Abstract
Objective:
In addition to negative impacts on clinical effectiveness in treating secondary hyperparathyroidism, low adherence to cinacalcet may have negative impacts on healthcare costs. This study assessed the relationship between medication adherence and healthcare costs among US patients on dialysis given cinacalcet to manage secondary hyperparathyroidism.
Methods:
Retrospective cohort study of patients who were receiving dialysis with an initial cinacalcet prescription between January 2004 and April 2010 and who survived ≥12 months. Longitudinal, integrated medical, and pharmacy claims data from the MarketScan® database were used to calculate medication possession ratios (MPR) over 12 months and to examine the association of adherence with inpatient, outpatient, emergency room, outpatient medication, and total costs while controlling for patient characteristics, co-morbid medical conditions, and concomitant medication MPR in a multivariate regression model. Patients were dichotomized as adherent (<180 days refill gap) or non-adherent (≥180 day refill gap). Adherent patients were further dichotomized as low adherent (<0.8 MPR) and high adherent (≥0.8 MPR).
Results:
The final study cohort included 4923 patients. After 12 months, 46% were non-adherent, 27% were low adherent, and 28% were high adherent. Greater cinacalcet adherence was associated with significantly lower inpatient costs with cost-savings of a greater magnitude than the increased medication costs.
Conclusions:
This study demonstrated that low adherence to cinacalcet, which may be associated with undesirable clinical and health-economic outcomes, is common. Despite limitations inherent in retrospective studies of claims databases, such as unobserved confounding, non-discrimination between prescription fill and actual use, and not knowing the reasons for non-adherence, these results suggest that inpatient cost savings of $8899, more than offset higher medication costs of $5858 associated with increased cinacalcet adherence.
Introduction
Uncontrolled secondary hyperparathyroidism (sHPT) in patients on dialysis can result in substantial morbidity and is associated with multiple adverse clinical outcomesCitation1, including bone disease, soft tissue and vascular calcification, parathyroidectomy, and increased risk of deathCitation2–4. Cinacalcet (Sensipar®/Mimpara®; Amgen Inc., Thousand Oaks, CA) is a first-in-class calcimimetic used with other therapies, such as phosphate binders and vitamin D sterols, for sHPT in patients on dialysis. Cinacalcet acts directly on calcium sensing receptors (CaSR) expressed in parathyroid glands and reduces parathyroid hormone (PTH) secretion by rendering parathyroid cells more sensitive to inhibitory actions of extracellular calciumCitation5. Cinacalcet treatment effectively lowers PTH levels among patients with sHPT and often concurrently reduces serum calcium and phosphorous concentrationsCitation6. As with most medications, cinacalcet cannot be effective if patients are non-adherent with the prescribed treatment regimen.
Pharmacy claims data provide a vast amount of information regarding medication dosing and refilling patterns commonly used as a tool for assessing medication adherence, and allow for large real-world populations to be studied. Other methods, such as detection of the drug or metabolite in biologic fluids, pill counts, monitoring devices, and direct observation are more labor intensiveCitation7. One widely used measure of medication adherence using pharmacy claims databases is the medication possession ratio (MPR), which represents the proportion of time a medication is received during a defined intervalCitation8. While an MPR of 100% is ideal, indicating that a medication was in the patient’s possession for each day of the treatment period, an MPR of ≥80% is considered a proxy for continuous useCitation7. MPR is useful in assessing adherence to medications intended for long-term use when prolonged lapses may lead to adverse outcomes. Adherence rates for oral prescription medications in the general population are reported as 50–76%Citation9,Citation10.
Little is known about rates of adherence to sHPT therapies, with limited information available about adherence to cinacalcet outside the clinical trial setting. In a study of 101 patients, adherence to cinacalcet was 29% over 12 monthsCitation11. Such low rates of medication adherence may have clinical and economic consequences, since high levels of PTH and elevated serum calcium and phosphorous have been associated with higher morbidity and mortalityCitation12–14. For example, increased adherence to cinacalcet and oral phosphate binders was shown to be associated with significantly better control of serum phosphorous and PTH levels without changes to other medicationsCitation15. This study assessed the association between adherence to cinacalcet for treatment of sHPT and healthcare costs. An assessment of an association of this nature could convey potential economic consequences of improved cinacalcet adherence to healthcare providers, administrators, and organizations making decisions regarding management of sHPT within a changing reimbursement and policy landscapeCitation16.
