Abstract
Background:
Personalized medicine requires diagnostic tests that stratify patients into distinct groups that may differentially benefit from targeted treatment approaches. This study compared the costs and benefits of two approaches for identifying those at high risk of developing type 2 diabetes for entry into a diabetes prevention program. The first approach identified high risk patients using impaired fasting glucose (IFG). The second approach used the PreDx Diabetes Risk Score (DRS) to further stratify IFG patients into high-risk and moderate-risk groups.
Methods:
A Markov model was developed to simulate the incidence and disease progression of diabetes and consequent costs and quality-adjusted life expectancy (QALY), comparing alternative approaches for identifying high-risk patients. We modeled direct medical costs, including the costs of the stratification testing, over a 10-year time horizon from a US payer perspective.
Results:
Stratification of IFG patients by the DRS method leads to improved identification and prevention among those at highest risk. At 5 years, the number needed to treat (NNT) in the IFG-only approach was 39 patients to prevent one case of diabetes compared to an NNT of 15 in the IFG + DRS approach. When compared to IFG alone, the IFG + DRS approach results in an incremental cost-effectiveness ratio (ICER) of $17,100/QALY gained at 5 years and would become cost saving in 10 years. In contrast and as compared to no stratification, the IFG-only approach would produce an ICER of $235,500/QALY gained at 5 years and $94,600/QALY gained at 10 years. The study findings are limited by the generalizability of the DRS validation study and uncertainty regarding the long-term effectiveness of diabetes prevention.
Conclusions:
The analysis indicates that the cost-effectiveness of diabetes prevention can be improved by better identification of patients at highest risk for diabetes using the DRS.
Introduction
There is a growing body of evidence that the onset of type 2 diabetes can be successfully delayed or prevented by therapeutic interventions, including lifestyle modifications and pharmacological treatmentCitation1–4. Targeting these programs to those at highest risk for developing diabetes may lead to cost-effective prevention strategies. Current risk assessment tools identify large segments of the population as ‘at risk’ for diabetes, making it difficult to implement cost-effective diabetes prevention programs. For example, the Centers for Disease Control and Prevention (CDC) has estimated that approximately 26% of US adults have impaired fasting glucose (IFG, fasting glucose of 100–125 mg/dL)Citation5 and approximately 34% of adults meet the criteria for metabolic syndromeCitation6. Although these individuals are considered ‘at risk’, the reported annual incidence of diabetes among IFG patients was 1.95%Citation7. Nonetheless, the American Diabetes Association (ADA) has recently recommended that all patients with IFG be referred to an effective ongoing support program for weight lossCitation8. Thus, more accurate tools for identifying those at highest risk for diabetes are needed to advance diabetes prevention.
The PreDx Diabetes Risk Score (DRS) is a multi-marker risk stratification tool that allows for more accurate assessment of diabetes riskCitation9. Based on serum biomarkers measured in a fasting blood sample, the DRS provides a validated and personalized risk score that distinguishes among people at high, moderate, and low risk of type 2 diabetesCitation10. This allows physicians to focus interventions on the relatively few patients who are genuinely at high risk of developing diabetes and to avoid unnecessary treatment and expenses for patients less likely to develop diabetes within the next five years.
The aim of this study was to compare the potential costs and health benefits of two approaches for identifying those at high risk of developing type 2 diabetes for entry into a diabetes prevention program. The first approach used fasting plasma glucose (FPG) and identified patients with IFG as high risk. The second approach used the DRS to further stratify patients with IFG into high-risk and moderate-risk groups. The potential cost-effectiveness of each of these two approaches was estimated using separate Markov models that placed the high-risk groups identified by each approach into a simulated prevention and surveillance program.
