Abstract
Objective:
The objective of this study was to examine the frequency of hypoglycemia among patients with type 2 diabetes who had concomitantly used exenatide BID (exenatide) and long-acting insulin and continued this combination vs those who continued long-acting insulin alone.
Methods:
Retrospective analyses, using a large managed care database, were used to estimate the frequency of hypoglycemia (episodes/patient/6 months) for patients who concomitantly used exenatide and long-acting insulin during a 6-month follow-up period.
Results:
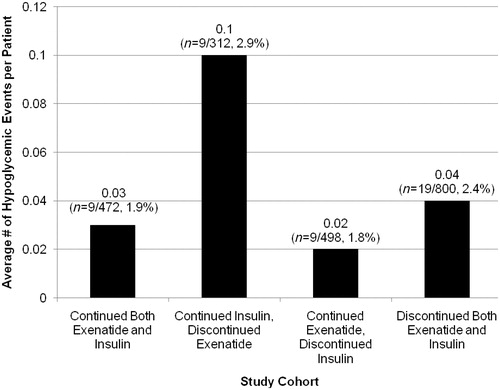
From among 2082 patients on concomitant exenatide and long-acting insulin, those who continued this combination (n = 472) had a lower frequency of hypoglycemia compared to those who remained on long-acting insulin alone (n = 312) (0.03 ± 1.9 vs 0.10 ± 1.01 [episodes/patient/6 months]; p < 0.0001).
Limitations:
Only hypoglycemia that required medical intervention (coded for hypoglycemia) was captured. The study could not evaluate any association between insulin dose titration and hypoglycemia or examine other outcomes such as HbA1c, weight, and body mass index, due to lack of data availability.
Conclusions:
Patients who concomitantly used exenatide BID and long-acting insulin experienced a lower rate of hypoglycemia.
Introduction
Exenatide, a glucagon-like peptide-1 (GLP-1) receptor agonist, improves glycemic control when used twice daily as monotherapy or in combination with oral anti-hyperglycemic medication therapy in patients with type 2 diabetesCitation1–3. Concomitant use of exenatide BID (exenatide) and insulin is not an approved indicationCitation4. Observational studies from clinical settings and randomized clinical trials (RCTs) reported that adding exenatide to existing insulin regimens resulted in additional HbA1c reduction, weight loss, and lower risk of hypoglycemiaCitation2,Citation5–8. Hypoglycemia concerns are considered a key barrier for physicians and patients to achieve glycemic goals, especially with insulin therapyCitation9,Citation10. The primary objective of this study was to examine the frequency of hypoglycemia (episodes/patient/6 months) over a 6-month period in patients with type 2 diabetes who were concomitantly using exenatide BID and long-acting insulin in real-world clinical practice settings. We were most interested in examining patients that continued both exenatide BID and insulin and those who continued insulin, but discontinued exenatide.
Methods
The HealthCore Integrated Research database was used for analyses. This study included patients with: (1) a claim of exenatide between 5/1/2005 and 10/1/2007, (2) at least 12 months pre- and 6 months post-index continuous eligibility (index date defined as the date of receiving first exenatide BID (exenatide) prescription), (3) diagnosis of type 2 diabetes, and (4) long-acting insulin use was defined as a claim for NPH or basal analog insulin (detemir or glargine) within 100 days before to 15 days following the index date. Patients <18 years old with a diagnosis of type 1 or gestational diabetes were excluded. Persistence on either or both therapies was defined as a <60-day gap between subsequent prescription claimsCitation11. This study identified that 99% of claims for insulin had days supply of ≤30 days. Hence, the Sikka et al.Citation11 method reported for measurement of persistence to medications was deemed appropriate for this study.