Methods
Study design and patient selection
This retrospective cohort study used longitudinal, integrated medical, and pharmacy claims data to assess cinacalcet MPR over 12 months, as a measure of adherence, and to examine the association between adherence and healthcare costs. Data were extracted from two Thomson Reuters MarketScan® Research Databases: the Commercial Claims and Encounters Database (employer and health plan sourced data for individuals under fee-for-service, fully capitated, and partially capitated health plans) and the Medicare Supplemental and Coordination of Benefit Database (healthcare experience of retirees with Medicare and supplemental coverage, including the Medicare-covered portion of payment, the employer-paid portion, and any patient out-of-pocket expenses). The MarketScan® databases provide detailed cost, use, and outcomes data for healthcare services in inpatient and outpatient settings. Medical claims are linked to outpatient prescription drug claims and individual-level enrollment data. All personal identifiers were de-identified and both databases are Health Insurance Portability and Accountability Act (HIPAA) compliant.
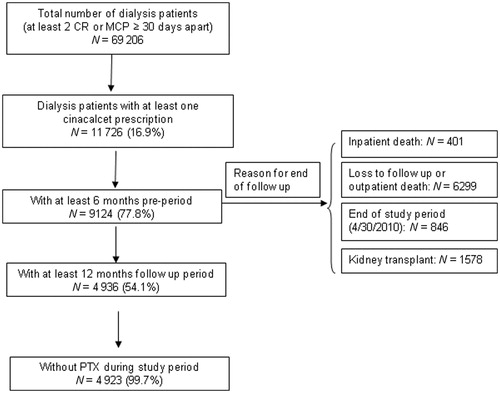
The study included all patients receiving dialysis who had ≥1 cinacalcet prescription from January 2004 through April 2010. Patients were extracted based on ≥2 claims specific to chronic dialysis reimbursement (Composite Rate revenue codes 821, 831, 841, and 851; Monthly Capitation Payment codes 90918–90925 or G0308–G0327) ≥30 days apart, and ≥1 claim for cinacalcet. The index date was defined as the date of the first claim for cinacalcet during the study period, providing for a 6 month cinacalcet-free baseline period. All patients were required to have continuous enrollment with drug coverage for at least 6 months prior to and 12 months after the index date. Patients who underwent a parathyroidectomy, a surgical procedure recommended for patients with severe hyperparathyroidism (persistent serum levels of intact PTH > 800 pg/mL [88.0 pmol/L]), associated with hypercalcemia and/or hyperphosphatemia that are refractory to medical therapyCitation1, during the 12-month follow-up period, were excluded from analysis.
Cinacalcet adherence
Adherence was dichotomized by classifying patients as adherent or non-adherent based on continuous exposure after the initial prescription, using a permissible gap of 180 days, informed by a preliminary examination of the distribution of gap days in cinacalcet prescriptions. This time window allowed for inclusion of patients with lower levels of compliance who made an effort to persist with therapy and for potential impact of intra-hospital drug use not captured by the claims database. Although, as defined, non-adherent patients were effectively discontinued, information on cinacalcet re-initiation was not assessed. Adherent patients were further dichotomized as low adherers or high adherers based on a cut-off of 80% MPR. MPR was defined as the ratio of number of days covered by cinacalcet prescription divided by the number of days during the 12-month post-index time period. Patients with an MPR > 100% because of over-supply had their MPR truncated at 100%.
Healthcare costs
Twelve-month healthcare costs were estimated using claims data and categorized as inpatient, emergency room (ER), outpatient, and pharmacy costs. Outpatient pharmacy costs included sHPT medication costs (cinacalcet, vitamin D sterols, and phosphate binders) and anti-hypertensive medication costs. Anti-hypertensive costs were included to control for concomitant medication MPR.
Costs were based on paid amounts of adjudicated claims, including insurer and health plan payments, copayments, and deductibles. Costs of services provided under capitated arrangements were estimated using payment proxies computed based on paid claims. All costs were converted to US$ 2010 values using the Medical Care Consumer Price Index (CPI).