Methods
Model overview
We developed a Markov model to simulate the incidence and disease progression of diabetes and the consequent costs and quality-adjusted life expectancy (QALY) comparing alternative approaches for identifying patients to be in a diabetes prevention and surveillance program. The perspective of a United States (US) healthcare system was used, i.e., including only direct medical costs. The cost-effectiveness of alternative scenarios was examined over 5–10 year time horizons with 5 years as the base-case horizon. All costs and utilities were discounted at a 3% annual rate. The model was implemented in TreeAge Pro Suite (R1.0.2 edn, TreeAge Software, Inc., Williamstown, MA, USA) in two modules: a decision-analytic model for patient stratification and a Markov model for the progression of health states (). The stratification was a single, initial event and the Markov model was implemented using a recurring 1-year cycle. Key model parameters are shown in .
Figure 1. Decision analytic model comparing different approaches for identifying patients at high risk for developing type 2 diabetes for entry into a diabetes prevention and surveillance program leading to a Markov model for the progression of diabetes related health states.
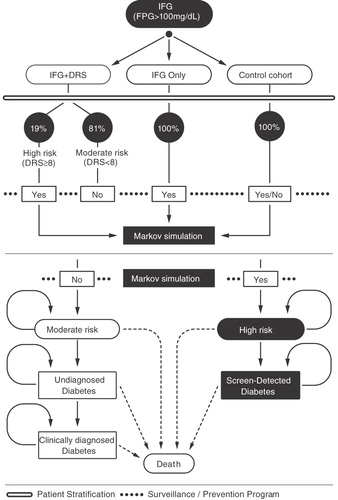
Table 1. Model parameters.
Population stratification and intervention pathways
Two diabetes prevention approaches were modeled: ‘IFG-only’ and ‘IFG + DRS’. The IFG-only approach placed all patients with IFG into a diabetes prevention and surveillance program. The IFG + DRS approach placed patients into a prevention and surveillance program who had IFG and also were identified as high risk by the DRS test. Both approaches were compared to a control cohort where only the surveillance component of the program was utilized. The control cohort thus represents a ‘wait and watch’ approach as a baseline against which to compare the incremental benefits of the two prevention scenarios.
The population modeled was non-diabetic patients with IFG, defined according to ADA guidelinesCitation8. Demographics and clinical characteristics of the population were modeled on the Inter99 study in which the DRS was previously developed and validatedCitation10.
Treatment benefit was modeled using the reported risk reduction in the metformin arm of the Diabetes Prevention Program (DPP) trialCitation3, i.e., 31% over three years among surviving patients. The metformin arm was chosen for modeling because the cost of metformin treatment is well documented and relatively inexpensive. Although the lifestyle arm of the DPP trial was more efficacious, interventions were fairly extensive and may not be realistic for general application. The efficacy of less intensive, presumably more sustainable, lifestyle interventions is not known. The efficacy of treatment was assumed to be constant throughout the maximum time horizon of 10 years. Surveillance for development of diabetes consisted of an annual oral glucose tolerance test (OGTT), a fasting plasma glucose (FPG) test and one additional primary care office visit per year. Additionally, the first year included a second office visit for treatment assessment and possible drug dose titration.
Disease progression in the Markov model
Probabilities of progression to diabetes were determined by (1) the performance of the risk stratification method employed as estimated by annualizing the observed 5-year risk in the Inter99 cohort and (2) the effectiveness of the prevention program. The overall 5-year prior risk of conversion to type 2 diabetes was 7.66%, from which progression probability to undiagnosed diabetes in the control cohort and to screen-detected diabetes in the IFG-only approach was computedCitation10. In the IFG + DRS approach, 5-year prior risk was 21.46% in the high-risk population and 4.4% in the moderate-risk population, from which probability of progression to screen-detected and undiagnosed diabetes was computed, respectively. All subjects were assigned to the prevention and surveillance program in the IFG-only approach. In the IFG + DRS approach, 19% of subjects were expected to have high DRS scores and were assigned to the prevention and surveillance program accordingly. All subjects were assigned to the surveillance program in the control cohort. Progression from high-risk to screen-detected diabetes in all scenarios was computed by multiplying the annualized prior risk of patients in the prevention program and the relative risk induced by the intervention. The risk of developing diabetes among patients in the prevention program was extracted from the metformin arm of the DPP trialCitation3. The progression probability from undiagnosed to clinically diagnosed diabetes was estimated as a rate with a mean duration of 5.2 yearsCitation11. Mortality was computed using age and gender specific all-cause mortality in US Life Tables (http://www.who.int/healthinfo/morttables/en/index.html) adjusted by health-state specific mortality. The relative risk of death for patients with screen-detected diabetes compared to patients without diabetes was 2.34Citation12. The relative risks of death in patients with undiagnosed and clinically diagnosed diabetes vs. patients with screen-detected diabetes were 1.231 and 1.098, respectivelyCitation12.
