Abstract
Background:
Omalizumab, licensed for patients with uncontrolled persistent allergic (IgE mediated) asthma, was found to be cost-effective based upon its clinical trial data. Observational studies have been undertaken to determine the real life outcomes of using omalizumab in the community.
Objective:
To determine the cost-effectiveness of omalizumab based upon observational data from the Netherlands and compare to its cost-effectiveness using clinical trial data.
Methods:
An observational study (eXpeRience) recruited allergic asthma patients eligible for Omalizumab therapy and followed them while on treatment. At 1 year, data from the Dutch patients enrolled in eXpeRience were examined to estimate the number of exacerbations and resource use while on omalizumab therapy compared to the year prior to omalizumab use. Observational data were used in a Markov model to calculate the lifetime cost-effectiveness ratios.
Results:
In the 1 year prior to omalizumab therapy the per-person rate of exacerbations was 3.39 compared to 1.07 in the year taking omalizumab. The discounted incremental lifetime additional costs for omalizumab were €55,865 for 1.46 additional quality-adjusted life years (QALY), resulting in €38,371/QALY. Using the INNOVATE clinical trial outcomes and current resource use, the prior ratio was €34,911/QALY, similar to the observational ratio. As in all observational studies, the main limitation is obtaining complete and accurate data. Patients with missing exacerbation or response data were excluded from this analysis.
Conclusion:
Non-clinical trial experience with omalizumab supported the finding of fewer exacerbations in the allergic asthma population while treated with omalizumab, and therapy was found to continue to have an attractive cost-effectiveness ratio.
Introduction
Asthma is a burdensome, chronic inflammatory disorder of the airways in which the majority of patients achieve good control with inhaled glucocorticosteroids (ICS) and long-acting inhaled beta 2-agonists (LABA). A small proportion of uncontrolled persistent asthma patients have IgE-mediated disease and the only approved anti-IgE therapy is omalizumab (OML), a humanized anti-IgE monoclonal antibody.
OML was originally assessed by the Netherlands’ health technology appraisal body in 2006, using an economic model based upon the INNOVATE pivotal trialCitation1 which supported the European registration. Clinical trials may not reflect the real-world benefit or costs of treatmentCitation2. To provide more insight into the real-life setting, a post-marketing, non-interventional, observational registry (eXpeRience) was establishedCitation3 to collect data on the effectiveness and safety of OML. These data are used in the study reported here to determine if OML is as cost-effective in the community setting as it was using the INNOVATE data. This economic analysis is one of the first reports to compare outcomes from both controlled and observational studies for OML in allergic asthma patients.
Methods
eXpeRience purpose and design
The observational eXpeRience registryCitation3 enrolled consenting patients (≥12 years) with uncontrolled allergic (IgE mediated) asthma despite treatment with high dose ICS (>1000 µg beclomethasone) and a LABA. Patients initiated OML therapy based on local standard practiceCitation3. Patients started OML treatment at their baseline visit and, after ∼16 weeks, the treating physician determined the patients’ response to treatment using the Global Evaluation of Treatment Effectiveness (GETE) instrumentCitation4. Lloyd et al.Citation4 explored the psychometric properties of the GETE and the relation to clinical measures, the AQLQ and asthma symptom scores. The GETE was found to be a valid measure of outcomes for patients with moderate-to-severe allergic asthma, and the OML label indicates that responders (good or excellent GETE scores) should remain on OML while the remainder are recommended to discontinue. Patients were asked to remain in the study for follow-up regardless of OML discontinuation.
The 1-year interim data from the Dutch eXpeRience population were analyzed to obtain inputs for the cost-effectiveness evaluation of OML. One-year retrospective resource use and clinical events were collected while patients were on standard therapy to serve as a comparison for their OML prospective treatment.
Model design
The validated and published lifetime Markov 5 state OML modelCitation5,Citation6 used in the prior Dutch submission was adapted to incorporate the clinical and resource outcomes from eXpeRience. In the ‘daily symptoms’ state, patients may have non-clinically significant asthma exacerbations in addition to their usual symptoms. As patients experience clinically significant (CS) exacerbations, they move from their daily symptoms state to either the CS or clinically significant severe (CSS) states. As they recover, they move back to the ‘daily symptoms’ state. The model includes two states for death: death from all causes and asthma-related death due to CSS exacerbations.
The model followed patients from age 40 for a lifetime and discounted future outcomes at 1.5% and future costs at 4%Citation7. Societal costs based on work days lost were included.