Four cohorts were defined based on use of insulin and exenatide in the post-index period. Patients on both therapies were defined based upon the persistency to both the therapies (i.e., a <60-day gap between subsequent prescription claims for both the medications concurrently). The cohorts were (1) patients continuing on both therapies (n = 472 [22.7%]), (2) patients continuing insulin and discontinuing exenatide (n = 312 [15.0%]), (3) patients continuing exenatide and discontinuing insulin (n = 498 [23.9%]), and (4) patients discontinuing both therapies (n = 800 [38.4%]). The current study focuses on hypoglycemia comparisons between only the first two cohorts. Hypoglycemic events were identified by the International Classification of Diseases, Ninth Revision (ICD-9) codes 250.8, 251.0, 251.1, and 251.2Citation12. The frequency of hypoglycemia was assessed using unadjusted Poisson regression.
Results
A total of 2082 patients met the study criteria of concomitantly using exenatide and long-acting insulin at baseline. Mean (standard deviation) age was 54 (9) years and 45% were females. Common comorbidities included hypertension (88–91%), dyslipidemia (84–90%), and any cardiovascular disease (21–26%). No significant differences were observed in demographic or clinical characteristics among the four cohorts except for dyslipidemia (p = 0.037). Demographic and clinical characteristics data are summarized in .
Table 1. Demographic and clinical characteristics of study population.
Among patients who were concomitantly using exenatide and long-acting insulin, 22.7% continued both treatments while 15% continued insulin but discontinued exenatide in the post-index period (). Patients who continued on both therapies had a lower frequency of hypoglycemia compared to those who remained on long-acting insulin alone (0.03 [1.9] vs 0.10 [1.01] episodes/patient/6 months, p < 0.0001). There was a decrease in use of sulfonylureas (SUs) in both cohorts. Subsequent SU use was lower in the insulin-only group compared to the exenatide plus insulin (38.7% vs 45.9%) group. The frequency of hypoglycemic events for patients who continued on exenatide alone and for those who discontinued both therapies (groups not considered for comparison in the current study) are shown in .
Discussion
The current study of frequency of hypoglycemia in 784 (out of the original 2082) patients using concomitant exenatide and long-acting insulin is the largest reported observational study of a US managed-care patient population receiving this treatment combination in real-world settings. This study reinforces the findings of previous clinical studies reporting that concomitant use of exenatide and long-acting insulin was associated with a lower incidence of hypoglycemiaCitation1,Citation2,Citation5,Citation6.
When metformin, thiazolidinediones, or exenatide are used either alone or in combination, hypoglycemia risk is lower than the risk with SUs and/or insulin, when used either alone or in combination with the above agentsCitation10. In this study, the use of SUs was lower in patients using insulin alone (38.7%) than in patients using exenatide and insulin (45.9%). Thus, SU use does not seem to explain the difference in hypoglycemia between the two study groups. The observational data presented here reinforce the concept that concomitant exenatide and insulin therapy was associated with a lower risk for hypoglycemia than continuing insulin therapy without exenatide. However, due to descriptive study design we cannot make any inferences about causal relationship between drug use and hypoglycemia. This study reports associations observed in the real world patient population using claims database.
Two retrospective analyses from selected clinical practice settings have found that use of exenatide combined with insulin was associated with further reductions in HbA1c and lower rates of hypoglycemiaCitation4,Citation5. Additionally, two RCTs of exenatide added to titrated basal insulin have been completed. Buse et al.Citation7 reported a greater reduction in HbA1c with no significant differences in hypoglycemia rates in patients treated with exenatide and titrated insulin glargine compared to titrated insulin glargine with placebo (1.4 vs 1.2 episodes/patient/year). In a smaller trial, Riddle et al.Citation8 also showed no significant differences in number of hypoglycemia episodes and greater efficacy with insulin plus exenatide compared to insulin plus placebo.
