time at less advanced health states).
One-way sensitivity analyses (OWSA)
The base case defines a clinically viable construct for the expected use of Duodopa within a clearly defined patient population; however, these results need to be considered in light of the uncertainty around key model parameters and can perhaps best be interpreted by considering a range of potential outcomes which are generated when key parameters are varied within plausible limits.
The results of the core sensitivity analyses are summarised in with findings discussed below in order of their impact on model outcomes; generated ICERs ranged from £32,127 to £66,421 with assumptions relating to treatment duration, clinical benefit and cohort definition contributing the most uncertainty to the analysis:
Table 6. One-way sensitivity analyses.
The model was most sensitive to assumptions relating to treatment duration (OWSA 1) with LCIG variable. The base case assumption is that patients will be maintained on LCIG for a maximum of 5 years and then convert to SC. If this assumption is not considered applicable and patients are treated with LCIG until death, then according to this analysis, the expected ICER would be in the region of £66,000 per QALY gained.
The model was also sensitive to clinical assumptions relating to short-term and long-term benefit assumptions of LCIG (OWSA 2). The differences in distribution of patients in OFF states I to IV for LCIG patients compared to SC are due to a combination of initial treatment effect and the additional long-term benefits of treatment with LCIG. When reducing the initial benefit of LCIG on OFF time by assuming that only half of the patients move to OFF I (instead of 100% as in base case) and the other 50% move to OFF II the ICER increases to £55,800/QALY.
A similar effect is also observed when the long-term benefit of LCIG on OFF time relative to SC is reduced to zero (OWSA 3). The ICER is estimated at £48,000/QALY gained.
Broadening of the patient cohort also impacts considerably on the model outputs. When including patients experiencing lower OFF time (<50% waking hours) in the analysis (OWSA 4), the impact on the ICER is substantial (moving from £36,000 to £52,000). This is not unexpected as costs are significantly higher for higher OFF states so capacity to benefit in economic terms is greater when more advanced health states are considered. LCIG is, however, indicated for treatment of advanced levodopa-responsive Parkinson’s disease with severe motor fluctuations and hyper-/dyskinesia. Those patients can therefore by definition be assumed to be spending increased time in OFF which is consistent with our base case analysis.
The model was also sensitive to the time frame of analysis (OWSA 5), when results were analysed over a shorter 5-year timeframe, the ICER generated increased to £50,948 per QALY. Shorter analysis time frames reflect the uncertainty around longer term outcomes, we might, however, argue that this timeframe is at the limit of plausibility as PD is a chronic disease and outcomes should ideally be considered over the lifetime of the patient even if assumptions relating to longer term events need to be made.
The model was less sensitive to health state costs in that use of observed cost (OWSA 6) data rather than corrected data generated an ICER of £43,060 per QALY. The model benefits from reference to a recent observational, cross-sectional study and a subsequent cost analysis, estimating the health state costs associated with the management of aPD from a UK perspectiveCitation19. There was, however, an absence of data for the costing of the more advanced health states due to small patient numbers resulting in non-robust estimates of cost at higher health statesCitation19. In order to smooth these data a standard regression model was applied to the costing study outputs. While statistically robust, this is a limitation of the analysis. The preferred method would be to rely solely on observed costs of a sufficient number of patients; however, due to current data constraints this is not practical. Therefore we might argue that the OWSA which considers the observed costs is not a reflection of expected cost in this patient cohort.
Table 7. Cost regression estimates.
Excluding patients with health state HY 3 did not have substantial impact on the overall results (OWSA 7); the ICER increased to £40,000 per QALY.
The model showed limited sensitivity to the assumptions around the drop-out rate (OWSA 8). Baseline data are estimated on the basis of long-term LCIG treatment data with per cycle drop out rates estimated at 15.4% (initial cycle) and 1.1% (subsequent cycles). These baseline data approximate the drop-out profile found in the Eggert studyCitation25 on which the initial effect assumptions were based. However, there is uncertainty around these data as the data source is from a relatively limited sample where longer term outputs are continually updated as patient cohorts progress through treatment. Ideally, drop-out rates would be obtained from prospective studies, but long-term RCT data are currently lacking. Assuming variation of drop out rates within clinically plausible limits, the ICERs generated ranged from 32,000 to 40,000. If higher drop-out rates are assumed, model outputs begin to approximate the ICER threshold at which NICE considers new interventions to be cost-effective. Only further exploration of continuation data can indicate which scenario is the more realistic.
