Abstract
Background:
Venous thromboembolism (VTE) incurs considerable socioeconomic costs, partly owing to the fact that the treatment and prevention of VTE via effective thromboprophylaxis remains suboptimal in the inpatient and outpatient settings of many healthcare systems. A number of organizations—including the National Quality Forum, The Joint Commission, and the Centers for Medicare and Medicaid Services—have established measures to assess and reduce the healthcare burden of VTE. These improvement strategies focus on increasing the use of thromboprophylaxis, implementing existing guidelines, and improving awareness.
Findings:
Based on clinical trial results, the oral anti-coagulants rivaroxaban, apixaban, and dabigatran etexilate have been approved in many countries for the prevention of VTE in patients after elective hip or knee replacement surgery. Recently, dabigatran etexilate and rivaroxaban have also been approved in the US for the prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation. In addition, rivaroxaban is currently the only newer anti-coagulant that has been approved in Europe for the treatment of deep vein thrombosis and for the long-term prevention of recurrent VTE. These oral anti-coagulants have several advantages over established anti-coagulants, including no need for routine coagulation monitoring and only minimal food and drug interactions. These characteristics, together with convenient oral administration, may improve adherence and quality of life for patients, which could result in reductions in the rate of VTE.
Conclusions:
These three oral agents have several advantages over established anti-coagulants and could, therefore, address the unmet needs of patients, physicians, and healthcare systems, with the potential to reduce the burden of anti-coagulant management and the occurrence of VTE.
Introduction
Venous thromboembolism (VTE), comprising deep vein thrombosis (DVT) and pulmonary embolism (PE), is a major burden to healthcare systems; up to 600,000 cases of VTE occur each year in the US, directly or indirectly causing at least 100,000 deathsCitation1. Population studies indicate that patients who have had a primary venous thromboembolic event are at an increased risk of recurrence and potentially life-threatening long-term complications, such as post-thrombotic syndrome and chronic thromboembolic pulmonary hypertensionCitation2,Citation3.
Although there has been an intense focus on ‘appropriate’ prevention of VTE within the US over the past decade, current initiatives center on the prevention of recurrence by treatment with anti-coagulants. The aim is to improve outcomes by reducing clot burden and by preventing thrombus propagation, embolization to the lungs, and the development of long-term complications. Without treatment, 50% of patients with symptomatic proximal DVT or PE have recurrent thrombosis within 3 monthsCitation4.
VTE incurs considerable economic costs: recent annual figures from the US estimate $9.8–$52 billion for DVT alone; as much as $3.4–$27 billion constitute preventable and avoidable costsCitation5. For patients readmitted to hospital for DVT, readmission costs ($11,862) were higher than those estimated for initial hospitalization ($9805), primarily because of a trend towards longer hospital stays. For patients readmitted to hospital for PE, total hospitalization costs for readmission and initial hospitalization were similar, at ∼$14,500Citation6.
Current treatment guidelines for VTE recommend initial therapy with parenteral agents followed by vitamin K antagonists (VKAs)Citation7. This review will discuss how new oral anti-coagulants—those currently in advanced development for the treatment of VTE and most likely to gain approval by the US Food and Drug Administration (FDA)—might address current challenges of standards of care, and improve patient outcomes in the US.
Challenges with established anti-coagulants
Unfractionated heparin (UFH) and low molecular weight heparin (LMWH) are indirect coagulation inhibitors with limitations that include parenteral administration, requirements for frequent laboratory monitoring (i.e., UFH), risk of decreasing bone formation, and the potential for causing heparin-induced thrombocytopeniaCitation8,Citation9. LMWHs have largely replaced UFH because of their lower risk of non-hemorrhagic side-effects, improved bioavailability, lower levels of binding to plasma proteins and endothelium, no requirement for routine coagulation monitoring, and generally superior pharmacokinetic propertiesCitation8.
In addition to a slow onset of action, VKAs are challenging to use in clinical practice because of their unpredictable pharmacokinetics and pharmacodynamics, narrow therapeutic window, considerable variability in patient dose-response, and multiple food–drug and drug–drug interactionsCitation10. These drawbacks necessitate frequent and costly coagulation monitoring and dose adjustments to ensure effective anti-coagulation by maximizing time in therapeutic range for the international normalized ratio (INR; usually target range of 2.0–3.0)Citation10. Product guidelines for the use of warfarin recommend daily INR measurement during warfarin initiation until the INR is stabilized within the therapeutic rangeCitation11. Thereafter, INR measurement should be performed at a frequency of every 1–4 weeks, depending on the individual patient’s situationCitation11. One recent Canadian study demonstrated that assessment of the warfarin dosing of patients every 12 weeks was non-inferior to assessment every 4 weeksCitation12. It is noteworthy that anti-coagulation with warfarin is effective in patients who have access to anti-coagulant monitoring services and are under the supervision of a dedicated physician. A recent large-scale study in Sweden showed that patients spent on average 76.2% of time within the therapeutic rangeCitation13. This is a much higher percentage than the 50–65% window reported in other studies, including the large-scale, international RE-LY (64%) and ROCKET AF (55%) studies of dabigatran etexilate and rivaroxaban, respectivelyCitation14,Citation15.
The challenges associated with anti-coagulation management using established anti-coagulants often result in poor adherence and discontinuation of therapyCitation16,Citation17, which places patients at increased risk of VTE. The simpler anti-coagulation management associated with novel oral anti-coagulants offers the potential for greater patient compliance, which would facilitate inpatient and outpatient prophylaxis and treatment, and be associated with corresponding cost reductions. However, owing to the relatively recent approval of the newer oral anti-coagulants, there is little published data to support greater compliance with these newer agents compared with traditional therapies.
Initiatives to improve thromboprophylaxis and treatment with anti-coagulants
Numerous strategies have been implemented by public health authorities to encourage appropriate thromboprophylaxis. Although clinical practice guidelines provide evidence-based recommendations that are broadly applicable, adherence to these guidelines has remained poorCitation18. Although two-thirds of at-risk patients received some form of thromboprophylaxis, fewer than one in seven received the appropriate type, dose, and duration of thromboprophylaxisCitation19. A number of organizations have established measures to assess and reduce the healthcare burden of VTE. The National Quality ForumCitation20 and the Centers for Medicare and Medicaid ServicesCitation21 have developed a list of ‘never events’, defining errors in medical care that are clearly identifiable and preventable, and have serious consequences for patients. For example, DVT or PE after total hip or total knee replacement surgery are now classified as hospital-acquired conditions. Care for these events will not be reimbursed by the Centers for Medicare and Medicaid Services, providing hospitals with a greater incentive to reduce the incidence of VTE and implement new quality initiatives.
