Abstract
Objective:
To evaluate clinical and economic outcomes in patients with type 2 diabetes mellitus (T2DM) who failed oral anti-diabetic drug (OAD) therapy and initiated either insulin glargine with disposable pen (GLA-P) or exenatide BID (EXE).
Research design and methods:
This retrospective study used data from a large US-managed care claims database and included adult T2DM patients initiating treatment with GLA-P or EXE in 2007 or 2008. Propensity score matching was used to control observed baseline differences between treatment groups. Primary study end-points included treatment persistence, A1C, healthcare utilization, and healthcare costs during the 1-year follow-up period.
Results:
Two thousand three hundred and thirty nine patients were included in the study (GLA-P: 381; EXE: 1958); 626 patients were in the 1:1 matched cohort (54% male; mean age: 54 years; mean A1C: 9.2%). At follow-up, patients in the GLA-P group were significantly more persistent in treatment than EXE patients (48% vs 15% in persistence rate and 252 vs 144 days in persistence days; both p < 0.001). GLA-P patients also had significantly lower A1C at follow-up (8.02% vs 8.32%; p = 0.042) and greater A1C reduction from baseline (−1.23% vs −0.92%; p = 0.038). There were no significant differences in claims-based hypoglycemia rates and overall diabetes-related healthcare utilization and cost.
Limitations:
Since this was a retrospective analysis, causality of treatment benefits cannot be established. The study was specific to two treatments and may not generalize to other models of insulin administration. Some of the results, although statistically significant, may not be found clinically important.
Conclusions:
In a real-world setting among T2DM patients who failed to achieve or sustain glycemic goal with OADs, initiation of GLA-P instead of EXE may be a more effective option because it was associated with greater treatment persistence, greater A1C reduction without a significantly higher rate of hypoglycemia, and similar healthcare costs.
Introduction
Type 2 diabetes mellitus (T2DM) is a progressively worsening disease that requires increasing medical intervention over timeCitation1. In 2007, the total estimated cost of diabetes in the US was $174 billion, including $116 billion in excess medical expenditures and $58 billion in reduced national productivityCitation2. Diabetes is associated with complications and comorbidities that increase healthcare costsCitation3,Citation4. Achieving and maintaining glycemic control can delay or prevent diabetes-related complicationsCitation5,Citation6 and reduce both short-Citation7–9 and long-termCitation10 healthcare costs.
When patients with T2DM do not achieve adequate glycemic control with lifestyle modification and metformin, the American Diabetes Association (ADA) recommends adding either another oral anti-diabetic drug (OAD) (usually a sulfonylurea) or insulin to the treatment regimenCitation1. Studies have shown that adding once-daily insulin glargine (Lantus, sanofi-aventis U.S. LLC) improves glycemic control in patients with T2DM not adequately treated with OADsCitation11–16. As an alternative to vial, syringe, and cartridges, a pre-filled disposable pen is available for the administration of insulin glargine (SoloSTAR, sanofi-aventis, U.S., LLC). Exenatide (EXE) (Byetta, Amylin Pharmaceuticals, Inc. and Lilly USA, LLC), an incretin mimetic administered only via disposable pen, is a newer therapy that is recognized by the ADA as a “less well-validated” alternative to insulin when initial therapies have failedCitation1.
When medications are first approved for use, knowledge about efficacy and safety comes primarily from randomized clinical trials. Now that both insulin glargine and EXE have been available for several years, observational studies can provide additional information about their use in real-world settings, specifically the routine clinical practice of physicians who manage the treatment of patients with T2DM. Such data can inform treatment decisions by providing integrated information about real-world clinical outcomes and economic costs associated with each therapy. This observational study of routine clinical practice compared clinical outcomes, including medication persistence, glycemic control, and hypoglycemia; healthcare utilization; and healthcare costs during the first year after initiating insulin glargine or EXE in patients with T2DM inadequately treated with OADs.
Patients and methods
This retrospective analysis was conducted using the Innovus IMPACT national managed care database from 2006 to 2009, which comprises ∼50 US healthcare plans and contains medical claims, pharmacy claims, eligibility data, and laboratory results for 86.4 million patients, of whom 74% had pharmacy benefits and 15% had laboratory results. Individual patients were not identifiable, in accordance with the Health Insurance Portability and Accountability Act (HIPAA) of 1996.
