Abstract
Objective:
A new and smaller percutaneous ventricular assist device (pVAD, Impella, Abiomed, Danvers, MA) has been developed to provide circulatory support in hemodynamically unstable patients and to prevent hemodynamic collapse during high-risk percutaneous coronary interventions (PCI). The objective of the study was to assess the cost-effectiveness of this specific device compared to the intra-aortic balloon pump (IABP) from the European perspective. Additional analysis on extracorporeal membrane oxygenation was conducted for observational purposes only, given its use in some European countries.
Methods:
A combination of a decision tree and Markov model was developed to assess the cost-effectiveness of the small, pVAD. The short-term (30-day) effectiveness and safety (early survival, risk of bleeding, and stroke) were modeled, as well as long-term risk of major adverse cardiovascular events (recurrent myocardial infarction, stroke, and heart failure). The short-term effectiveness and safety data for the device were obtained from two registries (the Europella and USpella), both of which are large multi-center studies in high-risk patient groups. Probabilities of long-term major adverse cardiovascular events were obtained from various published clinical studies. The economic analysis was conducted from a German statutory health insurance perspective and only direct medical costs were included. Cost-effectiveness was estimated over a 10-year time horizon.
Results:
Compared with IABP, the pVAD generated an incremental quality-adjusted life-year (QALY) of 0.22 (with Euro-registry data) and 0.27 (with US-registry data). The incremental cost-effectiveness ratio (ICER) of the device varied between €38,069 (with Euro-registry data) and €31,727 (with US-registry data) per QALY compared with IABP.
Key limitations:
Unadjusted, indirect comparisons of short-term effectiveness and safety between the interventions were used in the model. Cost and utility data were retrieved from various sources. Therefore, differences in patient populations may bias the estimated cost-effectiveness.
Conclusions:
Compared with IABP, the pVAD is a cost-effective intervention for high-risk PCI patients, with ICERs well-below the conventional cost-effectiveness threshold.
Introduction
Despite technological and procedural advances, high-risk percutaneous coronary intervention (PCI) still carries a high burden of morbidity and mortality. This is especially true for patients at high risk of left ventricular decompensation such as those presenting with a large acute myocardial infarction (AMI) with or without cardiogenic shock (CS), end-stage heart failure or cardiomyopathy of various etiology. Of the 2.5 million PCIs performed annually in Europe, 2–3% of patients (≈75,000) will experience life-threatening periprocedural complications such as CSCitation1,Citation2. The incremental cost of complications is as high as $15,437 in addition to the costs of the PCI and hospitalizationCitation3. Thus, the consequences of treatment are heavily associated with higher costs to the healthcare system for both providers and payers. Indeed, compared with patients without complications, the mean hospital costs of those experiencing complications are 56% higherCitation3.
Economic evaluations are essential tools for decision-makers who must choose the most effective healthcare interventions considering the available financial resources. The intra-aortic balloon pump (IABP) has been routinely used for circulatory support in patients with a high risk of hemodynamic collapse during PCI. The device has a relatively low cost per procedure but its role has been challenged as large-scale studies failed to show any clear benefits in patients with or without CSCitation4–8. For example, in patients without CS, the BCIS-1 trial reported hospital discharge rates of major adverse cardiac and cerebrovascular events (MACCEs) of 15.2% with elective IABP compared to 16.0% without planned IABP. Six-month all-cause mortality rates were also similar, at 4.6% and 7.4%, respectivelyCitation7. During long-term follow-up, however, patients treated with planned IABP had improved survival compared with patients without planned IABP supportCitation9, although the trial was not designed nor powered to demonstrate a difference in survival. Extracorporeal membrane oxygenation (ECMO) is another treatment option which is used mainly for patients with severe cardiac or pulmonary insufficiency. The new percutaneous ventricular assist device (pVAD, Impella, a registered trademark of Abiomed, Danvers, MA) is associated with a lower risk of periprocedural adverse eventsCitation10–17. It has also been associated with a shorter length of stay and less usage of blood products compared with ECMOCitation18.
The importance of health economics evaluation integration into the product development cycle has been highlighted previouslyCitation19,Citation20. In Europe, any new medical device is required to demonstrate safety and performance based on non-randomized clinical studies to get market authorization (European Conformity). The nature of medical devices and the regulatory process often result in the early approval of the products to ensure patients’ access to innovative technologies while randomized controlled trials are in progress. In such cases, early cost-effectiveness studies of new technologies provide additional information for preliminary clinical and financial decision-making.
