Abstract
Background:
Neuroendocrine tumours (NETs) are a rare form of neoplasm that can arise in most organs of the body and which share many common pathologic features. Although curative surgery can be conducted for patients with localised disease, once progression occurs and the disease becomes metastatic or un-resectable, treatment aims to extend life and maintain quality-of-life for as long as possible. The aim of the study was to elicit utilities for health state vignettes describing the burdens associated with receiving therapy for advanced NETs.
Methods:
Health state vignettes were developed by reviewing published literature and conducting in-depth interviews with patients and clinical experts. These states described the burden associated with both stable and progressive disease, in addition to the experience of a number of serious toxicities commonly associated with treatments (grade III/IV diarrhoea, hand-foot syndrome, hyperglycaemia, nausea/vomiting, pneumonitis, rash, stomatitis, and thrombocytopenia). One hundred members of the UK general public valued the states using the time trade-off methodology to determine utility values.
Results:
Stable disease had a utility value of 0.77 whilst disease progression was associated with a significant decline in health-related quality-of-life (HRQoL) and a value of 0.61. Toxicities experienced in the context of stable disease exhibited varying degrees of impact, with several being deemed as debilitating as disease progression (such as hand-foot syndrome [0.58] and stomatitis [0.56]).
Conclusion:
Although vignette studies have been criticised for the difficulty in establishing their validity, the collection of health utilities in rare populations is challenging. The findings from this study suggest that advanced NETs is associated with a considerable HRQoL burden, both as a direct result of the disease and the potential of experiencing a number of severe adverse events. These values could assist in future economic evaluation processes.
Introduction
Neuroendocrine tumours (NETs) consist of a spectrum of malignancies that originate from the neuroendocrine cells in a variety of anatomical locations throughout the bodyCitation1. NETs secrete a variety of metabolically active substances and can cause distinct clinical manifestationsCitation2. Approximately two-thirds of NETs occur in the gastrointestinal tractCitation3, with ∼50% of patients being diagnosed in the advanced stage of the diseaseCitation1.
NETs are reported as very uncommon, with estimates suggesting incidence rates of fewer than two per 100,000 per yearCitation4. Other recent studies have shown an increase in incidence, however, from ∼1.09 per 100,000 in 1973 to 5.25 per 100,000 in 2004Citation1. Five-year survival for patients with advanced NETs is ∼35%, with individuals who have pancreatic tumours faring more poorly with a 5-year survival of 27%Citation1. Whilst in early stage disease surgical resection can be performed with curative intent, once the disease becomes metastatic, the treatment focus becomes palliative and aimed at improving survival and preserving quality-of-life for as long as possibleCitation5. There are four classes of pharmacological agents currently available: biotherapeutics (which include somatostatin analogues and interferon-α), chemotherapeutics (including streptozotocin, temozolomide, and platinum-containing compounds), small molecules (for example sunitinib and everolimus), and biologicals (bevacizumab and other antibodies). To date there has been no proper evaluation of response, adverse events, or cost for competing treatments in advanced NETs, and this is recognised as essential for guiding future treatmentCitation6. This paucity of suitable data means that, although numerous treatment options are being used at present, the prospect of conducting economic evaluations of such agents is challenging.
When making decisions about allocation of healthcare resources it is essential to evaluate the impact on both costs and health outcomesCitation7,Citation8. Whilst prolongation of life constitutes a clear benefit of a given treatment, it is also necessary to quantify any changes in quality-of-life that result from the use of particular therapies. Survival and quality-of-life are combined into a single metric referred to as the quality-adjusted life year (QALY). QALYs permit effective comparisons of different treatment to be conducted across a spectrum of disease areas. QALYs are calculated by multiplying the time spent in a particular state of health by the HRQoL weight (i.e., utility score) associated with that health state.
Organisations involved in conducting assessments of cost-effectiveness have different stated requirements for the collection of utility data, but most such bodies consider benefits in terms of QALYsCitation9. The National Institute for Health and Clinical Excellence (NICE) for example stipulates that the EQ-5DCitation10 generic health status measure be used for assessment of HRQoL in adultsCitation8. Whilst the collection of such data may be desirable it is not always available or perhaps even appropriate. In these instances alternative methodologies must be employed. One approach that has been used previously to capture utilities is through the development of health state descriptions or ‘vignettes’ which attempt to depict the typical burdens experienced by patients in a particular condition. These descriptions are then valued using the Time Trade-off (TTO) interview procedureCitation11. Time Trade-off is a widely used technique for the valuation of health states and the preferred method of organisations such as NICECitation8. The method presents individuals with two alternative scenarios and asks individuals which they would prefer. One scenario offers the prospect of living with the burdens associated with a particular condition whilst the other offers a shorter period of life which is spent in ‘full health’ (i.e., free of problems). The amount of time spent in the full health state is then varied until the individual is indifferent between the two choices. The value assigned to this point forms the utility of the description which can be used as the HRQoL weight in the QALY calculation.