The model demonstrated no sensitivity to adverse event rates (OWSA 9) or the discount rate (OWSA 10).
Discussion
The base case analysis found a substantial incremental gain in QALYs for LCIG compared to standard care (1.1 QALYs) and an associated incremental cost per QALY gained of approximately £36,000/QALY. Improvements in QoL are substantial and should not be ignored despite the high attendant cost of LCIG. LCIG could be considered cost-effective in comparison to SC when other treatment options are either ineffective or unsuitable. Other treatments for aPD yield not dissimilar results – a recent cost-effectiveness analysis of deep brain simulation (DBS) compared to best medical treatment in Spain reported an ICER of approximately £29,000 (€34,389)Citation31. However, due to the high degree of uncertainty around key model parameters introduced through limited sample size and data availability, outputs might best be considered within the range of ICERs generated across both base case and plausible sensitivity analysis scenarios.
This model supersedes previous LCIG economic models in both structure and data inputs in order to provide a transparent, substantiated model that can be used to demonstrate the cost-effectiveness of LCIG in a UK setting. The benefits of this analysis include the use of new clinical and cost data for a population in an orphan drug setting. Despite these improvements the findings must be considered in the context of the study limitations which were mainly around limited data availability and the robustness of available sources. It was necessary to use data from disparate sources, mainly derived from studies with small numbers of patients. In addition, parts of the analysis needed to rely on currently unpublished data which may raise additional questions regarding credibility and applicability of the findings. It is recognised that these imposed limitations are not ideal for an economic analysis aimed at drawing conclusions on the cost-effectiveness of one treatment method compared to another. On the other hand, summarising existing evidence in an economic model and transparently explaining its limitations helps to increase the knowledge in this area and guides future research.
The model’s uncertainties were addressed in a series of comprehensive OWSAs which are discussed in detail in the Results section of this paper and a probabilistic sensitivity analysis (PSA) was not conducted. Definition of distributions around key parameters would have been difficult making implementation of a PSA module both challenging and complicated to interpret meaningfully. In the sensitivity analyses treatment duration and clinical and cohort assumptions were found to be most influential on the overall outcomes. Given the age and disease severity of the patients and limited data beyond 5 years, a 5 year treatment duration was thought to be a reasonable base case assumption. The model was also sensitive to long-term and short-term efficacy parameters. This is particularly important to know as the data used were based on clinical data from a very small clinical trial (OFF inputs based on Eggert et al.Citation25, n = 8) and yet to be published data (treatment improvement in H&Y based on meta-analysis by Neville et al.Citation26. Further clinical trials and publication of existing data will need to substantiate the currently used data on LCIG’s efficacy. Another influential variable in the model was the costs associated with each health state but again the data used were based on a small sample size and there were data gaps in the most severe states. Further studies to assess costs, with a particular emphasis on reaching higher sample sizes in more advanced OFF states would be helpful to better understand the true costs associated with these patients.
As additional clinical information and data on costs becomes available analysis updates are imperative in order to better perform a more robust analysis of the uptake of LCIG in this patient group. At the same time it would be interesting to include the costs and benefits to carers and families who may be able to decrease the quantity or intensity of care they provide as a result of LCIG treatment. Registries have been suggested as a method of obtaining clinical and cost data for orphan drugs. They also aid in identifying patients for future clinical trials and any proposed post-licensing studiesCitation16. For LCIG several registries have been started and their outcomes will be published upon completion.
In the UK, health technology assessment bodies such as the National Institute for Health and Clinical Excellence (NICE) in England and Wales, and the Scottish Medicines Consortium (SMC) in Scotland assess cost-effectiveness of technologies. Orphan drugs are currently subject to the same metrics for assessment of cost-effectiveness as conventional technologies despite the data limitations associated with these treatments. As found when developing this analysis, orphan drugs face the problem of very small patient populations that introduce wide ranges of uncertainty around effectiveness, limiting the ability to provide robust evidence of cost-effectiveness. In a cost-constrained NHS this can make it difficult for cost-effectiveness to be evaluated and for the treatments to be considered for funding. However, the innovative nature of orphan treatments alongside a considerable unmet need in extremely small patient populations could be argued to fall outside the remit of threshold-based decision making toolsCitation32. In addition it is suggested that the level of evidence required to support a decision to adopt a technology should depend on the consequences of the uncertainty – that is, if an uncertain decision proves to be wrong, how much will society lose in terms of resources and health outcomes forgoneCitation33? As the expected cost of uncertainty is largely determined by the number of patients affected this impact will be lower for orphan drugsCitation33 and definition of cost-effective interventions might be considered within the constructs of this paradigm.