The aim of the present study was to evaluate the cost-effectiveness of the short-term pVAD compared with IABP in high-risk PCI patients with severely compromised left ventricular function in the European healthcare system. Additional analysis on ECMO was conducted for observational purposes only, given its use in some European countries.
Methods
Model description
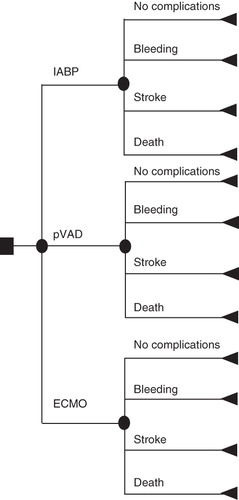
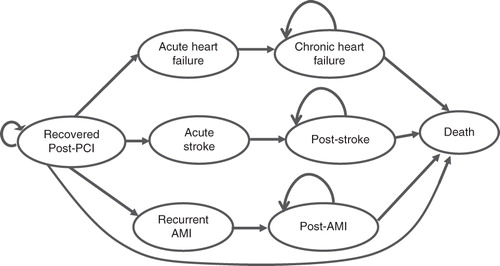
A decision tree and Markov model were constructed to assess the economic value of the pVAD’s circulatory support for high-risk patients undergoing PCI. The cycle length was 1 month. The decision tree structure included four branches for each intervention: no complications and the three most common acute complications (≤30 days) namely death, major bleeding, and stroke (). The decision tree covered the first month after the initial PCI, and all surviving patients were included in the Markov model. The three most common long-term (>30 days to 10 years) MACCE covered were heart failure, stroke and recurrent AMI (). During each cycle, patients who survived the initial PCI could either die, experience recurrent AMI, acute stroke, heart failure, or remain healthy. Patients who experienced acute adverse events could progress into chronic states (post-AMI, post-stroke, or chronic heart failure) or die. Subsequently, patients could remain in the chronic state or die.
The economic analysis was conducted from a German statutory health insurance perspective. Only direct medical costs related to the index intervention and short- and long-term MACCEs were included. Cost-effectiveness was estimated over a 10-year time horizon.
Input data
Clinical effectiveness and safety data
The clinical outcomes for the pVAD were derived from two registries (Europella; n = 144Citation15 and USpella; n = 175Citation11), both are large, multi-center, observational registries of high-risk patients including urgent PCI post-ST-elevation myocardial infarction (STEMI) as well as elective PCI. Mean left ventricular ejection fraction was low at 29% and ∼20% of patients in the US registry presented with CS of various etiologies at the time of the PCICitation11. Circulatory support improved various hemodynamic parameters significantly and the overall 30-day mortality rate was 4.8%, with rates of AMI and stroke of 0.6% and 0.7%, respectively. The studies’ designs, baseline characteristics, and clinical outcomes have been presented previouslyCitation11,Citation15. For the purpose of this study, only patients who underwent a high-risk PCI with the pVAD were included in the cost-effectiveness analysis. High-risk indications included multivessel disease in 86%, unprotected left main vessel in 52%, and sole remaining vessel in 10% (US registry)Citation11.
The IABP was used as the primary and relevant comparator. Effectiveness data for IABP were derived from a recent meta-analysis in patients with ST-elevation myocardial infarctionCitation8. Unadjusted, indirect comparisons were used for the 30-day effectiveness and safety of the two interventions in terms of mortality, bleeding, and stroke rates. There were no differences among the study arms in the probability of long-term MACCEs. Monthly probabilities were calculated for the data and were derived from published observational and controlled studies. Effectiveness data, transition probabilities, and parameter estimates for the Markov model are presented in .
Table 1. Effectiveness data, transition probabilities, and parameters estimates.
Survival
Disease-specific survival was adjusted by using the German life tablesCitation21. In patients that survived the first month after the index intervention, the remaining life expectancy was considered to be independent of the therapy received, although the per protocol analysis of the PROTECT II study showed that the pVAD was associated with a statistically significant reduction in the 90-day composite end-point of major adverse events (40% vs 51%, p = 0.023) compared with IABPCitation10.