The aim of the study was to elicit utilities for health state vignettes describing the burdens associated with receiving therapy for advanced NETs. These data could be used to support future economic evaluations of therapies for advanced NETs.
Patients and methods
The study protocol was submitted to the Independent Institutional Review Board (IIRB, Plantation, FL) for review prior to undertaking the study. Approval was granted in May 2011.
Development of the health states
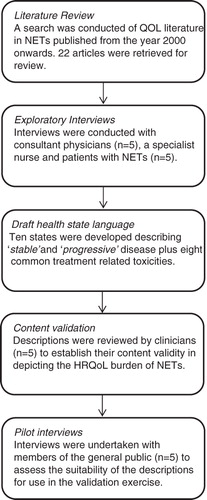
Two base health state descriptions were developed detailing the HRQoL impact of progression-free survival (‘stable’) and disease progression (‘progressive’) in NETs. These vignettes were produced on the basis of information provided by a literature review and input from both clinicians and patients. Additional states were produced which described the burden associated with a number of common treatment-related AEs by adapting the base ‘stable’ disease state. The development process is shown in .
Step 1: Literature review
The initial starting point for the development of the health states was a literature review examining existing quality-of-life research in NETs. Twelve databases (including MEDLINE, EMBASE, and the Cochrane Database of Systematic Reviews) were searched for articles published from the year 2000 onwards detailing studies where quality-of-life was a consideration. The review highlighted a relative paucity of existing literature, with 22 articles that met the criteria for extraction. These included a variety of different article types such as the development of a NET-specific module for the QLQ-C30Citation12, explorations of the HRQoL and psychosocial impacts of NETsCitation13, and a comparison of HRQoL in NET patients and members of the general publicCitation14. The findings from the literature demonstrated some support for the relationship between treatment efficacy and improvements in quality-of-lifeCitation15, but utility-type data suitable for use in supporting economic evaluations could not be identified.
Step 2: Exploratory interviews with clinicians and patients
Structured interview guides for use with clinicians and patients were developed based upon the findings from the earlier literature review. These guides aimed to explore the potential HRQoL impacts associated with NETs and their treatment. Interviews were conducted via telephone by an experienced interviewer.
Consultant physicians with significant experience of working with NET patients were recruited (n = 5) along with a specialist nurse. A number of issues were explored with these experts, such as symptom burden, adverse event experience, and perceived patient well-being. Experts reported that the condition could have very significant consequences for patients with a diverse range of potential symptoms dependent upon the disease characteristics. Treatment for the condition was also reported as often being burdensome and necessitating careful management of the toxicities associated with available therapies. Preservation of patient well-being was reported as a primary concern for experts but, nevertheless, patients often exhibited signs of poor psychological health.
After completion of the initial exploratory interview process experts were asked to characterise typical advanced NETs patients using the EQ-5D descriptive systemCitation10. The EQ-5D comprises five dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression), each with three levels of impact or experience (equating to none, limited, and severe). Individuals were asked to express in their opinion the level of burden that a typical patient with stable disease would experience on each of the included dimensions. The process was then repeated but this time considering patients with progressive disease. Results are reported in .
Table 1. Mode response of EQ-5D assessments by experts for stable and progressive disease.
Five patients with NETs were recruited through a specialist patient recruitment agency (pancreatic NET n = 3, gastrointestinal NET n = 2). Patients were encouraged to discuss their experiences of living with NETs and undergoing therapy for the condition. Individuals uniformly reported that the disease had presented a substantial challenge to their HRQoL. Despite best attempts to retain a relatively normal lifestyle patients invariably experienced functional limitations and often required assistance to perform activities of daily living. The most challenging episodes for patients typically occurred in the immediate post-retreatment period, with high levels of fatigue being particularly problematic. As with cancer generally, NETs appeared to place a significant psychological burden on patients with the extent dictated in part by an individual’s characteristics and coping mechanisms.