Selection criteria
Adult patients with T2DM, defined as having at least one inpatient visit or two physician visits dated at least 30 days apart with primary or secondary diagnosis of International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes 250.x0 (diabetes mellitus type II or unspecified type not stated as uncontrolled) or 250.x2 (diabetes mellitus type II or unspecified type uncontrolled) met initial eligibility criteria. The patient cohort included in this analysis had prescription claims for initiating treatment with either insulin glargine disposable pen (GLA-P) or EXE from 2007 through 2008. Patients were required to have continuous healthcare plan enrollment with both medical and pharmacy coverage during the 6 months prior to initiating GLA-P or EXE (baseline period) and during the 1 year following treatment initiation (follow-up period). Patients were included in this analysis if they received only OAD therapy for T2DM and did not achieve adequate glycemic control (glycated hemoglobin A1C [A1C] > 7.0%) during the baseline period.
Outcome measures
Measurement of treatment persistence is challenging for insulin therapies because of variable insulin dose titration schedules. Thus, the traditional measure of treatment discontinuation (i.e., certain number of days gap without refill) is not applicable to insulin. In this study, medication persistence was defined as continuous treatment with the index medication without discontinuation or switching during the 1-year follow-up period. Persistence days were the number of days until discontinuation of the index medication. Study medication was considered discontinued if the prescription was not refilled within the expected time of medication coverage, a duration defined as the 75th percentile of the time, stratified by metric quantity supplied, between first and second fills among patients with at least one refill.
Therapy switches were considered discontinuations of index therapy. Patients who restarted the initial medication during the follow-up period were still considered discontinuers. Because the perceived treatment “restart” may result from the definition of treatment discontinuation (e.g., the patient had a longer-than-usual time between refills), sensitivity analyses of medication persistence were conducted using 50th or 90th percentiles of time-to-refill among patients with at least one refill to define treatment discontinuation. Daily average consumption (DACON) of insulin was calculated as dividing the total number of units dispensed by number of days between index date and the date of the last fill.
Glycemic control was measured by A1C level at the end of 1-year follow-up and change from baseline A1C. Hypoglycemic events were defined as a healthcare encounter (outpatient, inpatient, or emergency department [ED] visit) coded to one of the following ICD-9-CM codes as a primary or secondary diagnosis for hypoglycemia: 250.8 (diabetes with other specified manifestations), 251.0 (hypoglycemic coma), 251.1 (other specified hypoglycemia), 251.2 (hypoglycemia, unspecified).
Healthcare utilization was measured as the number and total length of hospitalizations, and the number of ED, endocrinology, and office visits. Healthcare costs were assessed from the perspective of US healthcare plan administrators, and were measured as standardized, which allowed payment (in 2009 dollars) by the health plan to the provider. Total cost was the aggregate of inpatient, outpatient, ED, and pharmacy prescription costs. Diabetes-related cost included inpatient, outpatient, and ED care for T2DM; diabetes medications; and diabetes supplies.
Statistical analysis
Selection bias is inherent in real-world retrospective studies, because patients who were prescribed one treatment often differ systematically from patients prescribed a comparison treatment. To remove observed selection bias, a stringent 1:1 propensity score matching (PSM)Citation17 was implemented to ensure that patients prescribed GLA-P or EXE were comparable in observed baseline characteristics. Propensity scores were derived from a logistic regression model that included the following baseline characteristics: age, sex, comorbidities, region, health plan, A1C, treatment, and healthcare utilization and healthcare cost during the 6 months prior to initiation of GLA-P or EXE. We included several interaction terms, but they were not significant. Estimated C-statistics were over 0.80.
Descriptive analyses were conducted for baseline characteristics of the matched cohorts. Differences in treatment groups were assessed via chi-square tests for categorical variables and t-tests for continuous variables.
Results
Of the 2339 patients who met the study selection criteria (381 initiated GLA-P and 1958 initiated EXE), 626 patients were 1:1 matched by PSM (n = 313 in each group). Overall, the patients in the two treatment groups were well-matched. There were only two statistically significant differences in baseline characteristics between treatment groups: more insulin glargine patients than EXE patients had retinopathy and were enrolled in a health maintenance organization plan (). The matched cohort was 55% male, with an average age of 54 years and an average baseline A1C of 9.2%. Most patients had comorbid medical conditions (almost 80% had dyslipidemia) and were taking one or more OADs. Hypoglycemia occurred in 3% of patients during the 6 months prior to initiating study medication.