Resource utilization and cost data
German-specific resource utilization and cost data were used (). The costs of the interventions included the cost of PCI and single device cost (pVAD and IABP). The costs of ECMO services were included for the observational analysis only. The cost of the initial PCI with and without complications was derived from relevant German DRG tariffs. Because, in German settings, the percutaneous microaxial blood pumps, IABP and ECMO service are reimbursed through additional funding outside DRG system (ZE, Zusatzentgelt) and prices are negotiated at the hospital level, the costs for the pVAD, IABP, and 3-day ECMO support were obtained from a German hospital, in which all three technologies are actively used (Hannover Medical School, Hannover). As there might be significant variations between hospitals in the reimbursable price of the devices, prices from two additional hospitals (University Clinic Göttingen and University Clinic Bonn) were used in the sensitivity analysis.
Costs of health states were derived from published German economic evaluations. In the model, death was not associated with any cost. Cost data are presented in 2011 Euros. Inflation adjustment was performed using the German consumer price indexCitation38.
Utility data
Health-related quality-of-life (HRQoL) was expressed on the basis of the generic HRQoL instrument EQ-5D scores. Utilities were derived from German and, in the absence of German data, from the UK literatureCitation33–37 (). For major bleeding, a utility of 0.3 was used as no published utility sources were found. Patients’ HRQoL during the first month was calculated as a weighted sum of utilities for stroke, bleeding, and post-PCI condition with no complications.
Analysis
The incremental cost-effectiveness ratio (ICER) was calculated by comparing the difference in average total costs and the difference in average quality-adjusted life-years (QALY) and life-years gained (LYG) among the study cohorts with IABP as the primary comparator. Comparisons with ECMO were done for observational purposes only. The intervention was considered cost-effective if the ICER was below €50,000 per QALY. All costs and outcomes beyond the first year were discounted 3.5% annually based on the recommendations of the Institute for Quality and Efficiency in Health Care (IQWIG)Citation39. The model allowed age correlation for the data inputs for the cohorts with different mean age. The model was constructed in Microsoft Excel 2007 (Microsoft Corp., Redmond, WA).
Sensitivity analysis
One-way sensitivity analysis was performed to assess the impact of varying the model parameters while holding other variables fixed at base case values. Cost drivers (variables with the major input to the costs) were identified and results are presented by the means of a Tornado diagram. In addition, a probabilistic sensitivity analysis was performed with 10,000 Monte Carlo simulations. Beta distribution was used for the probabilities and utility variables, gamma distribution was used for the cost data, and uniform distribution was used for the variables based on expert or analyst assumptions.
Results
For this high-risk patient group, the pVAD was shown to be a cost-effective treatment. When compared with IABP, the incremental cost for the pVAD was €8377 (Euro-registry data) and €8599 (US-registry data). The pVAD generated an incremental QALY of 0.22 (Euro-registry data) and 0.27 (US-registry data). When the Euro-registry data were used, the pVAD was cost-effective against IABP with an ICER of €38,069/QALY. The ICER to achieve one additional LYG was €27,193/LYG. When the US-registry data were used, the pVAD was cost-effective against IABP with an ICER of €31,727/QALY. The ICER to achieve one additional LYG was €22,694/LYG.
When comparing the study arm interventions, ECMO generated less QALYs and life-years. Both the pVAD (ICER €10,168/QALY using Euro-registry data and €9944/QALY using US-registry data) and IABP (ICER €4326/QALY) were cost-effective compared to ECMO. The results of the cost-effectiveness analysis are presented in .
Table 2. Incremental cost-effectiveness ratio base case values.
Sensitivity analysis
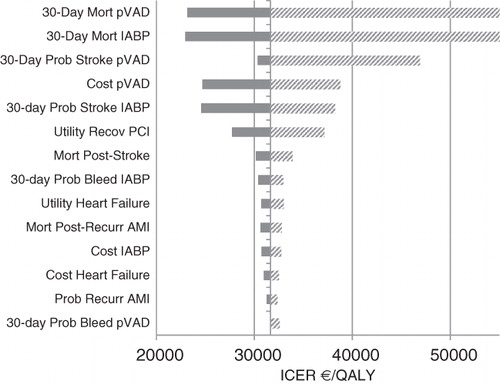
To verify the robustness of the analysis, one-way and probabilistic sensitivity analyses were carried out. In the one-way sensitivity analysis of the pVAD (using US-registry data) vs IABP, the ICER was the most sensitive to the changes of the 30-day mortality for the pVAD arm and the 30-day mortality for the IABP arm () with maximum tested value crossing the €50,000/QALY willingness-to-pay threshold. Other sensitive parameters included the utility of post-PCI recovered health state, the cost of the pVAD, and the 30-day probability of stroke in the pVAD and IABP arms.