The Joint Commission, in collaboration with the National Quality Forum, has developed a set of six performance measures ()Citation22 for prevention and treatment of VTE. Only one measure, VTE-6, tracks the incidence of ‘potentially preventable venous thromboembolism’. One recent pharmacy-led program reduced preventable VTE by 74% by increasing the appropriate type, dose, and duration of prophylaxis nearly 2-foldCitation5. Only about 60 of ∼5000 acute-care hospitals in the US are currently reporting on these measures because they are not mandatory at presentCitation23. However, it appears that these measures will be mandated by the Centers for Medicare and Medicaid Services in 2015Citation2Citation4. Mandating reporting of these measures would reduce morbidity, mortality, and healthcare costs at a time when savings are being desperately soughtCitation25,Citation26.
Table 1. The Joint Commission national hospital inpatient quality measures for venous thromboembolismCitation17.
New anti-coagulants: characteristics and clinical trials update
The difficulties surrounding the practicalities and clinical management of established anti-coagulants have led to the development of novel, oral agents. Unlike VKAs, which target multiple factors in the coagulation cascade, the newer oral agents target a specific coagulation factor, do not require routine coagulation monitoring, and could improve patient adherenceCitation27,Citation28.
The three most advanced clinical trial programs involve rivaroxaban, apixaban, and dabigatran etexilate; these oral anti-coagulants will be reviewed briefly.
Rivaroxaban
Rivaroxaban is an oral, direct Factor Xa inhibitor that has been approved in adult patients for the prevention of VTE after elective hip or knee replacement surgery; for the treatment of acute DVT and prevention of VTE recurrence; and for the prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation (AF) and at least one risk factor. Two-thirds of the drug undergoes metabolic degradation in the liver, half of which is excreted via the kidneys and half via the hepatobiliary route. One-third of the drug is eliminated as unchanged drug in the urine. Rivaroxaban has a fast onset of actionCitation29, with maximum concentrations occurring 2–4 h after tablet intake. For the 10 mg dose, bioavailability is high (80–100%) irrespective of fasting conditions, and the half-life is 5–13 h ()Citation30–32. The half-life and time to maximum plasma concentration of rivaroxaban are slightly prolonged in patients with renal impairment but, in general, the influence of renal function on drug clearance is moderate, even in patients with severe renal impairmentCitation33.
Table 2. Characteristics of rivaroxaban, dabigatran, and apixaban.
The RECORD program was a comprehensive clinical trial program that consisted of four phase III studies assessing the efficacy and safety of rivaroxaban for the prevention of VTE in adult patients undergoing elective total hip replacement surgery (RECORD 1 and RECORD 2) or elective total knee replacement surgery (RECORD 3 and RECORD 4)Citation34–37. RECORD1 compared rivaroxaban 10 mg once daily with the LMWH enoxaparin administered 40 mg once daily for 31–39 daysCitation34. RECORD2 compared rivaroxaban 10 mg once daily for 31–39 days with enoxaparin 40 mg once daily for 10–14 days followed by placeboCitation35. RECORD3 and RECORD4 compared rivaroxaban 10 mg once daily with enoxaparin 40 mg once daily or 30 mg twice daily, respectively, for 10–14 daysCitation36,Citation37. Rivaroxaban demonstrated superiority to the enoxaparin regimens in all four studies with respect to the primary efficacy end-point (composite of any DVT, non-fatal PE, and all-cause mortality), without significant differences in the rates of major bleedingCitation34–37. A pooled analysis of the four RECORD studies indicated that rivaroxaban regimens reduced symptomatic VTE plus all-cause mortality compared with enoxaparin regimens; this finding was consistent across patient sub-groups, irrespective of age, sex, body mass index, and renal functionCitation38. With rivaroxaban, there was a small, but statistically significant, increase in major plus non-major clinically relevant bleeding during the treatment periodCitation38.
The EINSTEIN phase III program comprises an acute DVT study (EINSTEIN DVT), an acute PE study (EINSTEIN PE), and a continued treatment study (EINSTEIN EXT). Results from EINSTEIN DVT and EINSTEIN EXT have been publishedCitation39. EINSTEIN DVT was a multi-center, randomized, open-label, event-driven, non-inferiority study comparing rivaroxaban (15 mg twice daily for the first 3 weeks followed by rivaroxaban 20 mg once daily) with standard-of-care therapy (enoxaparin and VKA, followed by dose-adjusted VKA only [INR target 2.5; range 2.0–3.0]) over a duration of 3, 6, or 12 months in more than 3400 patients with confirmed acute, symptomatic DVT without symptomatic PECitation39. Rivaroxaban showed non-inferiority for the composite of DVT, non-fatal PE, and fatal PE, compared with standard of care (2.1% vs 3.0%, respectively; hazard ratio [HR] = 0.68; 95% confidence interval [CI], 0.44–1.04; p < 0.001 for non-inferiority). The principal safety outcome, defined as clinically relevant bleeding, occurred in 8.1% of patients in both groups (HR = 0.97; 95% CI, 0.76–1.22; p = 0.77) ().
Table 3. Outcomes in the EINSTEIN acute DVT and continued treatment studiesCitation33.
The phase III EINSTEIN EXT studyCitation39 demonstrated that 20 mg once-daily rivaroxaban for 6 or 12 months was superior to placebo (1.3% vs 7.1%; HR = 0.18; 95% CI, 0.09–0.39; p < 0.001, relative risk [RR] reduction of 82%) in the secondary prevention of recurrent symptomatic VTE in patients with symptomatic DVT or PE who had already completed 6–12 months of treatment with a VKA or rivaroxaban (). The benefit of extended-duration rivaroxaban therapy was achieved irrespective of type of pre-treatment (rivaroxaban or VKA) or index event (DVT or PE). The incidence of major bleeding—the principal safety outcome—was rare (0.7% of patients receiving rivaroxaban; no patients receiving placebo). None of the major bleeding events was fatal, intracranial, or into a critical organ.