Table 1. Baseline characteristics of the insulin glargine disposable pen and exenatide matched groupsa.
At the end of 1 year, medication persistence was 48% among GLA-P patients and 15% among EXE patients; p < 0.0001. Average persistence days were 253 in the GLA-P group and 144 in the EXE group; p < 0.0001. Regardless of percentile cut-off (50% vs 75% vs 90%) used to define persistence, patients initiating GLA-P had a significantly lower rate of discontinuation and a greater number of persistence days than patients initiating EXE. Average daily consumption was 28.2 ± 19.0 units for GLA-P.
At 1-year follow-up, patients who initiated on GLA-P had a significantly greater reduction in A1C (−1.23%) than patients who initiated on EXE (−0.92%; p = 0.0384) and a significantly lower A1C value at 1-year follow-up (A1C: 8.02% for GLA-P and 8.32% for EXE; p = 0.0423), with a similar proportion of patients achieving A1C < 7% (26.8% vs 27.5%, p = 0.8551). Although patients treated with GLA-P were more likely to experience claims-recorded hypoglycemia during the 1-year follow-up than patients treated with EXE (6.7% vs 3.5%; p = 0.0771), the overall rates for hypoglycemic events were low (0.63 vs 0.23; p = 0.0625), and there were no statistically significant differences between the treatment groups ().
Table 2. Hypoglycemia during the 1-year follow-up period.
At 1-year follow-up, GLA-P initiators used significantly fewer OADs than EXE initiators (1.67 vs 1.98; p = 0.0027) but were more likely to use rapid-acting insulin (16.9% in GLA-P with DACON 37.8 ± 30.0 units per day, 2.5% in EXE with DACON 44.5 ± 28.3 units per day). Of patients who initiated EXE, 10.8% filled a prescription for insulin glargine during follow-up; only 3.5% of patients who initiated GLA-P later filled a prescription for EXE.
There were no significant differences between the GLA-P and EXE groups during follow-up in overall or diabetes-related healthcare utilization, including number of hospitalizations, hospitalization days, ED visits, or office visits (). There also were no statistically significant differences in total healthcare costs (inpatient, outpatient, ED, and pharmacy costs) between GLA-P ($15,327) and EXE ($14,829) users during the first year after initiating treatment; p = 0.4683 (). In addition, total diabetes-related costs were similar for both groups ($5894 GLA-P vs $5524 EXE; p = 0.9769). Specific diabetes-related costs were also compared. Average outpatient costs were greater for GLA-P ($1673) than for EXE users ($1473; p = 0.0331), as were average supply costs ($282 for GLA-P and $161 for EXE; p < 0.0001). However, prescription costs were greater for EXE ($2438 with EXE, $2106 with GLA-P; p = 0.0012), even after accounting for the rapid-acting insulin required by some insulin glargine users and the larger number of OADs needed with EXE. When diabetes-related outpatient, prescription, and supply costs were aggregated in each treatment group, the combined costs were nearly identical: $4061 for GLA-P and $4072 for EXE. Although healthcare costs were similar for the treatment groups, GLA-P was associated with a greater reduction of A1C, with better medication persistence.
Figure 1. (a) Total healthcare costs and (b) diabetes-related costs during the first year after initiating treatment with either insulin glargine disposable pen or exenatide in patients with T2DM. Significant differences between treatments were noted for diabetes-related outpatient, medication, and supply costs.
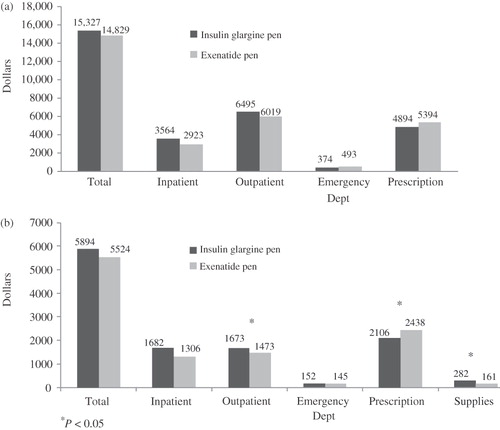
Table 3. Overall and diabetes-related healthcare utilization.