The pVAD was cost-effective compared with IABP when the cost of the three comparative technologies was taken from the Göttingen University Hospital (cost of the pVAD €8735, cost of IABP €792, and cost of 3-day ECMO support €5900; the ICER for comparison of the pVAD and IABP was €35,607/QALY using Euro-registry data and €29,680/QALY using US-registry data). The pVAD was also cost-effective when the cost was taken from the University Hospital Bonn (cost of the pVAD €9673, cost of IABP €809 and cost of 3-day ECMO support €9632; the ICER for comparison of the pVAD and IABP was €39,566/QALY using Euro-registry data and €32,930/QALY using US-registry data).
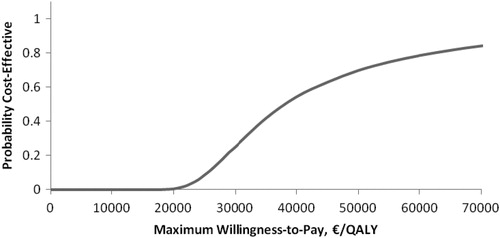
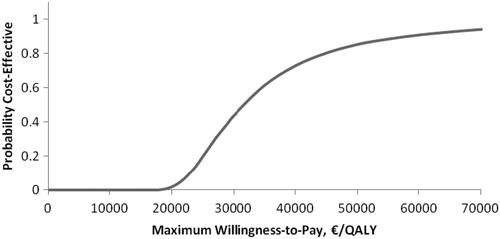
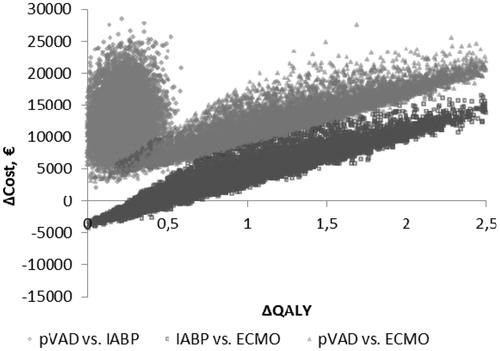
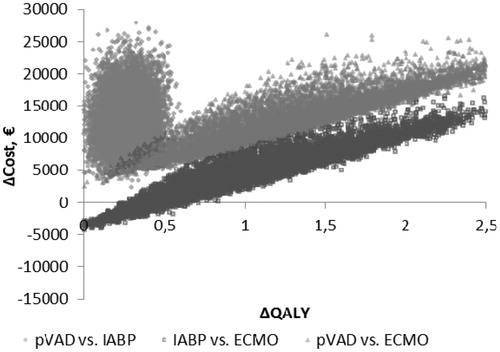
The probabilistic sensitivity analysis demonstrated that the pVAD device had a 69% (Euro-registry data, ) and an 85% (US-registry data, ) probability of being cost-effective when the cost-effectiveness threshold was considered to be €50,000/QALY. Cost-effectiveness acceptability planes ( and for Euro-registry data and US-registry data, respectively) demonstrated that the pVAD provides a stable beneficial effect in 10,000 simulations compared with IABP at an incremental cost in the range of €2049–€28,502 using Euro-registry data and in the range of €2351--€27,907 using US-registry data.
Discussion
Clinical decision-making is increasingly influenced by cost-effectiveness, which is a composite measure of both the costs and clinical effectiveness of a therapeutic intervention. Many interventions have typical initial costs that have definitive economic savings in prospective life in terms of life-years gained and improved quality-of-life to the patient and, consequently, to the healthcare system.
In the present cost-effectiveness analysis, the pVAD generated more QALY compared with IABP, although the initial cost of the pVAD was higher. Extracorporeal membrane oxygenation was analyzed for observational purposes only and, as expected, both the pVAD and IABP were cost-effective compared with ECMO due to the very high rate of complications with ECMO. The ICERs generated per QALY and LYG with the pVAD were well below the conventional cost-effectiveness threshold in most European countries.