These studies demonstrated that a single-drug approach with rivaroxaban is as effective as the current standard of care, and with similar safety, in the treatment of acute DVT. When treatment is extended, rivaroxaban is very effective in preventing recurrences, compared with placebo, and has an acceptable bleeding risk.
The MAGELLAN study was a large-scale phase III clinical trial that evaluated extended-duration (35 ± 4 days) rivaroxaban (10 mg once daily) compared with standard-duration (10 ± 4 days) enoxaparin (40 mg once daily) followed by placebo for the prevention of VTE in hospitalized, acutely ill medical patientsCitation40. The primary efficacy end-point was the composite of asymptomatic proximal DVT, symptomatic DVT (proximal or distal), symptomatic non-fatal PE, and VTE-related death. Non-inferiority was assessed at Day 10 ± 5 and superiority was assessed at Day 35 ± 6. Rates of VTE at Day 10 were 2.7% in both treatment groups (p = 0.0025), demonstrating the non-inferiority of rivaroxaban. At Day 35, rates of VTE were 4.4% and 5.7% in the rivaroxaban and the enoxaparin arms, respectively (p = 0.0211)Citation41. Overall rates of major bleeding and non-major clinically relevant bleeding between Day 1 and Day 35 were low but were significantly higher in the rivaroxaban group than in the enoxaparin group (4.1% vs 1.7%, respectively; p < 0.0001). In summary, despite the reduced rate of VTE for extended-duration rivaroxaban when compared with standard-duration enoxaparin, there was no overall net clinical benefitCitation41.
Apixaban
Apixaban is an oral, direct inhibitor of Factor Xa that has recently gained approval in the European Union for the prevention of VTE after elective hip or knee replacement surgery in adultsCitation42. Peak plasma levels are achieved in ∼3 h. The half-life is 8–11 h when administered twice daily and 12–15 h when administered once daily (). Apixaban is eliminated via multiple pathways, including renal and intestinal routesCitation43.
The ADVANCE program assessed apixaban in three phase III clinical trials for the prevention of VTE after hip or knee replacement surgeryCitation44–46. The primary efficacy end-point in all three studies was defined as the composite of asymptomatic and symptomatic DVT, non-fatal PE, and death from any cause during treatment. In the ADVANCE-1 study for the prevention of VTE after total knee replacement surgery, apixaban 2.5 mg twice daily and enoxaparin 30 mg twice daily were started 12–24 h after surgery and continued for 10–14 daysCitation44. The primary efficacy end-point occurred in 9.0% of patients in the apixaban group and in 8.8% of the enoxaparin group (RR, 1.02; 95% CI, 0.78–1.32); these rates were much lower than expected. The composite incidence of major bleeding and clinically relevant non-major bleeding was 2.9% with apixaban and 4.3% with enoxaparin (p = 0.03). Apixaban did not demonstrate non-inferiority to enoxaparin in ADVANCE-1, but it was associated with lower rates of bleedingCitation44. The ADVANCE-2 and ADVANCE-3 studies compared apixaban 2.5 mg twice daily with enoxaparin 40 mg once daily for the prevention of VTE after total knee replacement surgery (ADVANCE-2; 10–14 days) and total hip replacement surgery (ADVANCE-3; 35 days)Citation45,Citation46. In ADVANCE-2, the primary efficacy end-point occurred in 15% of patients in the apixaban group and in 24% of patients in the enoxaparin group (RR = 0.62 [95% CI, 0.51–0.74]; p < 0.0001; absolute risk reduction 9.3% [95% CI, 5.8–12.7]). Major or clinically relevant non-major bleeding occurred in 3.5% and 4.8% of patients receiving apixaban and enoxaparin, respectively (p = 0.09)Citation45. In ADVANCE-3, the primary efficacy outcome occurred in 1.4% and 3.9% of patients treated with apixaban and enoxaparin, respectively (RR with apixaban, 0.36; 95% CI, 0.22–0.54; p < 0.001 for both non-inferiority and superiority; absolute risk reduction, 2.5 percentage points; 95% CI, 1.5–3.5)Citation46. The composite outcome of major and clinically relevant non-major bleeding occurred in 4.8% of the apixaban group and in 5.0% of the enoxaparin group (absolute difference in risk, −0.2 percentage points; 95% CI, −1.4–1.0).
In summary, despite apixaban not meeting non-inferiority in the ADVANCE-1 study, which compared apixaban 2.5 mg bid with enoxaparin 30 mg twice daily after total knee replacement surgery, the ADVANCE-2 and ADVANCE-3 studies showed that apixaban 2.5 mg twice daily was superior to enoxaparin 40 mg once dailyCitation44–46. In all three studies, there were no significant differences in the rates of major bleeding between the studied treatment regimens. A meta-analysis of ADVANCE-1, ADVANCE-2, and a phase II study showed that apixaban was more effective than enoxaparin in reducing the risk of proximal DVT but no more effective in reducing the risk of PE or all-cause mortality in patients after total knee replacement surgeryCitation47.
The ADOPT study compared extended-duration (30 days) apixaban 2.5 mg twice daily with standard-duration (6–14 days) enoxaparin 40 mg once daily in acutely ill medical patientsCitation48, and was similar in design to the MAGELLAN study. The primary efficacy end-point of ADOPT was defined as the composite of VTE-related death, PE, symptomatic DVT (proximal or distal), or asymptomatic proximal-leg DVT (as detected by systematic bilateral compression ultrasonography) during the 30-day treatment period. The primary efficacy end-point occurred in 2.71% and 3.06% of patients in the apixaban and enoxaparin groups, respectively (RR with apixaban 0.87; 95% CI, 0.62–1.23; p = 0.44). The principal safety outcome (major bleeding) occurred in 0.47% and in 0.19% of patients receiving apixaban and enoxaparin, respectively (relative risk 2.58; 95% CI, 1.02–7.24; p = 0.04). In summary, ADOPT showed that extended-duration apixaban was not superior to standard-duration enoxaparin and that apixaban was associated with an increased risk of major bleeding.