Discussion
In this study of routine clinical practice, information was extracted from a US national managed care claims database for patients with T2DM inadequately treated with OADs. In contrast to previous comparisons of insulin glargine and EXE, patients were matched on baseline characteristics including glycemic control; equivalent medication delivery systems (disposable pens) were compared; and outcomes were obtained for glycemic control, as well as medication persistence, hypoglycemia, and healthcare utilization and costs.
In a previous real-world comparison of insulin glargine and EXE, there were significant differences between the two groups when treatment was initiatedCitation18. Patients prescribed insulin glargine had significantly higher baseline A1C levels and Charlson Comorbidity IndexCitation19 scores, indicating that they had more poorly controlled T2DM and poorer overall health than patients prescribed EXE. In this study, PSM analysis was used to address the problem of pre-treatment differences in patients initiating insulin glargine or EXECitation17. Propensity score matching (PSM) analysis matches patients on observable baseline characteristics and thus enables evaluation of treatment effects in more comparable groups of patients.
Method of drug delivery also can differ with insulin glargine and EXE. Both are administered via injection; however, insulin glargine may be administered using a syringe (filled via draw of the prescribed dose from a vial), a reusable pen, or a disposable penCitation20,Citation21. EXE is only available in a disposable penCitation21. The perceived complexity of administering the correct dose of insulin may contribute to patient (and physician) resistance to initiating insulin therapyCitation22. Administering insulin glargine using a disposable pen may enhance patient acceptance and facilitate diabetes managementCitation23,Citation24. Previous studies comparing insulin glargine and EXE did not account for differences in the medication delivery system because they were conducted prior to the availability of the insulin glargine disposable penCitation18,Citation25, or did not restrict the administration of insulin glargine to any particular delivery methodCitation26. This study controlled for method of insulin administration by including only those patients prescribed the disposable pen in comparison to EXE.
When baseline differences and method of drug delivery were controlled, medication persistence was more than 3-times more effective with insulin glargine than EXE. Only 15% of patients who initiated EXE continued to take the medication continuously through the 1-year follow-up. On average, patients discontinued EXE within 5 months after initiating treatment. Medication persistence for insulin glargine and EXE was much lower in this real-world study than has been observed in randomized clinical trials, which found rates of treatment withdrawal ranging from 0–10% for insulin glargine and 5–19% for EXECitation26–28. Results from this study differ from the findings of a similar analysis of a large US administrative claims database, which reported significantly greater persistence at 1 year with EXE than insulin glargineCitation18. However, results from the previous database analysis were influenced by baseline differences between the treatment groups: higher A1C and more comorbid conditions among insulin glargine users as noted aboveCitation18. Therefore, outcomes in the previous study for patients taking insulin glargine may be a function of their more advanced T2DM and poorer overall health, rather than their prescribed medication regimen.
This analysis did not employ medication possession ratio (MPR) to measure medication adherence, as has been done previouslyCitation18. Because calculation of MPR presumes knowledge of drug supply for a fixed period (e.g., 30 days), it is unreliable for a medication such as insulin, which is initially dosed based on patient weight and then titrated based on blood glucose readings. Medication supply for insulin varies, particularly during drug initiation when average insulin needs are being established. Therefore, MPR is not a reliable outcome measure when comparing insulin with a medication prescribed in a fixed dose (e.g., EXE).
In the current study, patients initiating insulin glargine had better glycemic control 1 year later without significantly more hypoglycemia and without incurring significantly greater healthcare costs than patients initiating EXE. Insulin glargine disposable pen users had significantly lower A1C at follow-up and significantly greater A1C reductions from baseline than EXE users; however, the majority of patients in both groups did not achieve target A1C ≤ 7.0%. These findings differ from those of randomized clinical trials, which showed comparable reduction in A1C for insulin glargine and EXE, with the exception of one study that favored EXECitation26–29. This dissimilarity might be explained by the observed difference in treatment persistence in the real-world setting. Although hypoglycemia is a commonly recognized side-effect of insulin therapyCitation20, this real-world analysis found no statistically significant differences in hypoglycemia events for patients treated with insulin glargine compared with patients treated with EXE.
Total medical costs at the baseline date were lower for the glargine group than the EXE group. After controlling baseline costs in the PSM model, total medical and diabetes-related costs were not significantly different for patients initiating insulin glargine or EXE.