Costs and utilities
All costs were standardized to 2007 US dollars. Costs were included for the initial stratification test (DRS, FPG) and one additional primary care office visit using laboratory list prices or Medicare reimbursement rates (http://www.cms.hhs.gov/Reimbursement/). After initial stratification, costs were accumulated on an annual basis depending on age, health state and participation in the prevention program.
The cost of the diabetes prevention and surveillance program was comprised of yearly FPG and OGTT tests in conjunction with a primary care office visit. Intervention costs were derived from the metformin arm of the DPP trial with the modification of the drug component to $4/month, reflecting the current average list price of generic metformin.
Average annual direct medical costs were estimated from the formula of Trogdon et al.Citation13. The baseline formula accounted for age, clinical diabetes status, and duration of diabetes state:
where DM = 1 if the patient had diabetes and DM = 0 if not. The factor of 1.08 is the correction from 2005 to 2007 US dollars using the medical care component of the Consumer Price Index.
For example, a 49-year-old patient without diabetes is expected to average $4970 per year in direct medical costs. A similar patient with newly diagnosed diabetes would have an average of $9590 per year in direct medical costs. A 59-year-old patient who has had diabetes for 10 years would have average annual costs of $12,130. These costs are similar to those reported recently in Dall et al.Citation14. The cost of undiagnosed diabetes was estimated to be 0.5815 times the incremental cost of diagnosed diabetes vs. diabetes-free as reported in Zhang et al.Citation15, which is consistent with the values reported in Palmer et al.Citation16 albeit slightly more conservative. The medical costs of an IFG as compared to normal fasting glucose (NFG) patient was 1.0867 as reported in Nichols et al.Citation17. The annual medical expenditure for patients varies with diabetes risk as a function of glucose status and other risk factorsCitation17–20. In order to estimate the cost of risk strata for novel risk stratification measures, for which no observational cost data exist, we assumed a risk-adjusted cost for each stratum constrained by the logical requirement that the total cost for the untreated population was the same for any stratification. This risk-adjusted cost was assumed to be linearly proportional to diabetes risk and was calibrated against reported cost differences for patients with IFG and NFG and known diabetes risk and population proportions for these two risk strataCitation17.
Estimates of health state utilities were derived from literature sources. Like utility estimates for other health states, estimates for diabetes vary widely based on setting, methodology used, and the specific health state description. From Grima et al., we used a value of 0.80 for the non-diabetic, 49-year-oldCitation21. This operated as a baseline where the critical differential was reduction in utility associated with being a newly diagnosed type 2 diabetes patient: the value of −0.14 was determined by calibration to the observed effectiveness in the DPP trial. The impact of the utility decrement on the cost-effectiveness was further assessed by examining results for utility reductions of −0.10 and −0.05. The model also differentiated between clinically diagnosed and new diabetes patients, with the latter having a slightly higher utility (by 0.017)Citation12.
Sensitivity analyses
To evaluate a scenario where IFG may be an alternative screening strategy, we computed the incremental cost-effectiveness ratios for IFG + DRS versus IFG only as a sensitivity analysis. In addition, we undertook a series of one-way sensitivity analyses to evaluate parameter uncertainty on modeled outcomes. One-way sensitivity analysis was performed on each input variable to determine which variable values had the largest impact on model results. Each variable was assessed at ±10% of the nominal value and ranked according to the spread of the incremental cost-effectiveness of the IFG + DRS scenario. Variables identified as accounting for 90% of the total spread in cost-effectiveness were selected for a multivariate probabilistic sensitivity analysis. Probabilistic sensitivity analysis also was performed with 1000 Monte Carlo simulations to estimate the probability of cost-effectiveness as a function of willingness-to-pay for an improvement in quality-adjusted survivalCitation22.