The model compared allergic asthma patients managed with OML plus standard therapy to those managed only with standard of care (SoC) therapy. OML therapy was assumed to be maintained for up to 5 years in those who demonstrated a response (good/excellent GETE).
Health outcome data
The annual exacerbation rates were calculated using the number of exacerbations occurring during the retrospective year for SoC and the prospective year for OML. All patients who received OML from baseline until determining response at week 16 were analyzed as the overall group. They were then divided into GETE good/excellent responders who remained on therapy, those who were determined not to respond who still remained on therapy, and those who were not responders who went off therapy.
Exacerbations were stratified according to the eXpeRience registry definitions as CS (worsening of asthma as judged clinically significant by the physician, requiring treatment with rescue oral or intravenous corticosteroids) or CSS (a CS exacerbation also resulting in peak expiratory flow (PEF) <60% of personal best or predicted and requiring treatment with systemic corticosteroids)Citation3. The annual exacerbation rates of the treated groups were transformed into relative risks in comparison to SoC exacerbations taken from the retrospective analysis of patients not on OML.
All-cause mortality rates for the lifetime model were taken from Dutch life tablesCitation8 appropriate for the gender mix of the observational study. A risk of asthma death associated with CSS exacerbations was applied based upon a UK studyCitation9 with sensitivity analyses conducted on this rate.
Resource use and exacerbations
Healthcare resource use was captured for 1 year prior to the baseline visit for SoC and prospectively for 1 year for OML. Hospital admissions, emergency room (ER) visits, and unscheduled physician visits relating to exacerbations were combined regardless of treatment and stratified by CS or CSS exacerbation. This assumed that resource use for exacerbations were not dependent upon the therapy received. The resources were costed using Dutch sourcesCitation10 and then inflated to 2010Citation11,Citation12 to produce CS and CSS exacerbation costs. Sensitivity analyses were conducted on CS and CSS resource costs.
The drug treatment costs were based on the weighted drug use observed. The model assumed that the doses and proportion of patients on therapy remained constant during the model and was the same for the SoC and OML arms.
The cost of monthly OML add-on therapy was based on the weighted average of OML dose regimens at the baseline visit. This dose was verified by comparing to that at the end of the first year of eXpeRience. Additional costs included prescription filling and OML administration in the doctor’s office ().
Table 1. Asthma model inputs.
Work loss
The eXpeRience study captured work days missed for all patients in the study for both the retrospective and observational study years. There was missing data for 77% of the retrospective patients (used as the SoC group). Thus, the prospective data was used to estimate the work loss and cost of productivity per hour was calculatedCitation11 and applied to patients regardless of the treatment arm for the entirety of a patient’s working life (). A sensitivity analysis was conducted using reported work days lost in the retrospective data and applied to the SoC group.
Utility values
Because eXpeRience did not collect utility values, the model used EQ-5D data from PERSIST, an observational study in patients with uncontrolled persistent asthma performed in BelgiumCitation13, along with Dutch tariffsCitation14, to adjust the utility calculations. Utilities for exacerbation events were taken from Lloyd et al.Citation15 because the PERSIST trial did not capture data at an exacerbation event ().
Results
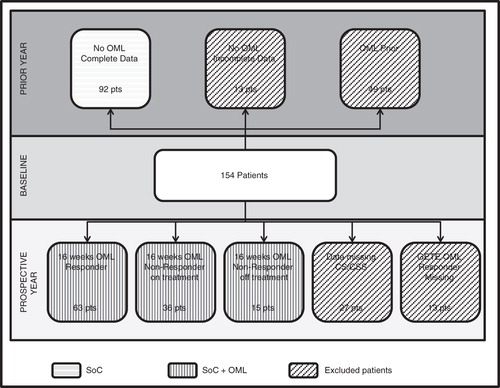
The registry followed 876 patients in 14 countries and data for 154 Dutch patients were used in this economic analysis. The average age of the Dutch sample was 43.9 (16–76) and 71.4% were women. Of the 154 patients, 92 patients had no OML therapy and complete data () for the retrospective study, thus their data provided the information for the SoC. For the prospective year on OML therapy, 114 of the 154 patients had complete data (27 patients had missing exacerbation event data and 13 patients were missing OML response data). Data from these 114 patients comprised the OML therapy group for the first 16 weeks. At 16 weeks, GETE response was evaluated and 63 of the 114 patients were considered good/excellent responders by the physicians (15 were considered non-responders). Although some patients without GETE-confirmed OML response remained on OML (n = 36), in the base case model these patients were returned to standard therapy alone with those costs and outcomes. In a sensitivity analysis, these so-called ‘non-responders’ who remained on OML were retained in the responder group and the exacerbation rates calculated based on this mixed group of patients.