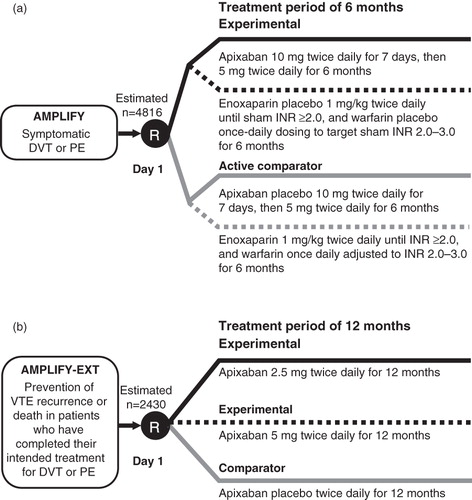
AMPLIFY (www.clinicaltrials.gov; NCT00643201) and AMPLIFY-EXT (NCT00633893) are phase III studies currently underway to evaluate the efficacy and safety of apixaban compared with current standard of care for the treatment of VTE and for the extended prevention of recurrent VTE and death, respectively (). Both studies are currently recruiting (www.clinicaltrials.gov; status as of February 7, 2012).
Dabigatran etexilate
Dabigatran is a reversible, competitive, direct thrombin inhibitor that is administered orally as the prodrug dabigatran etexilate. It has a fast onset of action of 1–2 h, a terminal half-life of 12–17 h, and ∼80% is excreted in the urine ()Citation49. Dabigatran is approved in more than 75 countries for the prevention of VTE in adult patients who have undergone elective total hip or knee replacement surgery, and has recently gained approval in the US for stroke prevention in patients with non-valvular AF.
Three double-blind, randomized phase III studies investigated the efficacy and safety of dabigatran etexilate for thromboprophylaxis after total hip and total knee replacement surgeryCitation50–52. Dabigatran etexilate had a similar safety profile to enoxaparin in all three trials. In the RE-NOVATE and RE-MODEL studies, dabigatran etexilate at doses of 150 mg or 220 mg once daily was started 1–4 h after total hip replacement surgery and total knee replacement surgery, respectivelyCitation50,Citation51. The comparator in both studies was enoxaparin 40 mg once daily, started the evening before surgery, and the primary efficacy end-point was defined as the composite of venographic or symptomatic VTE and death from all causes during treatment. In the RE-NOVATE study (median treatment duration: 33 days) the primary efficacy outcome occurred in 8.6%, 6.0%, and 6.7% of patients receiving dabigatran etexilate 150 mg once daily, dabigatran etexilate 220 mg once daily, and enoxaparin 40 mg once daily, respectively (absolute difference vs enoxaparin 1.9%; 95% CI, −0.6–4.4%, and –0.7%; 95% CI, –2.9–1.6%)Citation50,Citation51. In the RE-MODEL study (treatment period 6–10 days) the primary efficacy end-point occurred in 37.7% of the enoxaparin group, 36.4% of the dabigatran etexilate 220 mg group (absolute difference, –1.3%; 95% CI, –7.3–4.6), and 40.5% of the dabigatran etexilate 150 mg group (2.8%; 95% CI, –3.1–8.7)Citation50,Citation51. Both the RE-NOVATE and RE-MODEL studies demonstrated that dabigatran etexilate at doses of 150 mg or 220 mg once daily was non-inferior to enoxaparin (40 mg once daily)Citation50,Citation51.
In contrast, the RE-MOBILIZE (NCT00152971) study showed that, in patients undergoing total knee replacement surgery, VTE occurred within 12–15 days after surgery in 33.7% of the dabigatran etexilate 150 mg once daily group (p < 0.001 vs enoxaparin), in 31.1 % of the dabigatran etexilate 220 mg once daily group (p = 0.02 vs enoxaparin), and in 25.3% of patients receiving enoxaparin 30 mg twice daily with similar rates of bleeding between treatmentsCitation52. Therefore, in the RE-MOBILIZE study, dabigatran at either dose failed to meet non-inferiority compared with enoxaparin (30 mg twice daily)Citation52.
Four phase III clinical trials—RE-COVER (NCT00291330)Citation53, RE-COVER II (NCT00680186), RE-MEDY (NCT00329238), and RE-SONATE (NCT00558259)—have investigated the safety and efficacy of dabigatran for the treatment and secondary prevention of recurrent VTE.
RE-COVER and RE-COVER II compared dabigatran with warfarin and had the same primary efficacy and safety end-points. In contrast to the rivaroxaban and apixaban trials, these studies used a ‘dual-drug’ approach, with LMWH/UFH or fondaparinux in the initial phase (). RE-COVERCitation53 was a randomized, double-blind, non-inferiority trial that assessed the safety and efficacy of dabigatran (150 mg twice daily) compared with warfarin (INR 2.0–3.0) for 6 months in patients with acute symptomatic VTE after initial treatment (5–10 days) with an approved parenteral anti-coagulant. Dabigatran showed non-inferiority vs warfarin (p < 0.001), for the prevention of symptomatic recurrent VTE and VTE-related death within 6 months, but superiority was not achieved. Major bleeding occurred in 1.6% of patients receiving dabigatran and in 1.9% receiving warfarin (HR = 0.82; 95% CI, 0.45–1.48) (). RE-COVER II was completed in May 2011 (www.clinicaltrials.gov; status as of November 30, 2011).
Figure 2. Phase III VTE treatment trial designs for dabigatran etexilate: RE-COVER and RE-COVER II (a), RE-MEDY (b), and RE-SONATE (c). DVT, deep vein thrombosis; INR, international normalized ratio; PE, pulmonary embolism; R, randomization; VKA, vitamin K antagonist; VTE, venous thromboembolism.
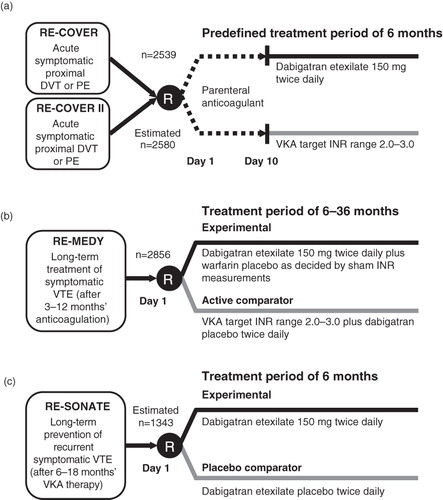
Table 4. Outcomes in the RE-COVER studyCitation40.
Preliminary results for the phase III studies RE-MEDYCitation54 (completed October 2010) and RE-SONATECitation55 (completed February 2011) have been presented. RE-MEDY demonstrated that dabigatran (150 mg twice daily) was as effective as warfarin (INR 2.0–3.0) in the extended treatment (6–36 months) of VTE in patients who had received 3–12 months of anti-coagulation therapy. When compared with warfarin there was a reduced risk of bleeding but an increase in acute coronary events in the dabigatran group ()Citation54.