Results
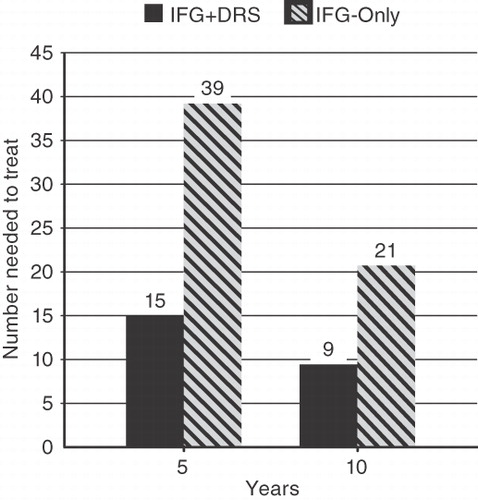
The NNT for each approach is shown in . After 5 years the IFG-only approach resulted in one case of diabetes prevented for every 39 patients in the prevention and surveillance program and the IFG + DRS approach resulted in one case of diabetes prevented for every 15 patients in the program. Clearly, subsequent stratification of IFG patients by DRS leads to greatly improved efficiency of diabetes prevention among those at highest risk. Although the NNT is reduced in both approaches for a 10 year period, the IFG + DRS resulted in a >2-fold reduction in the NNT for each case prevented.
Figure 2. Number of patients needed to treat among those identified as high-risk by the IFG-only approach and the IFG + DRS approach, for 5-year and 10-year model horizons.
The incremental cost-effectiveness ratios (ICER) as a function of time and the disutility of diabetes for the IFG-only and IFG + DRS approaches, relative to the control cohort, are shown in . At 5 years, the ICER of the IFG + DRS approach as compared to IFG alone is $17,400/QALY gained. The IFG + DRS approach would be cost saving by 10 years. In contrast, the IFG-only approach does not become cost-effective at a willingness-to-pay level of $50,000/QALY at any of the utility reductions evaluated throughout the 10-year time horizon of the model.
Table 2. Incremental cost-effectiveness of each strategy at 5 and 10 year horizons.
For the IFG versus IFG + DRS contrast, the IFG + DRS approach dominates the screen-only approach at 10 years and the incremental cost-effectiveness of treating all as compared to treating IFG + DRS positive is $218,200/QALY. At 5 years, the incremental cost-effectiveness of Treat-All vs. IFG + DRS is $432,700/QALY. The one-way sensitivity analysis indicated that four model parameters have a substantial impact on the estimated cost-effectiveness ratio. The parameters (variable range, cost-effectiveness) were: the 5-year risk of patients with high DRS scores (18–25%, $10,000–$26,700), the proportion of patients with high DRS scores (16%–22%, cost savings to $31,200), the intervention effectiveness (annualized relative risk of 0.58–0.72, $7300–$27,600) and the overall 5-year population risk of conversion to diabetes (6%–10%, $2200–$25,300).
A probabilistic sensitivity analysis was performed, simultaneously sampling the uncertainty distribution of the most important variables, for the 5-year analytic horizon. The willingness-to-pay threshold for a 50% likelihood of cost-effectiveness is approximately $240,000/QALY gained for the IFG-only approach and $18,000 for the IFG + DRS approach.
Discussion
Current ADA guidelinesCitation8 recommend that all subjects with IFG be referred to an effective ongoing support program for weight loss of 5–10% and that metformin be considered in subjects at very high risk for diabetes. According to the latest CDC estimatesCitation5 about 57 million US adults, or about 30% of adults, have IFG. A recent reportCitation23 estimates that one in 12 US adults has a combination of pre-diabetes and risk factors that may justify consideration of metformin therapy. Given the large proportion of the US population that meets these criteria, we sought to evaluate the cost-effectiveness of two approaches for identifying individuals who may develop diabetes in order to target interventions that have been shown to prevent the occurrence of diabetes. Our findings suggest that a simple risk stratification test when added to a standard IFG test can improve identification of individuals at high risk of developing diabetes and that such stratification may be cost-effective.