Statistical methods
Means and standard deviations for baseline continuous variables and frequencies and percentages for categorical variables were calculated. Baseline patient characteristics were described during the 6 month pre-index period. Ordered logistic regression models were utilized for each demographic and clinical variable, with adherence category as the ordinal dependent variable. Significant associations of increased adherence with baseline variables among the three groups were determined at an α level of 0.05. Unadjusted 12-month costs per patient by cost and adherence category were calculated as part of the descriptive analysis.
A generalized linear model (GLM) with log link and gamma distribution was used to assess associations between adherence levels and costs. The GLM model was adjusted for covariates including baseline patient characteristics () and co-morbid medical conditions (calculated Charlson Comorbidity Index and specific medical condition claims; ), MPR for concomitant medications (oral anti-hypertensive medications), patient’s out-of-pocket expense, and baseline costs. Concomitant medication MPR was estimated by calculating patients’ anti-hypertensive medication MPR during the post-index period for patients with baseline hypertension (>85% of all patients). Baseline costs were calculated from the 6-month pre-index time period.
Table 1. Baseline patient characteristics, baseline co-morbidities, and 12-month follow-up anti-hypertensive MPR by adherence category.
As sensitivity analyses, the study examined ICD-9 codes for inpatient claims to identify reasons for hospitalization. Finally, a rolling window model where the prior 6 month cinacalcet adherence was used to predict the costs in the following month was used as a check for the robustness of the GLM results. In these analyses, a patient generated a series of repeated measurements for each month of follow-up since the index date (excluding the initial 6 months for lagged exposure) until the end of available data, and thus could be greater than 12 months. A repeated measurement mixed-model with random coefficients was used for this analysis. Covariates in this model were the same as those for the previous GLM model.
Descriptive analyses were performed using the statistical software SAS version 9.2 (SAS Institute, Cary, NC) and multivariate analyses were conducted using Stata version 11.0 (StataCorp LP, College Station, TX). Statistical significance was determined at an α level of 0.05.
Results
Study population
Starting from 69,206 patients on chronic dialysis in the US, 11,726 patients had at least one cinacalcet prescription claim between 2004 and 2010 (). From that set, 4936 had both 6 months of pre-index and 12 months of post-index data. Thirteen patients underwent a parathyroidectomy during the 12-month study period. The final study population comprised 4923 patients on chronic dialysis newly treated with cinacalcet who met all eligibility criteria.
Demographic and clinical characteristics
The patient characteristics and co-morbid medical conditions across the three adherence categories () were broadly similar to the Medicare dialysis population as reported by the United States Renal Data System (USRDS)Citation17. High adherent patients were slightly older and had a higher anti-hypertensive medication MPR and cancer rates. Charlson Comorbidity Index, and congestive heart failure, cardiac, peripheral vascular disease, and dysrhythmia rates were lower with increased adherence.
Cinacalcet adherence
Overall, 30.2% of patients were non-adherent (discontinued cinacalcet) by the 6-month post-index date, and 45.6% by month 12. The proportion of patients with low adherence declined from 31.9% at month 6 to 26.5% at month 12. While 37.8% of patients were in the high adherence category at month 6, the proportion declined to 27.9% by month 12.
Healthcare costs associated with cinacalcet adherence
Unadjusted inpatient and emergency room costs decreased with improved cinacalcet adherence (). Outpatient medication costs, including cinacalcet costs, were higher among patients who were adherent than among those who were non-adherent, and highest for those who were high adherers. However, the increased medication costs were less than the inpatient cost savings (). The trend for inpatient costs observed suggests a negative association between inpatient costs and cinacalcet adherence. The inverse relationship between cinacalcet adherence and inpatient costs remained significant after adjusting for patient demographics, baseline co-morbidities, concomitant medication MPR, and baseline costs (). Decreasing ER costs with increased adherence were also statistically significant. While outpatient medication costs were greater for patients with low and high adherence than for non-adherent patients, the magnitude of cost increases were smaller than estimated cost savings from reduced inpatient costs.