Our results demonstrate that, by reducing periprocedural adverse events and subsequent costs and events during longer-term follow-up, the pVAD provided significant health and economic benefits to the healthcare system for high-risk patients undergoing a PCI. Numerous European and American studies have been published on the clinical impact of the pVAD support, and the only cost-effectiveness analysis has been limited to the US and Canada. To our knowledge, this is the first economic evaluation of the pVAD across a broad range of high-risk patients using results of large observational studies from the European perspective.
During high-risk PCI, the pVAD is deployed to allow the clinician more time to perform a more complete procedure while avoiding ischemic and hypotensive events, thus providing stability to patients who otherwise might not have the inherent ventricular function or ischemic reserve to tolerate the procedure. Our results are in agreement with two studies conducted in the US describing the cost-effectiveness of circulatory support with the pVAD in patients at high-risk of hemodynamic collapse during PCICitation40,Citation41. The economic evaluation of the PROTECT II study, a randomized trial that compared IABP and the pVAD in patients with severe left ventricular dysfunction and complex coronary anatomy undergoing PCI, revealed an ICER of $39,389/QALY, which is well below the conventional US threshold of $100,000/QALYCitation40 and in line with our cost-effectiveness analysis results.
Study limitations
Our analysis has limitations. The model used in this analysis was driven by unadjusted, indirect comparisons of short-term effectiveness and safety between the interventions (the pVAD, IABP, and ECMO) due to the absence of such data at the time of model development. Limitations of indirect comparisons were highlighted previouslyCitation42,Citation43. Ideally, the clinical effectiveness/safety and cost-effectiveness must be evaluated in a prospective randomized controlled trial which provides the greatest internal validity of the results. Recently, economic evaluations of the pVAD in a randomized comparison with IABP from the PROTECT II study were performed from the US perspective which may provide new insights into the cost-effectiveness of the evaluated technologiesCitation40,Citation41. Nevertheless, clinical studies have provided supportive data for the comparisons used in the current model. In the recently published PROTECT II studyCitation10, the per protocol analysis revealed that the pVAD was associated with a statistically significant reduction in the longer-term 90-day composite end-point of major adverse events (40% vs 51%, p = 0.023) compared with IABP. However, in the PROTECT II study, outcomes were reported at 90-day follow-up, whereas our model was built on 30-day effectiveness. The significant benefit demonstrated after hospital discharge (rates of death, stroke, AMI, and repeat revascularization) was 9.8% in the pVAD arm compared with 18.6% in the IABP arm (p = 0.009) and may be under-estimated in the current model, as our model assumed no differences in the probability of long-term outcomes. Thus, the lower rates of AMI and coronary interventions may lead to the prevention of heart failure which, in turn, may lead to further benefits by decreasing the use of implantable cardioverter-defibrillators, hospitalizations, etc. The burden of heart failure in Germany was estimated as €2.8 billion, of which €1.7 billion are spent on in-hospital servicesCitation44. The impact of longer-term outcomes and cost should be considered in future studies.
Also, because our analysis included various patient populations (including patients with CS in the ECMO arm), more recently published data since the completion of this study suggest that the QALY and LYG benefits of the pVAD may also have been over-estimated. Indeed, results of the EUROSHOCK registry, which retrospectively evaluated the safety and effectiveness of the pVAD in patients with AMI complicated by severe CS, revealed an overall 30-day mortality rate of 64.2%, with 40% of patients dying while on supportCitation45. Periprocedural complications with earlier versions of the pVAD and accessories were also significantly higher, with bleeding requiring transfusion and/or surgery occurring in 28.6% of patients compared with 6% in the high-risk PCI Euro-registry. Meanwhile, multiple changes were made to the pVAD system, which led to significant improvements in safety of the device. The ISAR-SHOCK trial, which randomized 26 patients to hemodynamic support with the pVAD compared to IABP, showed superior hemodynamic improvement with the pVAD, but had a similar 30-day mortality of 46% in both armsCitation46. Similarly, results from the recently published IABP-SHOCK II trialCitation4 failed to demonstrate the benefits of IABP in terms of 30-day mortality (39.7%) compared with optimal medical therapy (41.3%), although in the group of patients with high-risk elective PCI (Balloon Pump-Assisted Coronary Intervention Study), IABP demonstrated better short-term safetyCitation7. Regarding ECMO comparisons, while this analysis was carried out for observational purposes only, it should be noted that an over-estimation of the mortality and complication rates is possible due to the inclusion of patients with CS. Finally, the clinical effectiveness and safety, cost, and utility data were derived from multiple sources which may limit the consistency of the analysis. As the analysis was performed from the German statutory health insurance perspective, it was not possible to take into account the 2-day decrease in the length of hospital stay with the pVAD compared to IABP demonstrated in PROTECT II trial (7 vs 9 days, p = 0.048)Citation41. The decrease in the length of hospital stay from 9 to 7 days did not impact the tariff for the PCI procedure although, in countries with different payment systems, it may have an impact on the total cost of the pVAD treatment, which can be slightly over-estimated in the present study.