The SoC patients had 312 exacerbations, of which 223 were defined as CS and 89 defined as CSS from the retrospective year of study. The annual rate of any exacerbation was 3.39 (number of exacerbations divided by patient years), of which 28.5% were CSS, resulting in exacerbation rates of 0.97 for CSS and 2.42 for CS ().
The 114 OML patients had 123 exacerbations, of which 98 were CS and 25 were CSS for rates of 0.86 and 0.22, respectively. This population was separated into responders and non-responders. There were 63 responders with 48 total exacerbations during the year, resulting in a 0.22 relative risk of any exacerbation compared to the SoC group (calculated as 0.76/3.39). The exacerbation risk for the mixed group of OML patients was similarly calculated as 0.27.
The resources from all patients with complete data were used. There were 460 exacerbations, of which 27.4% were considered severe. Hospital admissions (128), ER visits (146), and unscheduled doctor visits (130) were assigned by the reported type of exacerbation. Unit costs (€70/physician visit; €154/ER visit; €3910/hospital admission) were taken from published sourcesCitation11 and the calculated costs by exacerbation (regardless of drug therapy) were €1316 for CS and €1308 for CSS.
Annual SoC drug costs were calculated at €1498 and annual OML costs were €16,766 based upon a price per mg and initial dose from prospective data. A sensitivity analysis was conducted using the cost of the soon-to-be available pre-filled syringe.
The deterministic results indicated that the OML responder cohort had greater life years (LYs) and quality-adjusted life years (QALYs) (1.82 and 1.46, respectively) than SoC due to the decreased number of CS and CSS exacerbations () in the OML group. The main cost driver was OML therapy, €55,865 more than SoC therapy. The higher drug costs were partially offset by the greater number of exacerbations in the SoC cohort. The resulting incremental cost-effectiveness ratio (ICER) was calculated by dividing the incremental costs by the incremental QALYs. At €38,371/QALY, the ratio is considered cost-effective for otherwise uncontrolled allergic asthma patients with relatively few therapy options.
Table 2. Lifetime cost effectiveness findings for omalizumab in the Netherlands.
Of the sensitivity analyses conducted (), the model was particularly sensitive to the time horizon (shorter time had higher ICERs), increasing the cost of a CSS exacerbation (decreased the ICER), and exacerbation-related mortality. Excluding asthma-related mortality increased the ICER to €80,332/QALY. Using the estimated work loss from the SoC retrospective data base compared to the prospective estimated work loss resulted in €14,504/QALY, the lowest ICER obtained in the analysis. Combing the outcomes from the OML patients regardless of response status had little impact on the ICER, thus patients who remained on OML had better outcomes than SoC patients, although not as ‘good’ as the patients determined to be good/excellent responders.
Table 3. Sensitivity analyses for omalizumab in the Netherlands.
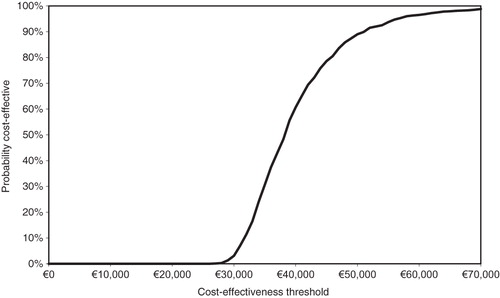
The probabilistic sensitivity analysis (PSA) found that, 75% of the time (), the ICER was less than €44,000/QALY, indicating that including ranges and distributions for all the parameters resulted in similar cost-effectiveness ratios as the deterministic analysis and is a relatively good value for the Dutch healthcare system.
Inserting the INNOVATE exacerbation rates into the economic model with eXpeRience resource use calculated €34,911/QALY compared to €38,371/QALY. Although there were more exacerbations reported in the eXpeRience study (435) than INNOVATE (229), the relative differences between the SoC and OML treatment groups were similar between the RCT and the observational study (17% of INNOVATE exacerbations were OML and 28% of eXpeRience exacerbations were OML).