Two recent studies based on data from the DPP trial evaluated the cost-effectiveness of applying lifestyle and pharmacological interventions to individuals with impaired glucose toleranceCitation24,Citation25. Although both studies found that the DPP lifestyle and pharmacological interventions provided important clinical benefits, they disagreed as to whether these interventions would prove cost-effective over the long term. While structural differences between the studies could explain their differing conclusions, one factor that certainly affected both studies was the initial selection of high-risk individuals based on blood glucose values alone. Consistent with our findings, a method for distinguishing those most likely to progress to diabetes among patients identified as ‘at risk’ could make the difference in determining whether or not these interventions prove cost-effective.
While metformin is considered generally safe, over half of patients receiving the medication suffer gastrointestinal side effects including diarrhea, nausea, and vomiting. We speculate that, due to these common and uncomfortable side effects, compliance would be an issue in attempting to treat the 30% of US adults who could be given metformin by current guidelines to prevent diabetes. Improved risk assessment information and targeting of intervention could improve the compliance rates and thus outcomes in diabetes prevention efforts.
Several limitations of this study should be noted. While costs, conditional mortality rates, and intervention efficacy were drawn from studies on US cohorts, stratification estimates for the DRS test were based on the Inter99 cohort, a non-US population. The effectiveness of the prevention program from the DPP trial was assumed to be the same regardless of patient stratification, as data on treatment effectiveness and adherence to a primary prevention strategy among patients with high DRS test results is not yet available. In addition, for simplicity and transparency our model was designed to simulate only pre-diabetic and diabetic states and did not explicitly include co-morbidities. The effects of the co-morbidities on incremental cost and QALYs were captured in the average values for the population not in the prevention program. However, the effects of prevention and surveillance on the rates and severity of co-morbidities were not accounted for in this analysis and the cost-effectiveness may be underestimated for this reason. The cost formula from Trogdon and Hylands may introduce a possible bias. The formula stratifies diabetes as dichotomous (Yes/No), but our target population (patients at risk of diabetes) may have higher costs than those without diabetes. If this is true, then our incremental cost-effectiveness ratios may be biased downward. Finally, while our intent was to evaluate the cost-effectiveness of DRS, we acknowledge that other risk scoring tools have been reported in the literature and that our findings do not necessarily apply to these other methods of risk stratificationCitation26,Citation27. The findings suggest that modeled outcomes are sensitive to the proportion of patients with high DRS and the overall population risk indicate. Further investigation of different screening or pre-screening strategies may be a promising avenue for further optimizing cost-effectiveness and savings.
In conclusion, the analyses indicate that the cost-effectiveness of diabetes prevention programs can be greatly improved by the identification of those patients at highest risk for diabetes using the DRS test. As compared to treating all patients with IFG, an approach that is unlikely to result in net cost savings in this population, risk stratification using the DRS may ultimately result in net cost savings.
Transparency
Declaration of funding
The research reported in this paper was funded by a grant from Tethys Bioscience, Emeryville, CA, USA.
Declaration of financial/other relationships
S.D.S., L.P.G. and H.R. are consultants to Tethys Bioscience. J.K. and E.J.M. are employees of Tethys Bioscience.
Acknowledgments
We thank Bryan Walton for graphic illustrations and Linda Wuestehube for assisting with manuscript preparation.