After completion of the exploratory interview process patients were asked to complete an EQ-5D assessment exercise similar to that previously undertaken by experts. In this instance patients were asked to rate their own health on the five dimensions. Results are reported in .
Table 2. EQ-5D assessments of NET patients own health.
Step 3: Draft health state language
Based upon the review of literature and interviews with patients and clinicians, a set of draft health states was produced. These descriptions were designed to depict the typical HRQoL challenges faced by patients with NETs without explicit mention of the type of condition they represented. The information obtained from the three sources suggested that HRQoL impact of tumours in both the GI tract and pancreas were sufficiently similar so that a single set of states could adequately characterise their associated burden. In an attempt to retain consistency, the states were based around the skeleton of the 5 EQ-5D dimensions. Ten states were developed in total describing stable disease, stable disease accompanied by one of eight AEs (either Grade III/IV diarrhoea, hand-foot syndrome, hyperglycaemia, nausea/vomiting, pneumonitis, rash, stomatitis, or thrombocytopenia), and progressive disease.
Step 4: Content validation
Once the draft health state descriptions had been developed they underwent a thorough review process. Three of the clinicians who had previously provided input into the state development were asked to examine the descriptions to ensure that they were accurate in depicting the experience of NET patients. Two additional clinicians who had no previous involvement in the study were asked to comment further on the accuracy, representativeness, and comprehensiveness of the health states. A number of minor revisions were made to the descriptions to better characterise symptoms (e.g., the insertion of ‘dry’ into the description of hand-foot syndrome).
Step 5: Pilot interviews
A series of pilot interviews were undertaken with members of the general public (n = 5) to determine whether the descriptions were fit for purpose. A tailor-made discussion guide was used to explore the extent to which the states were comprehensible to participants and their ability to readily distinguish between the different descriptions. The pilot interviews revealed no issues and so the health states were finalised for use in the TTO interviews.
Health state valuation
The health state valuation process was undertaken with members of the UK general public (n = 100). Participants were recruited from a number of locations around the UK (Berkshire, Oxfordshire, and Yorkshire), with the intent of providing a representative sample as described by the available census dataCitation16. Potential participants were provided with detailed study information and were given the opportunity to ask questions regarding any aspect of the study. Full written informed consent was obtained prior to interviews taking place which were conducted in suitable private locations by experienced interviewers. Participants received £25 as compensation for their time and travel expenses.
Before undertaking the valuation exercise participants completed a basic sociodemographic questionnaire, the EQ-5D generic health status assessment instrument, and a simple ranking exercise using the NET health states. This exercise was designed to determine the order of preference for the different descriptions and to familiarise individuals with both the concept of rating health states and the health state descriptions themselves. Once complete the participants undertook the TTO exerciseCitation17, which involved the following for each health state: participants were required to choose whether they preferred: (1) to live in the health state for a period of 10 years followed by death; (2) to live in the health state for 10-x years in full health; or (3) to indicate that the two previous options were equally desirable. Time in full health alternated between high and low values to avoid anchoring bias until the participant was indifferent between the options presentedCitation17. The data collected for each state was in the form of a utility value ranging from 0 [dead] to 1 [full health].
Statistical analysis
A planned sample size of 100 participants was deemed sufficient to provide robust estimates given that there were no specific hypotheses upon which power calculations could be based. Participant characteristics were summarised and mean utility values were calculated for the individual health states along with a measure of dispersion and interval estimate.
Results
Participant characteristics
Participant characteristics were compared with available census dataCitation16. The study sample had a fairly representative mean age (35.9 vs 38.2) and occupational make-up (53% vs 44%). The ethnicity of the sample slightly under-represents ethnic minorities (4% non-white vs 7.9%) and shows a bias towards female participants (61% vs 51%) ().
Table 3. Participants’ demographic profile (n = 100).
Participants’ EQ-5D ratings () were compared to the results from a previous national postal surveyCitation18. The number of significant problems experienced by participants across dimensions was lower than previously found. Given that the interviews were conducted face-to-face this may explain why fewer individuals with severe problems were willing to participate in the study.
Table 4. Participants’ EQ-5D ratings compared with a national surveyCitation18.