Due to the above-mentioned limitations, the results of the modeling should be interpreted with caution and further update of the model with data from controlled studies is required.
Conclusion
On the basis of the present analysis, the pVAD is a cost-effective intervention compared with the IABP for high-risk patients undergoing a PCI. Both data sources (Euro-registry and US-registry) showed cost-effectiveness by producing ICERs well below the European conventional willingness-to-pay threshold and by gaining more life-years and quality-adjusted life-years with minimal incremental costs.
Transparency
Declaration of funding
This study was supported by Abiomed Inc.
Declaration of financial/other relationships
JBR and OVB have disclosed that they are employees of Synergus AB, a MedTech consulting firm that received funding from Abiomed to help conduct this study. SND has disclosed that he is a consultant to Abiomed. TK has disclosed that he has received a research grant and speaker’s fees from Abiomed. IP has disclosed that he is an advisor to Abiomed, MedTronic, St. Jude, Ample Medical, Vortex, and Seimans. TS has no relevant financial relationships to disclose. JPSH has disclosed that he has been on the speakers’ bureau of Abiomed. JME peer reviewers on this manuscript have no relevant financial relationships to disclose.
Acknowledgments
We thank Danielle Libersan, PhD for her help in the development of the manuscript and Staffan Bjessmo, MD, PhD for his review of the manuscript and valuable comments. The conceptual model was developed and data inputs were provided by Sarowar Golam, MD, who was employed at Synergus AB at the time of model development, while analysis was performed by Johanna B. Roos.
References
- Moschovitis A, Cook S, Meier B. Percutaneous coronary interventions in Europe. EuroIntervention 2010;6:189-94
- Lindholm MG, Køber L, Boesgaard S, et al. Cardiogenic shock complicating acute myocardial infarction; prognostic impact of early and late shock development. Eur Heart J 2003;24:258-65
- Jacobson KM, Hall Long K, McMurtry EK, et al. The economic burden of complications during percutaneous coronary intervention. Qual Saf Health Care 2007;16:154-9
- Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012;367:1287-96
- Curtis JP, Rathore SS, Wang Y, et al. Use and effectiveness of intra-aortic balloon pumps among patients undergoing high risk percutaneous coronary intervention: insights from the National Cardiovascular Data Registry. Circ Cardiovasc Qual Outcomes 2012;5:21-30
- Patel MR, Smalling RW, Thiele H, et al. Intra-aortic balloon counterpulsation and infarct size in patients with acute anterior myocardial infarction without shock. The CRISP AMI randomized trial. JAMA 2011;306:1329-37
- Perera D, Stables R, Thomas M, et al. Elective intra-aortic balloon counterpulsation during high-risk percutaneous coronary intervention. A randomized controlled trial. JAMA 2010;304:867-74
- Sjauw KD, Engström AE, Vis MM, et al. A systematic review and meta-analysis of intra aortic balloon pump therapy in ST-elevation myocardial infarction: should we change the guidelines? Eur Heart J 2009;30:459-68
- Press-release: elective use of an intra-aortic balloon pump during high-risk PCI is associated with a 34 percent reduction in all-cause mortality, 2012. http://ca.maquet.com/file_assets/therapies-solutions/en/BCIS-1-Balloon-Pump-Assisted-Coronary-Intervention.pdf. Accessed December 10, 2012
- O’Neill WW, Kleiman NS, Moses J, et al. A prospective randomized clinical trial of hemodynamic support with Impella 2.5 versus intra-aortic balloon pump in patients undergoing high-risk percutaneous coronary intervention: the PROTECT II study. Circulation 2012;126:1717-27
- Maini B, Naidu SS, Mulukutla S, et al. Real-world use of the Impella 2.5 circulatory support system in complex high-risk percutaneous coronary intervention: the USpella registry. Catheter Cardiovasc Interv 2012;80:717-25