This study has several limitations. First, as with any retrospective analysis, it cannot establish causality of the treatment benefits of insulin glargine disposable pen or EXE. Second, results for insulin glargine administered via disposable pen might not be applicable to other modes of insulin administration. Also, information typically evaluated in randomized clinical studies was unavailable. The database did not provide duration of diabetes; however, based on other information available (number of OADs taken previously, baseline A1C, comorbidities), these patients have probably had T2DM for some time, and previous treatments have failed to provide adequate management. Although statistically significant, the observed 0.3% difference in A1C reduction between these two cohorts may not be clinically significant. Hypoglycemia was the only adverse event studied, and healthcare encounter data were used, therefore it may under-estimate the true rate of hypoglycemia and bias against insulin glargine due to the well-known association of insulin with hypoglycemia; information was not available regarding effects commonly observed with EXE, such as nausea, vomiting, and diarrheaCitation26. No weight data were available, although previous studies showed weight gain with insulin glargine and weight loss with EXECitation26,Citation28.
Because differences in hypoglycemic events and micro- and macro-vascular complications were not statistically significant between the groups prior to matching, they were not included in the PSM model. Patients’ duration of diabetes was not available in this observational data set and could not be included in the model. Although we have used stringent 1:1 PSM, there were differences in HMO participation between the insulin glargine and EXE groups. To the extent that these variables are related to treatment choice, this analysis does not account for their effects on study endpoints.
Additionally, this study does not explain why medication persistence was so low for EXE. Patients could restart EXE or insulin glargine after discontinuation; however, analysis of the patients’ drug prescription data in the last quarter of the 1-year follow-up period still showed a significantly lower refill rate of EXE than of the insulin glargine disposable pen (68.3% vs 43.1%, p < 0.001). Perhaps EXE was not tolerated because of gastrointestinal side-effects or because it failed to provide adequate glycemic control. EXE acts in part by stimulating endogenous insulin secretionCitation1; thus, when pancreatic function is severely compromised, EXE may be unable to increase insulin production sufficiently to provide glycemic control. The matched sub-group of EXE patients used in this analysis was in poorer health (higher A1C, more comorbidities) than other EXE patients in the database, which suggests that EXE may not be the optimal treatment for long-term management in these patients with more severe T2DM. Combinational use of insulin glargine and EXE may further improve their glycemic control without increased hypoglycemia or weight gainCitation30. Finally, the analysis was conducted using an intent-to-treat approach and did not take into account what treatments patients received after they discontinued index therapy and whether index therapy was restarted after discontinuation. Therefore, initiation of insulin therapy during the study period by a substantial number of EXE patients may have masked potential differences between insulin glargine and EXE in terms of healthcare costs and other outcome measures.
Conclusions
When patients with T2DM fail to achieve glycemic control with OAD therapy alone, the most validated next step in guidelines-based treatment is insulin, such as once-daily insulin glargineCitation1. EXE, a non-insulin injectable, is a recognized alternative treatment according to the ADACitation1. However, in this analysis, medication persistence with EXE was much lower than with the insulin glargine disposable pen at 1-year follow-up. This observational analysis of routine clinical practice showed that, compared with EXE, insulin glargine administered via disposable pen may be a more effective option due to its association with greater treatment persistence, greater A1C reduction without a significantly higher rate of hypoglycemia, and similar healthcare costs. These findings may help inform healthcare plan administrators about effectiveness in the treatment of patients with T2DM not adequately controlled by OADs alone.
Declaration of interest
Dr W. Wei is an employee of sanofi-aventis U.S. Dr O. Baser, E. Baser, and L. Xie were consultants for sanofi-aventis for this project.
Transparency
Declaration of funding
Study funding provided by sanofi-aventis U.S.
Acknowledgments
Editorial support was provided by Nancy S. Holland, PhD, of Embryon, and was funded by sanofi-aventis U.S.