Health state utilities
The TTO derived utilities show the value that participants assigned to the health states (). The results demonstrated a very similar ordering to the warm-up VAS ranking exercise. Approximately half of the utility values fall within a narrow range of 0.583 (SD = 0.23) to 0.623 (SD = 0.23), reflecting that there is relatively little to differentiate between the severe burden that these states are associated with. The states with the highest values were stable disease 0.77 (SD = 0.20) and stable disease with hyperglycaemia 0.78 (SD = 0.19). The lowest values were associated with the hand-foot syndrome 0.58 (SD = 0.23) and stomatitis 0.56 (SD = 0.24) AEs, as well as progressive disease 0.61 (SD = 0.24) which has a fairly typical value for advanced oncological conditions such as breast cancer (0.61)Citation19, non-small cell lung cancer (0.6)Citation20, and non-Hodgkin’s lymphoma (0.61)Citation21.
Table 5. Mean health state utility values.
Discussion
This study aimed to elicit utility values for health states which described the typical HRQoL of individuals undergoing therapy for advanced NETs. The extent of disease was captured in two states (stable and progressive) along with the potential impact of a number of common treatment-related AEs. The states were developed through a rigorous process which included a review of available literature and interviews with both clinical experts and patients. After being reviewed to ensure their accuracy, the states were pilot tested before data collection was undertaken with the UK general public.
The results reveal that advanced NETs is associated with a considerable deterioration in HRQoL. Whilst disease-related burdens account for a substantial proportion of this disutility, treatment-related toxicities appear to play a significant role in determining the perceived severity of individual health states. The least burdensome AE was reported as hyperglycaemia in the context of stable disease which possessed a mean utility slightly (but not significantly) higher than stable disease alone. The apparent lack of severity in the context of the disease (and of other AEs) may have resulted in participants viewing its impact as negligible. The prospect of experiencing a number of AEs such as stomatitis and pneumonitis was viewed by the general public as being equivalent or potentially worse than experiencing disease progression. Whilst this may appear to be unexpected there are a number of potential explanations. First, it should be noted that the descriptions attempt to capture the HRQoL impact at the moment of AE experience. Whilst symptoms such as nausea and vomiting or diarrhoea can be extremely unpleasant for patients, they are usually only experienced for a limited period of time. Grade III/IV AEs present a serious (and sometimes potentially fatal) threat to patients and can often lead to a change in treatment regimen or discontinuation. Individuals undertaking the TTO exercise are asked to consider spending the remainder of their lives in this narrow window which cannot be claimed to be clinically realistic. Furthermore, as individuals are asked to rate health states with no indication as to the disease stage they depict, the greatly worsened prognosis associated with disease progression may be largely ignored. The values elicited in this study however do closely correspond with utility figures obtained through mapping from EORTC QLQ-C30 data collected in a large NET trialCitation22.
There are a number of limitations to the study that are worth noting. The use of the health state vignette approach for capturing utilities has been criticised in-part because of the difficulty associated with establishing the validity of health state descriptionsCitation8. A significant effort was made in this study to ensure that the vignettes were produced in a rigorous fashion. The development methodology used incorporated information from a variety of sources to ensure the accuracy and comprehensiveness of the descriptions which were constructed around an EQ-5D based skeleton. Such an approach should provide a solid foundation for the validity of the health states in depicting the experience of those with NETs. Moreover, the decision to adopt a vignette-based approach for this study was driven by the significant number of practical and ethical constraints involved in collecting this type of data. Attempting to elicit utilities directly from patients experiencing severe AEs poses a significant challenge as the incidence of such events is generally low. It is also uncertain as to what percentage of patients would be capable of providing data should they even wish to, given their current condition.
Conclusion
In conclusion the findings suggest that advanced NET is associated with a substantial HRQoL burden for individuals even where they exhibit signs of stable disease. The potential for experiencing a number of grade III/IV treatment-related toxicities could be particularly debilitating for patients in the short-term, and may lead to treatment discontinuation. Disease progression is associated with notably poorer quality-of-life, and illustrates the significant value to be gained in extending the progression-free survival period. The health utility values provided by this study could inform assessments of cost-effectiveness for therapies for advanced NETs.
Transparency
Declaration of funding
This study was funded by Novartis.
Declaration of financial/other interests
Paul Swinburn and Andrew Lloyd are employees of Oxford Outcomes, an ICON plc company, who were paid for designing and conducting the study. Jenny Wang and David Chandiwana are employees and shareholders of Novartis, and both authors were involved in the writing of the manuscript, and the decision to submit the paper for publication. Was Mansoor is the recipient of an honorarium from Novartis.
Acknowledgements
The authors acknowledge the contributions made by: Andrea Burgess, Royal Hampshire County Hospital, Winchester, UK; Dr David Farrugia, Cheltenham General Hospital, Cheltenham, UK; Amir Khan, Manor Hospital, Walsall, UK; Dr Denis Talbot, Churchill Hospital, Oxford, UK; and Dr Juan Valle, The Christie, Manchester, UK, for providing their clinical expertise. Honoraria for those contributions were provided by Novartis.