Figure 2. Mean unadjusted costs by adherence. Data represent mean (SE) for the costs attributable to cinacalcet and inpatient expenses associated with cinacalcet adherence.
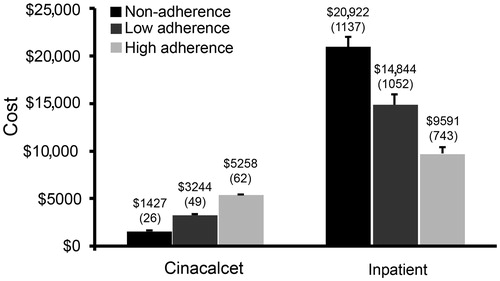
Table 2. Unadjusted 12-month mean costs by adherence category.
Table 3. Incremental cost associated with increased adherence from multivariate models.
Sensitivity analysis
In order to better understand the inpatient cost drivers, this study examined the inpatient claims’ ICD-9 codes at the 3-digit level. Among the 4618 IP claims, the top five most frequent primary diagnosis codes were related to complications from a specific procedure (15.4%), heart failure (6.6%), hypertensive renal disease (6.1%), diabetes mellitus (5.1%), and acute renal failure (3.9%). Cardiovascular-related primary diagnoses codes as a group accounted for 26.8% of the claims and translated to 40.9% of observed IP costs. The relationship between adherence and inpatient costs was further supported by similar results using the rolling window model.
Discussion
There was an inverse relationship between cinacalcet adherence and inpatient costs in this analysis of longitudinal, patient-level prescription claims data for patients on chronic dialysis from real-world practice settings. The high levels of non-adherence observed in this study (30% at 6 months and 45% at 12 months) are consistent with rates reported from a smaller claims databaseCitation11. Reasons for why cinacalcet adherence is lower than adherence rates for other medications may be 2-fold: (1) chronic dialysis patients as a group may encounter multiple life and clinical challenges where medication adherence might be expected to be lower than the general population, and (2) sHPT as a disease, where the effects of non-adherence are not necessarily immediately noticeable to the patient.
Although clinical consequences of improved adherence to sHPT medications have been previously shownCitation15, our study assessed the impact of adherence on economic consequences. The study estimated inpatient cost-savings of ∼$4000–$8900/patient/year associated with low and high cinacalcet adherence, respectively, compared with non-adherence. Although the inpatient cost-savings associated with high adherence to cinacalcet therapy was significant in two different multivariate analyses, these savings did not translate into significant differences in total costs. Total costs may have been affected, in part, by increased cinacalcet costs with higher adherence. However, the total outpatient pharmacy costs for high adherence patients compared to non-adherence patients increased by ∼$7100, whereas cinacalcet costs only increased by ∼$3800 between the two adherence groups. Combined vitamin D and phosphate binder costs made up a larger fraction of the outpatient pharmacy costs than cinacalcet within each category, and non-cinacalcet costs were therefore important drivers of increased pharmacy costs associated with increased cinacalcet adherence. The positive significant correlation between cinacalcet and anti-hypertension medication MPR suggests that adherence may in part be due to patient behavior. Further studies are needed to determine what accounts for the increased outpatient pharmacy costs. Regardless, even though more adherent patients had higher outpatient drug and sHPT drug costs, due, in part, to the increased purchase of cinacalcet, inpatient cost-savings related to cinacalcet adherence outweighed the costs of the medications.