In patients who had received 6–18 months of anti-coagulation therapy, the RE-SONATE phase III trial showed that dabigatran (150 mg twice daily) for 6 months reduced recurrent venous thromboembolic events compared with placebo (0.4% vs 5.6%; p < 0.0001), with clinically relevant bleeding in 5.3% of patients receiving dabigatran vs 1.8% receiving placebo (p = 0.001) ()Citation55.
Real-world scenarios with new oral anti-coagulants
Once a drug has been approved and reached the market, institutions such as the FDA and the European Medicines Agency (EMA) will monitor the safety of a drug in the real-world setting. Such reporting systems are valuable to identify hazards with drugs that were not apparent at time of approval.
Rivaroxaban
A recent retrospective cohort studyCitation56 reported a statistically significant increase (p = 0.046) in the rate of patients who returned to hospital with wound complications within 30 days after total hip or knee replacement surgery when thromboprophylaxis with tinzaparin (1.8%; 95% CI, 0.9–3.5) was compared with rivaroxaban (3.9%; 95% CI, 2.6–5.9). Real-world evaluation of rivaroxaban and other anti-coagulants will assess the incidence rate for this type of complication, although a pooled analysis of the RECORD program (RECORD1–4) showed no significant differences in the rate of serious adverse events related to surgery—including post-procedural infection, operative hemorrhage, wound dehiscence, post-operative wound infection, incision site hemorrhage, and hemarthrosis—with enoxaparin and rivaroxaban therapy (0.6% and 0.5%, respectively)Citation57.
Dabigatran etexilate
A recent, small-scale prospective study (n = 56) assessed wound complication in patients who received dabigatran etexilate after hip replacement surgery and compared results with a retrospectively matched patient group (n = 67) who had received subcutaneous dalteparinCitation58. In patients receiving dabigatran etexilate, the rate of delayed discharges owing to wound discharge was significantly increased from 7% in the dalteparin group to 27% in the dabigatran etexilate group (n = 15, p = 0.004). In addition, 7% of patients who received dabigatran etexilate returned to theatre with a wound complication compared with 1% of patients who received dalteparin (p = 0.18)Citation58. Furthermore, a retrospective analysis of real-world use of dabigatran etexilate for stroke prevention in patients with AF showed that the rates of hospitalization for bleeding and stroke/systemic embolism were higher than expected. These adverse events are consistently observed in elderly patients who have a reduced renal function and inherently a higher risk of bleedingCitation59.
The higher-than-expected (based on clinical trial outcomes) rates of adverse events reporting with rivaroxaban and dabigatran etexilate in clinical practice may reflect the use of these agents in high-risk patients, non-adherence of patients, or simply generally higher levels of reporting seen with newly approved drugs relative to established agents. Results of these real-world reports demonstrate the need for further investigation of the novel oral anti-coagulants in clinical practice.
Practical and pharmacoeconomic considerations
Drawbacks associated with established anti-coagulants (including UFH, LMWH, fondaparinux, and VKAs) lead to suboptimal patient adherence and have significant cost implications. A recent study reported that the cost of one INR test with VKA therapy ranged from $6.19–$145.70Citation60. Therefore, in addition to improved efficacy and patient adherence, the introduction of new anti-coagulants could significantly reduce the costs of drug administration and monitoring. A study in Canada reported savings of CAD300 per patient when comparing rivaroxaban and enoxaparin in patients undergoing total hip replacement surgery, owing to increased quality-adjusted life years (0.0006) and fewer symptomatic venous thromboembolic events (0.0061)Citation61. Similarly, in patients undergoing total knee replacement surgery, savings of CAD129 per patient were reportedCitation61. In another study, from the US payer perspective, rivaroxaban was associated with cost savings of US$511.93 and US$465.74 per patient after total hip replacement and total knee replacement, respectively, compared with enoxaparinCitation62. A similar study conducted in Sweden reported incremental cost per additional quality-adjusted life-years was SEK29,400 for extended prophylaxis (35 days) with rivaroxaban after total hip replacement and SEK35,400 for standard-duration enoxaparin or dalteparin (14 days)Citation63. Therefore, independent of the healthcare system, rivaroxaban is a cost-effective alternative to enoxaparin for thromboprophylaxis in patients undergoing total hip or knee replacement surgery. Evaluation of the cost-effectiveness of dabigatran etexilate in the UK showed that dabigatran etexilate was more cost-effective than enoxaparin after total knee replacement surgery; and was substantially more cost-effective after total hip replacement surgery (GBP137 vs GBP237 per patient, respectively)Citation64. Therefore, recent pharmacoeconomic assessments showed that rivaroxaban and dabigatran were more cost-effective than enoxaparin for thromboprophylaxis in patients undergoing elective hip or knee replacement surgeryCitation65–67.
In patients with AF, a cost-simulation study showed that dabigatran was more cost-effective in patients with a high risk of hemorrhage or stroke unless INR control with warfarin was excellent. Warfarin was more cost-effective in moderate-risk patients but only if INR control was goodCitation68. Cost simulations within the Canadian healthcare system indicated that dabigatran was estimated to be highly cost-effective compared with warfarin for stroke prevention in patients with AFCitation69. Further cost–efficacy analyses are required to determine the true costs of anti-coagulation therapy within the setting of different healthcare systems.
In addition to reducing costs associated with routine coagulation monitoring, oral agents such as rivaroxaban, apixaban, and dabigatran will eliminate the costs associated with injectable agents (e.g., nurse administration or training a patient to self-administer). Furthermore, simple and convenient oral administration of the new agents without routine coagulation monitoring may improve adherence and quality of life for patients, which could result in further reductions in the rate of VTE.