Limitations of claims databases should be noted. Only hypoglycemia that required medical intervention (coded for hypoglycemia) was captured. This study was of short duration. A hypoglycemic claim suggests that an episode was of clinical impact sufficient to bill for services; however, hypoglycemic events may have been under-reported. Hypoglycemia frequency was recorded only for a 6-month period and time-to-event analyses were not performed. The study could not evaluate any association between insulin dose titration and hypoglycemia or examine other outcomes such as HbA1c levels, weight, and body mass index, due to lack of data availability. Hence, results may not be generalizable to various patient populations. We could not identify total duration of insulin therapy prior to initiating exenatide as it was not reported in the claims database. Another limitation of this study is the availability of NPH insulin over-the-counter without a prescription; therefore, if patients were using this medication there would have been no claim(s).
Conclusions
This study reinforces the clinical and RCT observations that concomitant use of exenatide BID and long-acting insulin is associated with a lower frequency of hypoglycemia in a large, diverse, type 2 diabetes patient population from real-world clinical practice settings. Further study is still necessary to determine any association between the lower risk of hypoglycemia seen with continuing exenatide BID with insulin vs discontinuing exenatide BID and possible adverse outcomes or mortality.
Transparency
Declaration of funding
This research was funded by Amylin Pharmaceuticals, Inc., San Diego, CA and by Eli Lilly and Company, Indianapolis, IN.
Declaration of financial/other relationships
Amy Blickensderfer, PharmD, is an employee of Amylin Pharmaceuticals, Inc., and owns stock in Amylin Pharmaceuticals. Ralph Quimbo is an employee of HealthCore, Inc. Rolin Wade, RPh, is an employee of Cerner Corporation. Byron J. Hoogwerf, MD, and Manjiri Pawaskar, PhD, are employed by Eli Lilly and Company and own stock in Eli Lilly and Company.
Acknowledgments
The authors thank Rebecca McCracken of i3 for her writing contributions to the preparation of the manuscript. The authors also thank Derek Misurski of GSK, US Health Outcomes and Rosalind Fabunmi formerly of Amylin Pharmaceuticals, Inc. for their contribution in the concept development and study design, and Likun Hou of HealthCore, Inc. for her contribution in the analysis.
References
- Cvetković RS, Plosker GL. Exenatide: a review of its use in patients with type 2 diabetes mellitus (as an adjunct to metformin and/or a sulfonylurea). Drugs 2007;67:935-54
- Tzefos M, Olin JL. Glucagon-like peptide-1 analog and insulin combination therapy in the management of adults with type 2 diabetes mellitus. Ann Pharmacother 2010;44:1294-300
- Norris SL, Lee N, Thakurta S, et al. Exenatide efficacy and safety: a systematic review. Diabet Med 2009;26:837-46
- Highlights of prescribing information Byetta. San Diego, CA: Amylin Pharmaceuticals, Inc., 2009. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/021773s9s11s18s22s25lbl.pdf Accessed September 2010
- Sheffield CA, Kane MP, Busch RS, et al. Safety and efficacy of exenatide in combination with insulin in patients with type 2 diabetes mellitus. Endocr Pract 2008;14:285-92
- Yoon NM, Cavaghan MK, Brunelle RL, et al. Exenatide added to insulin therapy: a retrospective review of clinical practice over two years in an academic endocrinology outpatient setting. Clin Ther 2009;31:1511-23
- Buse JB, Bergenstal RM, Glass LC, et al. Use of twice-daily exenatide in Basal insulin-treated patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med 2011;154:103-12
- Riddle M, Ahmann A, Basu A, et al. Metformin + exenatide + basal insulin vs metformin + placebo + basal insulin: reaching A1c < 6.5% without weight gain or serious hypoglycemia [abstract]. Presented at 70th American Diabetes Association Scientific Sessions, Orlando, FL, 25-29 June 2010
- Tibaldi J. Initiating and intensifying insulin therapy in type 2 diabetes mellitus. Am J Med 2008;121:20-9
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32:193-203
- Sikka R, Xia F, Aubert RE. Estimating medication persistency using administrative claims data. Am J Manag Care 2005;11:449-57
- Ginde AA, Blanc PG, Lieberman RM, et al. Validation of ICD-9-CM coding algorithm for improved identification of hypoglycemia visits. BMC Endocr Disord 20081;8:4
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613-9