Despite limitations inherent in retrospective studies of claims databases, such as unobserved confounding, non-discrimination between prescription fill and actual use, and lack of control for causes of non-adherence, medical claims data for chronic-use medications have been shown to correlate well with patient drug exposureCitation7, and a number of studies of adherence and outcomes have been conducted using claims dataCitation7,Citation11,Citation15,Citation18,Citation19. However, caution is warranted in interpretation of these results. Although the multivariate analysis adjusted for differences in observed patient characteristics, adherence and costs may be impacted by unobserved characteristics, such as race. African-Americans have higher cinacalcet use patterns than other racial groupsCitation20. A higher proportion of African-Americans may be expected to be on cinacalcet at any given point in time, but little is known about African-American’s adherence patterns and how that may or may not confound the associations explored in this study. Although claims data do not contain laboratory parameters routinely used in clinical practice to inform treatment choices and assess success of the interventions in sHPT, all patients in this study cohort had been prescribed cinacalcet, which is a prescription driven by laboratory levels. Thus, the laboratory levels at the index date of initial cinacalcet prescription were not expected to differ by adherence groups and, therefore, were not viewed as confounding factors. Since it was not possible to control for reasons for non-adherence, the observed patterns of no drug possession may not indicate non-adherence; the patient may have received samples precluding the need for a prescription refill or the provider may have discontinued the prescription based on clinical indications (e.g., hypocalcaemia), attainment of laboratory goals, or failure to respond. Lastly, the results from the study population used in this study, a sample of patients who did not undergo parathyroidectomies and received primary coverage through a commercial plan and US Medicare patients who continued secondary coverage via commercial plans, may not be generalizable to the typical chronic dialysis Medicare population within the US or dialysis patients in other countries. However, mean baseline patient demographics and patient characteristics of this study did not differ substantially from those reported in the USRDS Annual Data ReportCitation17. Thus, the different patient population can also be interpreted as a specific strength of this study as previous analyses of chronic dialysis patients were primarily based on the US Medicare covered patients.
Evaluation of adherence to sHPT therapies in dialysis patients is increasingly important in the changing reimbursement environment with the ongoing bundling of costs for dialysis treatment by Centers for Medicare and Medicaid Services (CMS) in the US. With bundling, dialysis providers will incur more financial risk and increased complexities in administering care for their patients. In this context, it is useful for providers to consider and implement treatment options that provide the most overall economic benefit while delivering optimal care. Thus, understanding the costs, economic benefits, and potential overall cost offsets from oral therapies in dialysis will be critical in informing decision-making for providers and payers in this changing and increasingly resource-constrained reimbursement environment.
Treatment with cinacalcet has repeatedly been shown to improve PTH, Ca, and P, and adherence with therapy is crucial for long-term control of these parametersCitation6,Citation21–23. Recent results from the ADVANCE study indicating that cinacalcet therapy may be associated with attenuated cardiovascular calcificationCitation24 may help explain, in part, the reduced inpatient costs associated with cardiovascular-related hospitalizations seen in the sensitivity analysis. Lastly, a recent observational study by Block et al.Citation20 reported an association between cinacalcet use and improved survival. While the totality of the evidence seems to support overall beneficial effects of cinacalcet, the true associations between cinacalcet adherence and healthcare cost associated with clinical outcomes need to await completion of the ongoing randomized Evaluation of Cinacalcet Therapy to Lower Cardiovascular Events (EVOLVE) trialCitation25. This study’s findings could be further strengthened by the replication of the results using additional datasets and by a more thorough examination of the reasons for non-adherence with sHPT therapies.
Conclusions
The current study is, to the authors’ knowledge, the first observational study to examine cinacalcet adherence and its relationship with healthcare costs in patients on chronic dialysis with sHPT. Non-adherence with cinacalcet, which could lead to undesired clinical and economic outcomes, appears to be prevalent. While interpretative caution is needed, these results suggest improving adherence may reduce the economic burden of sHPT with possible implications for providers and payers considering sHPT treatment regimens within a resource-constrained environment.
Transparency
Declaration of funding
Funding for this research was provided by Amgen, Inc., Thousand Oaks, CA, USA.
Declaration of financial/other relationships
A.L., V.B., I.K., and W.G. are employees of Amgen, Inc., Thousand Oaks, CA. X.S., N.F., and D.D. are employees of Thomson Reuters, Cambridge, MA, which received funding for this study.
Acknowledgments
The authors gratefully acknowledge the assistance of Jon Nilsen, PhD, Amgen, Inc., in drafting and editing this manuscript.