All three of the newer oral anti-coagulants discussed in this review are eliminated renally to varying degrees, and more clinical studies are needed to determine how best to utilize the agents in patients with moderate-to-severe renal impairmentCitation33,Citation70. Currently, no clinically approved antidotes exist for these new agents, but, owing to their relatively short half-life (compared with warfarin and fondaparinux), an antidote may be less of a requirement. The respective summary of product characteristics for each new oral agent provides guidanceCitation30,Citation42,Citation71 should patients present with an acute bleeding episode, require emergency surgery, or in case of acute renal dysfunction. In such cases, strategies such as delaying the next drug dose or discontinuation of anti-coagulation, activated charcoal (in case of overdose or very recent administration), mechanical compression, surgical intervention, or blood product transfusion should be considered and are thought to control bleeding in most cases. If these strategies are unsuccessful, a reversal agent may be helpful. A recent phase I study with healthy male volunteers showed that prothrombin complex concentrate completely restored coagulation parameters after administering rivaroxaban (20 mg twice daily) but not dabigatran (150 mg twice daily) and, therefore, might be a viable antidote for rivaroxabanCitation72. For apixaban and rivaroxaban, a newly developed recombinant Factor Xa may prove an effective antidoteCitation73. Dabigatran is partially dialyzableCitation71, in contrast to rivaroxaban and apixaban, for which plasma protein binding is high (92–95% and 87%, respectively)Citation30,Citation42. The outcomes of current or future clinical studies will provide further insight into the feasibility of reversal approaches for the new oral anti-coagulants.
Perioperative and procedural ‘bridging’ or ‘interruption’ will also change from the current standard of care because of the quicker onset and offset of action of these newer agents. It is likely that rivaroxaban, dabigatran, and apixaban therapy can be interrupted 1–2 days prior to the procedure, depending on the patient’s renal function, and be restarted when hemostasis is achieved, probably within 1–3 days depending on whether the procedural bleeding risk is low or high. It is unlikely any overlap of parenteral LMWH or UFH is beneficial, although further clinical trials are still needed to guide clinicians in this areaCitation74.
Conclusion
Approximately two-thirds of all venous thromboembolic events are associated with recent hospitalization, and VTE has been identified as the most common preventable cause of in-hospital deathCitation75. National and local quality improvement strategies have been developed to close the gap between expert recommendations and clinical practice. Strategies focus on increasing the use of thromboprophylaxis and ensuring that patients with VTE are treated according to existing guidelines, in addition to improving awareness of thromboembolic risks among healthcare practitionersCitation75,Citation76. These strategies are rapidly gaining importance as the population ages and the number of hospitalized patients with multiple risk factors for VTE increases.
Prevention and treatment of VTE is entering a new era, with the availability of novel, target-directed, oral anti-coagulants that do not require routine monitoring. Clinical trial results for the prevention of VTE in patients after elective hip or knee replacement surgery have led to the approval in some countries of three agents for this indication: the direct thrombin inhibitor dabigatran etexilateCitation50–52 and the direct Factor Xa inhibitors rivaroxabanCitation34–37 and apixabanCitation42. Following the RE-LY trialCitation14, dabigatran has also been approved in the US, Canada, and Japan for the prevention of stroke and systemic emboli in patients with non-valvular AF. Rivaroxaban has also gained recent approval for this indication in the US, following the ROCKET AF trial. Oral agents are available as alternatives to the current standard of care for patients at risk of thromboembolism and there is an expectation that their use will expand to include further indications. Given the advantages of these new agents over established anti-coagulants, they could potentially reduce the burden of anti-coagulant management and may lead to higher rates of VTE prophylaxis, thereby reducing the number of ‘never events’ occurring in hospitals.
Transparency
Declaration of funding
This paper was sponsored with funding from Bayer HealthCare Pharmaceuticals and Janssen Research & Development, L.L.C. (formerly Johnson & Johnson Pharmaceutical Research & Development, L.L.C.)
Declaration of financial/other relationships
Within the last year, CM has received traveling fellowship funds from the North American Thrombosis Forum and honoraria as a consultant and speaker from Boehringer Ingelheim and Sanofi-Aventis Pharmaceuticals and as a consultant from Polymedix Inc. and Leo Pharmaceuticals. CM is an unpaid consultant for Janssen Research & Development, L.L.C. (formerly Johnson & Johnson Pharmaceutical Research & Development, L.L.C.) and Janssen Scientific Affairs, L.L.C. (formerly Ortho-McNeil Janssen Scientific Affairs). AS has received honoraria as a consultant for Bayer Healthcare Pharmaceuticals, Eisai, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, and Sanofi-Aventis. AS is also on the Drug Safety Monitoring Board for Astellas and on the Steering Committee for Bayer Healthcare Pharmaceuticals.
Acknowledgments
The authors wish to acknowledge Claudia Wiedemann, PhD, who provided editorial support with funding from Bayer HealthCare Pharmaceuticals and Janssen Research & Development, L.L.C.