Discussion
This analysis of the OML eXpeRience observational study found OML cost-effective in the Dutch setting. The base case ICER was €38,371 and the sensitivity analyses ranged between €32,375–€80,332. The model findings using eXpeRience agree with the initial cost-effectiveness analysis (€39,215) based upon the exacerbation rates and resource use from the INNOVATE RCT. Applying the resource use from eXpeRience registry and the INNOVATE exacerbation rates also has similar findings (€34,911). This analysis provides the Dutch health authorities evidence of the cost-effectiveness of OML in allergic asthma patients in the Dutch practice setting.
An interesting finding in the observational study is the continuation of OML therapy in some patients who did not show GETE good/excellent response at 16 weeks. These patients had fewer relapses than the SoC patients and fewer than the non-responders who were taken off OML. It appears that, where response may be limited (not reaching good or excellent according to the physician’s assessment), continued use of OML improved patient outcomes, but this use requires further investigation. It is possible that patients were maintained on OML because the improvement was considered sufficient by the physician, although not reaching the GETE good/excellent response level, and the patients appeared to have benefited. The GETE instrument is meant to restrict OML use to patients most likely to improve, but clearly in eXpeRience a relaxation of the good/excellent assessment allowed a sub-set of patients to continue therapy and they had an improved response. If the data were correctly recorded and analyzed further research may be necessary with these patients
As in all observational studies, there are limitations to the data. Inaccuracies and missing data are possible. Patients missing exacerbations data or GETE response were excluded from the cost-effectiveness study and no data were imputed. An examination of patients missing either exacerbation or GETE response information indicated they were similar to patients with that data.
Regardless of the limitations, this observational study provided ‘real–life’ use of OML in the allergic asthma population in the Netherlands and verified the decrease of both exacerbations and resource use compared to the prior year without OML therapy.
The variable with the greatest impact on the ICER is the potential for asthma-related deaths. There is some evidence that patients with uncontrolled asthma have a greater risk of death than the general population. Watson et al.Citation9 reported age-related risk of asthma deaths for individuals hospitalized with acute severe asthma (J46 hospital code) ranging between 0.097% (0–11 years of age) and 2.478% (45 years and older). A Swedish studyCitation16 of community-based severe exacerbations found a 3.109% probability of death. Thus, in a lifetime analysis of asthma management, it is reasonable to apply some additional risk for exacerbation-related deaths. In this analysis, the rate from Watson et al. was applied when CSS exacerbations occurred. In a sensitivity analysis, the risk of exacerbation-related deaths was eliminated, resulting in an ICER of €80,332 when excluding the 2.8 asthma deaths in the SoC treatment group.
Model duration also causes variation in the ICER. As in most chronic diseases, examining costs and outcomes over shorter timeframes results in higher ICERs than the longer term analyses. Shorter timeframes do capture the full outcomes associated with treatment.
This study is one of the first in allergic asthma patients to compare the cost-effectiveness ratios between the RCT and an observational study. An Italian study compared the retrospective resource use and clinical outcomes with prospectively collected data for a group of OML patientsCitation17. This study found that patient clinical and health-related quality-of-life improved while medical resources decreased with the addition of OML to SoC. Another observational retrospective study conducted in IrelandCitation18 also found a substantial reduction in hospital admissions (66% vs 24%) and bed days (2.4 ± 0.41 vs 0.8 ± 0.37) when OML was added to SoC. Our study further compared the ICER found using the RCT with the observational data, showing that the findings were translatable into the day-to-day practice in allergic asthma patients.
Conclusion
The financial burden of uncontrolled allergic asthmatic patients is high due to hospitalizations, exacerbations, and unscheduled visits to physicians and hospitals. Anti-IgE treatment offers substantial clinical improvement to a majority of IgE-mediated allergic patients at reduced resource use. Currently, there are no alternative therapies for this patient category with similar clinical efficacy and safety profiles. Therefore, GINA guidelinesCitation19 have proposed OML as Step 5 treatment in severe asthmatic patients when no asthma control is obtained with high doses of ICS and LABA. Cost-effectiveness studies like the eXpeRience study add new ‘real-life’ information to the growing body of evidence supporting the additional value of anti-IgE treatment for patients with uncontrolled allergic asthma.
Transparency
Declaration of funding
This study was sponsored by Novartis.
Declaration of financial/other relationships
FvN, SS, Ms. CT, and RB were employed by the United BioSource Corporation (UBC) during the execution of this study, which provides consulting and other research services to pharmaceutical, device, government, and non-government organizations. In this salaried position, they work with a variety of companies and organizations. They receive no payment or honoraria directly from these organizations for services rendered. G-JB was a paid investigator in the original observational study. MG is an employee of Novartis B.V.