References
- Eknoyan G, Levin A, Levin NW, K/DOQI. clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003;42(4 Suppl 3):1‐201
- Block GA, Klassen PS, Lazarus JM, et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 2004;15:2208‐18
- Kalantar-Zadeh K, Kuwae N, Regidor DL, et al. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int 2006;70:771‐80
- St Peter WL, Li Q, Liu J, et al. Cinacalcet use patterns and effect on laboratory values and other medications in a large dialysis organization, 2004 through 2006. Clin J Am Soc Nephrol 2009;4:354‐60
- Goodman WG, Hladik GA, Turner SA, et al. The Calcimimetic agent AMG 073 lowers plasma parathyroid hormone levels in hemodialysis patients with secondary hyperparathyroidism. J Am Soc Nephrol 2002;13:1017‐24
- Block GA, Martin KJ, de Francisco AL, et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med 2004;350:1516‐25
- Andrade SE, Kahler KH, Frech F, et al. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf 2006;15:565‐74; discussion 75–7
- Sclar DA, Skaer TL, Chin A, et al. Utility of a transdermal delivery system for antihypertensive therapy. Part 1. Am J Med 1991;91:50‐6
- Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database of Systematic Reviews 2008, Issue 2. Art. No.: CD000011. DOI: 10.1002/14651858.CD000011.pub3 [Last accessed October 2011]
- Rosen MI, Rigsby MO, Salahi JT, et al. Electronic monitoring and counseling to improve medication adherence. Behav Res Ther 2004;42:409‐22
- Gincherman Y, Moloney K, McKee C, et al. Assessment of adherence to cinacalcet by prescription refill rates in hemodialysis patients. Hemodial Int 2010;14:68‐72
- Block GA, Hulbert-Shearon TE, Levin NW, et al. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis 1998;31:607‐17
- Ganesh SK, Stack AG, Levin NW, et al. Association of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol 2001;12:2131‐8
- Leggat JE Jr, Orzol SM, Hulbert-Shearon TE, et al. Noncompliance in hemodialysis: predictors and survival analysis. Am J Kidney Dis 1998;32139‐45
- Pruijm M, Teta D, Halabi G, et al. Improvement in secondary hyperparathyroidism due to drug adherence monitoring in dialysis patients. Clin Nephrol 2009;72:199‐205
- Department of Health and Human Services: Centers for Medicare & Medicaid Services, End Stage Renal Disease Prospective Payment System: Final Rule and Proposed Rule, CMS-1418-F, RIN 0938-AP57, 42 CFR Parts 410, 413 and 414. August 12, 2010
- US Renal Data System. USRDS 2009 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2009
- Philipneri MD, Rocca Rey LA, Schnitzler MA, et al. Delivery patterns of recommended chronic kidney disease care in clinical practice: administrative claims-based analysis and systematic literature review. Clin Exp Nephrol 2008;12:41‐52
- Pinsky BW, Takemoto SK, Lentine KL, et al. Transplant outcomes and economic costs associated with patient noncompliance to immunosuppression. Am J Transplant 2009;9:2597‐606
- Block GA, Zaun D, Smits G, et al. Cinacalcet hydrochloride treatment significantly improves all-cause and cardiovascular survival in a large cohort of hemodialysis patients. Kidney Int 2010;78:578‐89
- Lindberg JS, Culleton B, Wong G, et al. Cinacalcet HCl, an oral calcimimetic agent for the treatment of secondary hyperparathyroidism in hemodialysis and peritoneal dialysis: a randomized, double-blind, multicenter study. J Am Soc Nephrol 2005;16:800‐7
- Sprague SM, Evenepoel P, Curzi MP, et al. Simultaneous control of PTH and CaxP is sustained over three years of treatment with cinacalcet HCl. Clin J Am Soc Nephrol 2009;4:1465‐76
- Strippoli GF, Palmer S, Tong A, et al. Meta-analysis of biochemical and patient-level effects of calcimimetic therapy. Am J Kidney Dis 2006;47:715‐26
- Raggi P, Chertow GM, Torres PU, et al. The ADVANCE study: a randomized study to evaluate the effects of cinacalcet plus low-dose vitamin D on vascular calcification in patients on hemodialysis. Nephrol Dial Transplant 2010;26:1327-39
- Chertow GM, Pupim LB, Block GA, et al. Evaluation of Cinacalcet Therapy to Lower Cardiovascular Events (EVOLVE): rationale and design overview. Clin J Am Soc Nephrol 2007;2:898‐905