References
- Buchanan TA, Xiang AH, Peters RK, et al. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk hispanic women. Diabetes 2002;51:2796-803
- Gerstein HC, Yusuf S, Bosch J, et al. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet 2006;368:1096-105
- Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393-403
- Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343-50
- National Diabetes Information Clearinghouse, National Diabetes Statistics, 2007. NIH Publication No. 08-3892. Available at: http://diabetes.niddk.nih.gov/DM/PUBS/statistics/DM_Statistics.pdf [Last accessed 29 September 2010]
- Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003–2006. Natl Health Stat Report 2009;13:1-7
- Nichols GA, Hillier TA, Brown JB. Progression from newly acquired impaired fasting glusose to type 2 diabetes. Diabetes Care 2007;30:228-33
- American Diabetes Association. Standards of medical care in diabetes – 2010. Diabetes Care 2010;33:S11-61
- Urdea M, Kolberg J, Wilber J, et al. Validation of a multimarker model for assessing risk of type 2 diabetes from a five-year prospective study of 6784 Danish people (Inter99). J Diabetes Sci Technol 2009;3:748-55
- Kolberg JA, Jorgensen T, Gerwien RW, et al. Development of a type 2 diabetes risk model from a panel of serum biomarkers from the Inter99 cohort. Diabetes Care 2009;32:1207-12
- Harris MI, Klein R, Welborn TA, et al. Onset of NIDDM occurs at least 4–7 yr before clinical diagnosis. Diabetes Care 1992;15:815-19
- Gillies CL, Lambert PC, Abrams KR, et al. Different strategies for screening and prevention of type 2 diabetes in adults: cost effectiveness analysis. BMJ 2008;336:1180-5
- Trogdon JG, Hylands T. Nationally representative medical costs of diabetes by time since diagnosis. Diabetes Care 2008;31:2307-11
- Dall TM, Mann SE, Zhang Y, et al. Distinguishing the economic costs associated with type 1 and type 2 diabetes. Population Health Management 2009;12:103-10
- Zhang Y, Dall TM, Mann SE, et al. The economic costs of undiagnosed diabetes. Population Health Management 2009;12:95-101
- Palmer AJ, Roze S, Valentine WJ, et al. Intensive lifestyle changes or metformin in patients with impaired glucose tolerance: modeling the long-term health economic implications of the diabetes prevention program in Australia, France, Germany, Switzerland, and the United Kingdom. Clin Ther 2004;26:304-21
- Nichols GA, Brown JB. Higher medical care costs accompany impaired fasting glucose. Diabetes Care 2005;28:2223-9
- Boudreau DM, Malone DC, Raebel MA, et al. Health care utilization and costs by metabolic syndrome risk factors. Metab Syndr Relat Disord 2009;7:305-14
- Nichols GA, Arondekar B, Herman WH. Complications of dysglycemia and medical costs associated with nondiabetic hyperglycemia. Am J Manag Care 2008;14:791-8
- Nichols GA, Arondekar B, Herman WH. Medical care costs one year after identification of hyperglycemia below the threshold for diabetes. Med Care 2008;46:287-92
- Grima DT, Thompson MF, Sauriol L. Modelling cost effectiveness of insulin glargine for the treatment of type 1 and 2 diabetes in Canada. Pharmacoeconomics 2007;25:253-66
- Briggs A, Claxton K, Sculpher M. Decision Modelling for Health Economic Evaluation. New York: Oxford University Press, 2008
- Rhee MK, Herrick K, Ziemer DC, et al. Many Americans have pre-diabetes and should be considered for metformin therapy. Diabetes Care 2009;33:49-54
- Eddy DM, Schlessinger L, Kahn R. Clinical outcomes and cost-effectiveness of strategies for managing people at high risk for diabetes. Ann Intern Med 2005;143:251-64
- Herman WH, Hoerger TJ, Brandle M, et al. The cost-effectiveness of lifestyle modification or metformin in preventing type 2 diabetes in adults with impaired glucose tolerance. Ann Intern Med 2005;142:323-32
- Wilson PW, Meigs JB, Sullivan L, et al. Prediction of incident diabetes mellitus in middle-aged adults. Arch Intern Med 2007;167:1068-74
- Collins GS, Altman DG. External validation of the QDScore for predicting the 10-year risk of developing type 2 diabetes. Diabetic Med 2011;28:599-607