Patients with advanced PD who are not eligible for alternate treatments are currently maintained on standard care and show a clear unmet need for alternative effective treatment options. Our model found a substantial incremental gain in QALYs for LCIG compared to standard care (1.1 QALYs) and an associated incremental cost per QALY gained of approximately £36,000/QALY. LCIG could therefore be considered cost-effective in the limited cohort of patients for whom it is indicated. The findings of this analysis must be considered in the light of the limitations outlined but also in the context of the orphan status of LCIG. Although orphan drugs are expensive in comparison to standard treatments, the small number of eligible patients restricts the total costs and consequently the total budget impact is limited.
Transparency
Declaration of funding
This study was funded by Abbott Healthcare Products Ltd, manufacturer of LCIG, marketed as Duodopa. The funding body gave access to currently unpublished data and provided assistance in study design, data analysis and result interpretation.
Declaration of financial/other relationships
J.L., C.R., and E.W. are employees of IMS Health who received consultancy fees from Abbott. A.B. and M.S. were/are employees of Abbott and contributed to manuscript and review before submission. K.R.C., L.J.F., and S.M. supported the study with their clinical and health economic expertise, contributed to manuscript and review, and received honoraria for this work.
Acknowledgements
No assistance in the preparation of this article is to be declared.
Notes
*Duodopa is a registered trade name of Abbott, UK.
References
- Dewey RB Jr. Management of motor complications in Parkinson's disease. Neurology 2004;62:S3-7
- Factors impacting on quality of life in Parkinson's disease: results from an international survey. Mov Disord 2002;17:60-7
- Chrischilles EA, Rubenstein LM, Voelker MD, et al. The health burdens of Parkinson's disease. Mov Disord 1998;13:406-13
- Keranen T, Kaakkola S, Sotaniemi K, et al. Economic burden and quality of life impairment increase with severity of PD. Parkinsonism Relat Disord 2003;9:163-8
- Schrag A, Jahanshahi M, Quinn N. How does Parkinson's disease affect quality of life? A comparison with quality of life in the general population. Mov Disord 2000;15:1112-18
- Carter JH, Stewart BJ, Archbold PG, et al. Living with a person who has Parkinson's disease: the spouse's perspective by stage of disease. Mov Disord 1998;13:20-8
- Martinez-Martin P, ito-Leon J, Alonso F, et al. Quality of life of caregivers in Parkinson's disease. Qual Life Res 2005;14:436-72
- Martinez-Martin P, Forjaz MJ, Frades-Payo B, et al. Caregiver burden in Parkinson's disease. Mov Disord 2007;22:924-31
- Martinez-Martin P, Arroyo S, Rojo-Abuin JM, et al. Burden, perceived health status, and mood among caregivers of Parkinson's disease patients. Mov Disord 2008;23:1673-80
- National Collaborating Centre for Chronic Conditions. Parkinson's Disease: National Clinical Guideline for Diagnosis and Management in Primary and Secondary Care. London: Royal College of Physicians, 2006. Report No.: CG35
- Department of Health & Human Services. Levodopa/carbidopa as orphan drug, application #99-1294, 2000
- European Commission – Enterprise and Industry Directorate General – Consumer goods – Pharmaceuticals. Levodopa and Carbidopa (Gastroenteral use) EU Orphan Designation number: EU3/01/035. 2008. Available http://ec.europa.eu/health/documents/community-register/html/o035.htm (Last accessed 8 December 2011)
- Department of Health of Ageing Therapeutic Goods Administration (2006). Duodopa (levodopa 20 mg/mL and carbidopa 5 mg/mL) intestinal gel suspension – Orphan Drug Application, Submission No. 2006/0636/1. 2006
- Department of Health, Labor and Welfare. Designation number 224. 2009
- European Commission. The Orphan Drugs Strategy. 2007
- Drummond MF, Wilson DA, Kanavos P, et al. Assessing the economic challenges posed by orphan drugs. Int J Technol Assess Health Care 2007;23:36-42
- Frankel J. Verification of epidemiological and clinical assumptions for Duodopa Budget Impact Model. 2006 (personal communication)