References
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32:193-203
- American Diabetes Association. Economic costs of diabetes in the U.S. in 2007. Diabetes Care 2008;31:596-615
- Brown JB, Pedula KL, Bakst AW. The progressive cost of complications in type 2 diabetes mellitus. Arch Intern Med 1999;159:1873-80
- Simpson SH, Corabian P, Jacobs P, et al. The cost of major comorbidity in people with diabetes mellitus. CMAJ 2003;168:1661-7
- Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405-12
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837-53
- Menzin J, Langley-Hawthorne C, Friedman M, et al. Potential short-term economic benefits of improved glycemic control: a managed care perspective. Diabetes Care 2001;24:51-5
- Wagner EH, Sandhu N, Newton KM, et al. Effect of improved glycemic control on health care costs and utilization. JAMA 2001;285:182-9
- Shetty S, Secnik K, Oglesby AK. Relationship of glycemic control to total diabetes-related costs for managed care health plan members with type 2 diabetes. J Manag Care Pharm 2005;11:559-64
- Eastman RC, Javitt JC, Herman WH, et al. Model of complications of NIDDM. II. Analysis of the health benefits and cost-effectiveness of treating NIDDM with the goal of normoglycemia. Diabetes Care 1997;20:735-44
- Blickle JF, Hancu N, Piletic M, et al. Insulin glargine provides greater improvements in glycaemic control vs. intensifying lifestyle management for people with type 2 diabetes treated with OADs and 7-8% A1c levels. The TULIP study. Diabetes Obes Metab 2009;11:379-86
- Davies M, Lavalle-Gonzalez F, Storms F, et al. Initiation of insulin glargine therapy in type 2 diabetes subjects suboptimally controlled on oral antidiabetic agents: results from the AT.LANTUS trial. Diabetes Obes Metab 2008;10:387-99
- Schreiber SA, Haak T. Insulin glargine benefits patients with type 2 diabetes inadequately controlled on oral antidiabetic treatment: an observational study of everyday practice in 12,216 patients. Diabetes Obes Metab 2007;9:31-8
- Swinnen SG, Dain MP, Aronson R, et al. A 24-week, randomized, treat-to-target trial comparing initiation of insulin glargine once-daily with insulin detemir twice-daily in patients with type 2 diabetes inadequately controlled on oral glucose-lowering drugs. Diabetes Care 2010;33:1176-8
- Rosenstock J, Sugimoto D, Strange P, et al. Triple therapy in type 2 diabetes: insulin glargine or rosiglitazone added to combination therapy of sulfonylurea plus metformin in insulin-naive patients. Diabetes Care 2006;29:554-9
- Meneghini LF, Taylor L, Schwartz S. Improved glycemic control with insulin glargine vs pioglitazone as add-on therapy to sulfonylurea or metformin in patients with uncontrolled type 2 diabetes. Endocr Pract 2010;16:588-99
- Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983;70:41-55
- Fabunmi R, Nielsen LL, Quimbo R, et al. Patient characteristics, drug adherence patterns, and hypoglycemia costs for patients with type 2 diabetes mellitus newly initiated on exenatide or insulin glargine. Curr Med Res Opin 2009;25:777-86
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613-9
- Lantus [prescribing information]. Bridgewater, NJ: Sanofi-Aventis U.S. LLC, 2009
- BYETTA [prescribing information]. San Diego, CA: Amylin Pharmaceuticals, Inc., 2009
- Brunton SA, Davis SN, Renda SM. Overcoming psychological barriers to insulin use in type 2 diabetes. Clin Cornerstone 2006;8(Suppl 2):S19-S26
- Carter J, Roberts A. Usability of a pre-filled insulin injection device in a 3-month observational survey of everyday clinical practice in Australia. Curr Med Res Opin 2008;24:2741-9
- Fischer JS, Edelman SV, Schwartz SL. United States patient preference and usability for the new disposable insulin device Solostar versus other disposable pens. J Diabetes Sci Technol 2008;2:1157-60
- Misurski D, Lage MJ, Fabunmi R, et al. A comparison of costs among patients with type 2 diabetes mellitus who initiated therapy with exenatide or insulin glargine. Appl Health Econ Health Policy 2009;7:245-54
- Heine RJ, Van Gaal LF, Johns D, et al. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2005;143:559-69
- Diamant M, Van Gaal L, Stranks S, et al. Once weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION-3): an open-label randomised trial. Lancet 2010;375:2234-43
- Barnett AH, Burger J, Johns D, et al. Tolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open-label, two-period, crossover noninferiority trial. Clin Ther 2007;29:2333-48
- Bunck MC, Diamant M, Corner A, et al. One-year treatment with exenatide improves beta-cell function, compared to insulin glargine, in metformin treated type 2 diabetes patients: a randomized, controlled trial. Diabetes Care 2009;32:762-68
- Buse JB, Bergenstal RM, Glass LC, et al. Use of twice-daily exenatide in basal insulin-treated patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med 2011;154:103-112