References
- US Department of Health and Human Services. The Surgeon General's call to action to prevent deep vein thrombosis and pulmonary embolism. 2008. http://www.surgeongeneral.gov/topics/deepvein/calltoaction/call-to-action-on-dvt-2008.pdf. Accessed February 6, 2012
- Heit JA, Mohr DN, Silverstein MD, et al. Predictors of recurrence after deep vein thrombosis and pulmonary embolism: a population-based cohort study. Arch Intern Med 2000;160:761-8
- Prandoni P, Lensing AWA, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med 1996;125:1-7
- Kearon C. Natural history of venous thromboembolism. Circulation 2003;107:I22-I30
- Mahan CE, Holdsworth MT, Welch SM, et al. Deep-vein thrombosis: a United States cost model for a preventable and costly adverse event. Thromb Haemost 2011;106:405-15
- Spyropoulos A, Lin J. Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: an administrative claims analysis from 30 managed care organizations. J Manag Care Pharm 2007;13:475-86
- Kearon C, Kahn SR, Agnelli G, et al. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians evidence-based clinical practice guidelines, 8th edn. Chest 2008;133:454S-545S
- Hirsh J, Bauer KA, Donati MB, et al. Parenteral anticoagulants: American College of Chest Physicians evidence-based clinical practice guidelines, 8th edn. Chest 2008;133:141S-59S
- Rajgopal R, Bear M, Butcher MK, et al. The effects of heparin and low molecular weight heparins on bone. Thromb Res 2008;122:293-8
- Ansell J, Hirsh J, Hylek E, et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians evidence-based clinical practice guidelines, 8th edn. Chest 2008;133:160S-98S
- Taro Pharmaceuticals U.S.A. I. (Warfarin sodium) Full prescribing information. 2011. http://www.drugs.com/pro/warfarin.html. Accessed February 6, 2012
- Schulman S, Parpia S, Stewart C, et al. Warfarin dose assessment every 4 weeks versus every 12 weeks in patients with stable international normalized ratios: a randomized trial. Ann Intern Med 2011;155:653
- Wieloch M, Sjalander A, Frykman V, et al. Anticoagulation control in Sweden: reports of time in therapeutic range, major bleeding, and thrombo-embolic complications from the national quality registry AuriculA. Eur Heart J 2011;32:2282-9
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139-51
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883-91
- Oake N, Fergusson DA, Forster AJ, et al. Frequency of adverse events in patients with poor anticoagulation: a meta-analysis. CMAJ 2007;176:1589-94
- Rose AJ, Ozonoff A, Grant RW, et al. Epidemiology of subtherapeutic anticoagulation in the United States. Circ Cardiovasc Qual Outcomes 2009;2:591-7
- Tapson VF, Hyers TM, Waldo AL, et al. Antithrombotic therapy practices in US hospitals in an era of practice guidelines. Arch Intern Med 2005;165:1458-64
- Amin A, Spyropoulos AC, Dobesh P, et al. Are hospitals delivering appropriate VTE prevention? The venous thromboembolism study to assess the rate of thromboprophylaxis (VTE start). J Thromb Thrombolysis 2010;29:326-39
- National Quality Forum. National voluntary consensus standards for prevention and care of venous thromboembolism: additional performance measures. Washington, DC: National Quality Forum, 2008. http://www.qualityforum.org/Publications/2008/10/National_Voluntary_Consensus_Standards_for_Prevention_and_Care_of_Venous_Thromboembolism__Additional_Performance_Measures.aspx. Accessed February 6, 2012
- Centers for Medicare and Medicaid Services. MA Payment guide for out of network payments. 2010. http://www.cms.gov/MedicareAdvtgSpecRateStats/Downloads/oon-payments.pdf. Accessed February 6, 2012
- The Joint Commission. National patient safety goals effective. 2012. http://www.jointcommission.org/assets/1/6/NPSG_Chapter_Jan2012_HAP.pdf. Accessed February 8, 2012
- Mahan C, Hussein MA, Amin AN, et al. Venous thromboembolism pharmacy intervention management program with an active, multifaceted approach reduces preventable venous thromboembolism and increases appropriate prophylaxis. Clin Appl Thromb Hemost 2012;18:45-58
- Centers for Medicare & Medicaid Services. Factsheet: improving quality of care during inpatient hospital stays. 2011. http://www.cms.gov/apps/media/press/factsheet.asp?Counter=4040&intNumPerPage=10&checkDate=&checkKey=&srchType=1&numDays=3500&srchOpt=0&srchData=&keywordType=All&chkNewsType=6&intPage=&showAll=&pYear=&year=&desc=false&cboOrder=date. Accessed February 9, 2012
- The Joint Commission. Hospital: 2011 National patient safety goals. 2011. http://www.jointcommission.org/hap_2011_npsgs/. Accessed February 6, 2012
- Centers for Medicare & Medicaid Services. CMS proposes to expand quality program for hospital inpatient services in FY 2009. 2007. http://www.cms.gov/apps/media/press/release.asp?Counter=3041. Accessed February 6, 2012
- Weitz JI. Emerging anticoagulants for the treatment of venous thromboembolism. Thromb Haemost 2006;96:274-84
- Borris LC. Barriers to the optimal use of anticoagulants after orthopaedic surgery. Arch Orthop Trauma Surg 2009;129:1441-5
- Tersteegen A, Schmidt S, Burkhardt N. Rivaroxaban - an oral, direct Factor Xa inhibitor - binds rapidly to Factor Xa. J Thromb Haemost 2007;5(2 Suppl):Abstract P-W-651
- Bayer Pharma AG. Xarelto® (rivaroxaban) summary of product characteristics. 2011. http://www.xarelto.com/html/downloads/Xarelto_Summary_of_Product_Characteristics_Dec2011.pdf. Accessed February 6, 2012
- Kubitza D, Becka M, Wensing G, et al. Safety, pharmacodynamics, and pharmacokinetics of BAY 59-7939 - an oral, direct Factor Xa inhibitor - after multiple dosing in healthy male subjects. Eur J Clin Pharmacol 2005;61:873-80
- Kubitza D, Becka M, Roth A, et al. Dose-escalation study of the pharmacokinetics and pharmacodynamics of rivaroxaban in healthy elderly subjects. Curr Med Res Opin 2008;24:2757-65
- Kubitza D, Becka M, Mueck W, et al. Effects of renal impairment on the pharmacokinetics, pharmacodynamics and safety of rivaroxaban - an oral, direct Factor Xa inhibitor. Br J Clin Pharmacol 2010;70:703-12
- Eriksson BI, Borris LC, Friedman RJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 2008;358:2765-75
- Kakkar AK, Brenner B, Dahl OE, et al. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet 2008;372:31-9
- Lassen MR, Ageno W, Borris LC, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 2008;358:2776-86
- Turpie AGG, Lassen MR, Davidson BL, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet 2009;373:1673-80
- Turpie AGG, Lassen MR, Kakkar AK, et al. A pooled analysis of four pivotal studies of rivaroxaban for the prevention of venous thromboembolism after orthopaedic surgery: effect on symptomatic venous thromboembolism and death, and bleeding. Haematologica 2009;94(2 Suppl):212, Abstract 0522