- Schrag A, Quinn N. Dyskinesias and motor fluctuations in Parkinson's disease: a community based study. Brain 2000;123:2297-305
- Findley L, Wood E, Lowin J, et al. The economic burden of advanced Parkinson's disease: an analysis of a UK patient dataset. J Med Econ 2011;14:130-9
- Willis M, Persson U, Zoellner Y, et al. Reducing uncertainty in value-based pricing using evidence development agreements. The case of continuous intraduodenal infusion of levodopa/carbidopa (Duodopa®) in Sweden. Applied Health Economics & Health Policy 2010;8:1-10
- Davey P, Rajan N, Lees M, et al. Cost-effectiveness of pergolide compared to bromocriptine in the treatment of Parkinson's disease: a decision-analytic model. Value Health 2001;4:308-15
- Palmer CS, Schmier JK, Snyder E, et al. Patient preferences and utilities for 'off-time' outcomes in the treatment of Parkinson's disease. Qual Life Res 2000;9:819-27
- Palmer CS, Nuijten MJ, Schmier JK, et al. Cost effectiveness of treatment of Parkinson's disease with entacapone in the United States. Pharmacoeconomics 2002;20:617-28
- Antonini A, Isaias IU, Canesi M, et al. Duodenal levodopa infusion for advanced Parkinson's disease: 12-month treatment outcome. Mov Disord 2007;22:1145-9
- Eggert K, Schrader C, Hahn M, et al. Continuous jejunal levodopa infusion in patients with advanced parkinson disease: practical aspects and outcome of motor and non-motor complications. Clin Neuropharmacol 2008;31:151-66
- Neville A, Parson R, Nyhom D. Treatment of advanced Parkinson's disease with levodopa/carbidopa intestinal gel is associated with improvements in Hoehn and Yahr stage. Submitted 2011
- Nilsson D, Nyholm D, Aquilonius SM. Duodenal levodopa infusion in Parkinson's disease – long-term experience. Acta Neurol Scand 2001;104:343-8
- Nyholm D, Lewander T, Johansson A, et al. Enteral levodopa/carbidopa infusion in advanced Parkinson disease: long-term exposure. Clin Neuropharmacol 2008;31:63-73
- Nyholm D, Remahl N, Dizdar N, et al. Duodenal levodopa infusion monotherapy vs oral polypharmacy in advanced Parkinson's disease. Neurology. 2005 Jan 25;64(2):216-23
- Anderson P, Benford M, Harris N, et al. Real-world physician and patient behaviour across countries: Disease-Specific Programmes – a means to understand. Curr Med Res Opin 2008 Nov;24(11):3063-72
- Valldeoriola F, Morsi O, Tolosa E, et al. Prospective comparative study on cost-effectiveness of subthalamic stimulation and best medical treatment in advanced Parkinson's disease. Mov Disord 2007;22:2183-91
- National Institute for Health and Clinical Excellence. Appraising Orphan Drugs (Draft 3). Available at: http://www.nice.org.uk/niceMedia/pdf/smt/120705item4.pdf [Last accessed 13 December 2010]
- McCabe C, Claxton K, Tsuchiya A. Orphan drugs and the NHS: should we value rarity? BMJ 2005;331:1016-19
- Department of Health. NHS Reference Costs Appendix NSRC04 NHS Trust and PCT Combined Reference Cost Schedules. Available at: http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_098945 (Last accessed 13 December 2010)
- Honig H, Antonini A, Martinez-Martin P, et al. Intrajejunal levodopa infusion in Parkinson's disease: a pilot multicenter study of effects on nonmotor symptoms and quality of life. Mov Disord 2009;24:1468-74
- Curtis L. Unit Costs of Health and Social Care. Personal Social Services Research Unit, University of Kent. 2008. Available at: http://www.pssru.ac.uk/uc/uc2008contents.htm (Last accessed 15 December 2010)
- Joint Formulary Committee. British National Formulary [57]. London: British Medical Association and Royal Pharmaceutical Society of Great Britain, 2009
Appendix
The majority of patients in the Adelphi dataset (68%) had an H&Y score of III or IV and reported 0–25% of their time in an OFF state. Data to directly inform some of the more advanced model health states were limited. In order to estimate resource use in the more severe states, a GLM regression model with gamma distribution was developed to estimate the relationship between H&Y score and OFF time to resource use costs based on Adelphi data for patients in H&Y 3,4 or 5 (n = 302)Citation19.