References
- Yao JC, Hassan M, Phan A, et al. One-hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:2063‐3072
- Kaltsas GA, Besser GM, Grossman AB. The diagnosis and medical management of advanced neuroendocrine tumors. Endocr Rev 2004;25:458‐511
- Yim KL. Role of biological targeted therapies in gastroenteropancreatic neuroendocrine tumours. Endocr 2011; 40:181--186
- Buchanan KD, Johnston CF, O’Hare MM, et al. Neuroendocrine tumors. A European view. Am J Med 1986;81:14‐22
- Kulke MH, Siu LL, Tepper JE, et al. Future directions in the treatment of neuroendocrine tumors: Consensus report of the National Cancer Institute neuroendocrine tumor clinical trials planning meeting. J Clin Oncol 2011;29:934‐43
- Rindi G, Wiedenmann B. Neuroendocrine neoplasms of the gut and pancreas: new insights. Nat Rev Endocrinol 2012;8:54--64
- Drummond MF, Sculpher MJ, Torrance GW, et al. Methods for the Economic Evaluation of Health Care Programmes. 3rd edn. Oxford: OUP, 2005
- National Institute for Health & Clinical Excellence (NICE). Guide to the methods of technology appraisal, NICE: London, UK June 2008 http://www.nice.org.uk/niceMedia/pdf/TAP_Methods.pdf. Accessed January 15, 2010
- Lloyd A, Wild D, Gallop K, et al. Reimbursement agency requirements for health related quality-of-life data: a case study. Expert Rev Pharmacoecon Outcomes Res 2009;9:527‐37
- Brooks R. EuroQol: the current state of play. Health Policy 1996;7:53‐72
- Torrance GW, Thomas WH, Sackett DL. A utility maximization model of evaluation of health care programs. Health Serv Res 1972;7:118‐33
- Davies AH, Larsson G, Ardill J, et al. Development of a disease-specific Quality of Life questionnaire module for patients with gastrointestinal neuroendocrine tumours. Eur J Cancer 2006;42:477‐84
- Fröjd C, Larsson G, Lampic C, et al. Health related quality of life and psychosocial function among patients with carcinoid tumours. A longitudinal, prospective and comparative study. Health Qual Life Outcomes 2007;5:18
- Haugland T, Vatn MH, Veenstra M, et al. Health related quality of life in patients with neuroendocrine tumors compared with the general Norwegian population. Qual Life Res 2009;18:719‐26
- Teunissen JJ, Kwekkeboom DJ, Krenning EP. Quality of life in patients with gastroenteropancreatic tumors treated with [177Lu-DOTA0, Tyr3]octreotate. J Clin Oncol 2004;22:2724‐9
- Office of National Statistics. Population of the United Kingdom: by ethnic group. National Statistics website, 2001. http://www.statistics.gov.uk/CCI/nugget.asp?ID¼273. Accessed January 15, 2010
- Torrance GW. Measurement of health state utilities for economic appraisal. J Health Econ 1986;5:1‐30
- Kind P, Dolan P, Gudex C, et al. Variations in population health status: results from a United Kingdom national questionnaire survey. BMJ 1998;316:736‐41
- Dedes KJ, Matter-Walstra K, Schwenkglenks M, et al. Bevacizumab in combination with paclitaxel for HER-2 negative metastatic breast cancer: an economic evaluation. Eur J Cancer 2009;45:1397‐406
- Esnaola NF, Lazarides SN, Mentzer SJ, et al. Outcomes and cost-effectiveness of alternative staging strategies for non-small-cell lung cancer. J Clin Oncol 2002;20:263‐73
- Lyman G, Lalla A, Barron R, et al. Cost-effectiveness of pegfilgrastim versus 6-day filgrastim primary prophylaxis in patients with non-Hodgkin's lymphoma receiving CHOP-21 in United States. Curr Med Res Opin 2009;25:401‐11
- Scottish Medical Consortium. Advice for the use of Sutent in the treatment of unresectable or metastatic, well-differentiated pancreatic neuroendocrine tumours with disease progression in adults. 2011 http://www.scottishmedicines.org.uk/SMC_Advice/Advice/698_11_sunitinib_Sutent_with_PAS/sunitinib_Sutent_with_PAS. Accessed September 30, 2011