- The EINSTEIN Investigators. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010;363:2499-510
- Cohen AT, Spiro TE, Büller HR, et al. Extended-duration rivaroxaban thromboprophylaxis in acutely ill medical patients: MAGELLAN study protocol. J Thromb Thrombolysis 2011;31:407-16
- Cohen AT, Spiro TE, Büller HR, et al. Rivaroxaban compared with enoxaparin for the prevention of venous thromboembolism in acutely ill medical patients. American College of Cardiology Congress 60th Annual Scientific Session. New Orleans, LA, April 2-5, 2011; Oral presentation. http://my.americanheart.org/idc/groups/ahamah-public/@wcm/@sop/@scon/documents/downloadable/ucm_425442.pdf. Accessed February 8, 2012
- Bristol-Myers Squibb, Pfizer EEIG. Eliquis (apixaban) summary of product characteristics. 2011. http://www.eliquis.com/PDF/ELIQUIS%20®%20(apixaban)%20SmPC.pdf. Accessed February 6, 2012
- Carreiro J, Ansell J. Apixaban, an oral direct Factor Xa inhibitor: awaiting the verdict. Expert Opin Investig Drugs 2008;17:1937-45
- Lassen MR, Raskob GE, Gallus A, et al. Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med 2009;361:594-604
- Lassen MR, Raskob GE, Gallus A, et al. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomised double-blind trial. Lancet 2010;375:807-15
- Lassen MR, Gallus A, Raskob GE, et al. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med 2010;363:2487-98
- Huang J, Cao Y, Liao C, et al. Apixaban versus enoxaparin in patients with total knee arthroplasty. A meta-analysis of randomised trials. Thromb Haemost 2011;105:245-53
- Goldhaber SZ, Leizorovicz A, Kakkar AK, et al. Apixaban versus enoxaparin for thromboprophylaxis in medically ill patients. N Engl J Med 2011;365:2167-77
- Stangier J. Clinical pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor dabigatran etexilate. Clin Pharmacokinet 2008;47:285-95
- Eriksson BI, Dahl OE, Rosencher N, et al. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost 2007;5:2178-85
- Eriksson BI, Dahl OE, Rosencher N, et al. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet 2007;370:949-56
- The RE-MOBILIZE Writing Committee. Oral thrombin inhibitor dabigatran etexilate vs North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty 2009;24:1-9
- Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009;361:2342-52
- Schulman S, Ericsson H, Goldhaber SZ, et al. Dabigatran or warfarin for extended maintenance therapy of venous thromboembolism. J Thromb Haemost 2011;9(2 Suppl):731, Abstract O-TH-033
- Schulman S, Baanstra D, Eriksson H, et al. Dabigatran versus placebo for extended maintenance therapy of venous thromboembolism. J Thromb Haemost 2011;9(2 Suppl):22, Abstract O-MO-037
- Jensen CD, Steval A, Partington PF, et al. Return to theatre following total hip and knee replacement, before and after the introduction of rivaroxaban: a retrospective cohort study. J Bone Joint Surg Br 2011;93:91-5
- Gent M, Kakkar AK, Eriksson BI, et al. A pooled analysis of surgical complications: results from the RECORD program. Annual Meeting of the American Academy of Orthopaedic Surgeons. New Orleans, LA, March 9-13, 2010; P096. http://www3.aaos.org/education/anmeet/anmt2010/poster/poster.cfm?Pevent=P096. Accessed February 8, 2012
- Gill SK, Theodorides A, Smith N, et al. Wound problems following hip arthroplasty before and after the introduction of a direct thrombin inhibitor for thromboprophylaxis. Hip Int 2011;21:678-83
- Stanek EJ, Agatep BC, Herrera V, et al. Assessment of initial US dabigatran utilization and short-term persistence. Circulation 2011;124:Abstract A16675
- Chambers S, Chadda S, Plumb JM. How much does international normalized ratio monitoring cost during oral anticoagulation with a vitamin K antagonist? A systematic review. Int J Lab Hematol 2010;32:427-42
- Diamantopoulos A, Lees M, Wells PS, et al. Cost-effectiveness of rivaroxaban versus enoxaparin for the prevention of postsurgical venous thromboembolism in Canada. Thromb Haemost 2010;104:760-70
- Duran A, Sengupta N, Diamantopoulos A, et al. Cost effectiveness of rivaroxaban versus enoxaparin for prevention of post-surgical venous thromboembolism from a US payer's perspective. Pharmacoeconomics 2012;30:87-101
- Ryttberg L, Diamantopoulos A, Forster F, et al. Cost-effectiveness of rivaroxaban versus heparins for prevention of venous thromboembolism after total hip or knee surgery in Sweden. Expert Rev Pharmacoecon Outcomes Res 2011;11:601-15
- Wolowacz SE, Roskell NS, Maciver F, et al. Economic evaluation of dabigatran etexilate for the prevention of venous thromboembolism after total knee and hip replacement surgery. Clin Ther 2009;31:194-212
- McCullagh L, Tilson L, Walsh C, et al. A cost-effectiveness model comparing rivaroxaban and dabigatran etexilate with enoxaparin sodium as thromboprophylaxis after total hip and total knee replacement in the Irish healthcare setting. Pharmacoeconomics 2009;27:829-46
- Friedman RJ, Sengupta N, Lees M. Economic impact of venous thromboembolism after hip and knee arthroplasty: potential impact of rivaroxaban. Expert Rev Pharmacoecon Outcomes Res 2011;11:299-306
- Wolowacz SE. Pharmacoeconomics of dabigatran etexilate for prevention of thromboembolism after joint replacement surgery. Expert Rev Pharmacoecon Outcomes Res 2011;11:9-25
- Shah SV, Gage BF. Cost-effectiveness of dabigatran for stroke prophylaxis in atrial fibrillation. Circulation 2011;123:2562-70
- Sorensen SV, Kansal AR, Connolly S, et al. Cost-effectiveness of dabigatran etexilate for the prevention of stroke and systemic embolism in atrial fibrillation: a Canadian payer perspective. Thromb Haemost 2011;105:908-19
- Raghavan N, Frost CE, Yu Z, et al. Apixaban metabolism and pharmacokinetics after oral administration to humans. Drug Metab Dispos 2009;37:74-81
- Boehringer Ingelheim International GmbH. Pradaxa (dabigatran etexilate) summary of product characteristics. 2011. http://www.medicines.org.uk/emc/medicine/20760. Accessed February 6, 2012
- Eerenberg ES, Kamphuisen PW, Sijpkens MK, et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation 2011;124:1573-9
- Lu G, Luan P, Hollenbach S, et al. Reconstructed recombinant Factor Xa as an antidote to reverse anticoagulation by Factor Xa inhibitors. J Thromb Haemost 2009;7(2 Suppl):Abstract OC-TH-107
- Mahan CE, Spyropoulos AC. Peri-operative antithrombotic management and anticoagulant bridging. Can J Gen Intern Med 2011;6(1 Suppl):13-21
- Bratzler DW. Development of national performance measures on the prevention and treatment of venous thromboembolism. J Thromb Thrombolysis 2010;29:148-54
- Amin AN, Deitelzweig SB. Optimizing the prevention of venous thromboembolism: recent quality initiatives and strategies to drive improvement. Jt Comm J Qual Patient Saf 2009;35:558-64