Acknowledgments
The authors thank the physicians and patients participating in the eXpeRience observational study.
References
- Humbert M, Beasley R, Ayres J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy 2005;60:309-16
- Vignola AM, Humbert M, Bousquet J, et al. Efficacy and tolerability of anti-immunoglobulin E therapy with omalizumab in patients with concomitant allergic asthma and persistent allergic rhinitis: SOLAR. Allergy 2004;59:709-17
- Braunstahl GJ, Leo J, Thirlwell J, et al. Uncontrolled persistent allergic asthma in practice: eXpeRience registry baseline characteristics. Curr Med Res Opin 2011;27:761-7
- Lloyd A, Turk F, Leighton T, et al. Psychometric evaluation of global evaluation of treatment effectiveness: a tool to assess patients with moderate-to-severe allergic asthma. J Med Econ 2007;10:285-96
- Brown R, Turk F, Dale P, et al. Cost-effectiveness of omalizumab in patients with severe persistent allergic asthma. Allergy 2007;62:149-53
- Dewilde S, Turk F, Tambour M, et al. The economic value of anti-IgE in severe persistent, IgE-mediated (allergic) asthma patients: adaptation of INNOVATE to Sweden. Curr Med Res Opin 2006;22:1765-76
- College voor zorgverzekeringen (CVZ). Guidelines for pharmacoeconomic research, updated version. Diemen, The Netherlands: College voor zorgverzekeringen, 2006. http://www.cvz.nl/binaries/content/documents/cvzinternet/en/documents/procedures/guidelines-pharmacoeconomic-research.pdf. Accessed August 1, 2011
- Centraal Bureau voor de Statistiek. Centraal Bureau voor de Statistiek [web page]. http://www.cbs.nl/nl-NL/menu/home/default.htm. Accessed December 14, 2010.
- Watson L, Turk F, James P, et al. Factors associated with mortality after an asthma admission: a national United Kingdom database analysis. Respir Med 2007;101:1659-64
- Nederlandse Zorgautoriteit (NZA). Zoekprogramma DBC bedragen en overige bedragen medisch specialistische zorg door of vanwege de zorginstelling. Utrecht, The Netherlands: Nederlandse Zorgautoriteit (NZA), 2010. http://ctg.bit-ic.nl/Nzatarieven/top.do. Accessed December 20, 2010
- Hakkaart-van Roijen L, Tan SS, Bouwmans CCA. Handleiding voor kostenonderzoek: Methoden en standaard kostprijzen voor economische evaluaties in de gezondheidszorg (Geactualiseerde versie 2010). Diemen, The Netherlands: College voor Zorgverzekeringen, 2010. http://www.cvz.nl/binaries/content/documents/cvzinternet/nl/documenten/rubriek+zorgpakket/cfh/handleiding-kostenonderzoek-2010.pdf. Accessed February 18, 2011
- Price Index. 2010. http://www.nza.nl/regelgeving/prijsindexcijfers/. Accessed December 14, 2010.
- Brusselle G, Michils A, Louis R, et al. "Real-life" effectiveness of omalizumab in patients with severe persistent allergic asthma: the PERSIST study. Respir Med 2009;103:1633-42
- Lamers LM, Stalmeier PF, McDonnell J, et al. [Measuring the quality of life in economic evaluations: the Dutch EQ-5D tariff]. Ned Tijdschr Geneeskd 2005;149:1574-8
- Lloyd A, Price D, Brown R. The impact of asthma exacerbations on health-related quality of life in moderate to severe asthma patients in the UK. Prim Care Respir J 2007;16:22-7
- Lowhagen O, Ekstrom L, Holmberg S, et al. Experience of an emergency mobile asthma treatment programme. Resuscitation 1997;35:243-7
- Dal Negro RW, Pradelli L, Tognella S, et al. Cost-utility of add-on omalizumab in difficult-to-treat allergic asthma in Italy. Eur Ann Allergy Clin Immunol 2011;43:45-53
- Costello RW, Long DA, Gaine S, et al. Therapy with omalizumab for patients with severe allergic asthma improves asthma control and reduces overall healthcare costs. Ir J Med Sci 2011;180:637-41
- Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. Cape Town, South Africa: Global Initiative for Asthma (GINA), 2011. http://www.ginasthma.org/guidelines-gina-report-global-strategy-for-asthma.html. Accessed August 1, 2011