- Alasnag MA, Gardi DO, Elder M, et al. Use of the Impella 2.5 for prophylactic circulatory support during elective high-risk percutaneous coronary intervention. Cardiovasc Revasc Med 2011;12:299-303
- Iliodromitis KE, Kahlert P, Plicht B, et al. High-risk PCI in acute coronary syndromes with Impella LP 2.5 device support. Int J Cardiol 2011;153:59-63
- Dixon SR, Henriques JPS, Mauri L, et al. A prospective feasibility trial investigating the use of the Impella 2.5 system in patients undergoing high-risk percutaneous coronary intervention (The PROTECT I Trial). Initial U.S. experience. JACC Cardiovasc Interv 2009;2:91-6
- Sjauw KD, Konorza T, Erbel R, et al. Supported high-risk percutaneous coronary intervention with the Impella 2.5 device. The Europella Registry. J Am Coll Cardiol 2009;54:2430-4
- Burzotta F, Paloscia L, Trani C, et al. Feasibility and long-term safety of elective Impella-assisted high-risk percutaneous coronary intervention: a pilot two-centre study. J Cardiovasc Med 2008;9:1004-10
- Henriques JP, Remmelink M, Baan J, Jr et al. Safety and feasibility of elective high-risk percutaneous coronary intervention procedures with left ventricular support of the Impella Recover LP 2.5. Am J Cardiol 2006;97:990-2
- Cheung A. Temporary circulatory support: Impella. ASAIO 58th Annual Conference. San Francisco, CA. June 13–16, 2012
- Vallejo-Torres L, Steuten LM, Buxton MJ, et al. Integrating health economics modeling in the product development cycle of medical devices: a Bayesian approach. Int J Technol Assass Health Care 2008;24:459-64
- Sculpher M, Drummond M, Buxton M. The iterative use of economic evaluation as part of the process of health technology assessment. J Health Serv Res Policy 1997;2:26-30
- The Federal Statistics Office, German Life Tables, 2008-2010. http://www.destatis.de/EN/FactsFigures/SocietyState/Population/Deaths/Deaths.html. Accessed November 24, 2012
- Formica F, Avalli L, Martino A, et al. Extracorporeal membrane oxygenation with a poly-methylpentene oxygenator (Quadrox D). The experience of a single Italian centre in adult patients with refractory cardiogenic shock. ASAIO J 2008;54:89-94
- Smedira NG, Moazami N, Golding CM, et al. Clinical experience with 202 adults receiving extracorporeal membrane oxygenation for cardiac failure: survival at five years. J Thorac Cardiovasc Surg 2001;122:92-102
- Ezekowitz JA, Kaul P, Bakal JA, et al. Declining in-hospital mortality and increasing heart failure incidence in elderly patients with first myocardial infarction. J Am Coll Cardiol 2009;53:13-20
- Witt BJ, Ballman KV, Brown RD, Jr et al. The incidence of stroke after myocardial infarction: a meta-analysis. Am J Med 2006;119:354 e1-9
- Fokkema ML, van der Vleuten PA, Vlaar PJ, et al. Incidence, predictors, and outcome of reinfarction and stent thrombosis within one year after primary percutaneous coronary intervention for ST-elevation myocardial infarction. Catheter Cardiovasc Interv 2009;73:627-34
- Adabag AS, Therneau TM, Gersh BJ, et al. Sudden death after myocardial infarction. JAMA 2008;300:2022-9
- Wang Y, Lim LL, Heller RF, et al. A prediction model of 1-year mortality for acute ischemic stroke patients. Arch Phys Med Rehabil 2003;84:1006-11
- Kolominsky-Rabas PL, Heuschmann PU, Marschall D, et al. Lifetime cost of ischemic stroke in Germany: results and national projections from a population-based stroke registry: the Erlangen Stroke Project. Stroke 2006;37:1179-83
- Friedel H, Delges A, Clouth J, et al. Expenditures of the German statutory health insurance system for patients suffering from acute coronary syndromes and treated with percutaneous coronary intervention. Eur J Health Econ 2010;11:449-55
- McMurray JJ, Andersson FL, Stewart S, et al. Resource utilization and costs in the Candesartan in Heart failure: assessment of reduction in mortality and morbidity (CHARM) programme. Eur Heart J 2006;27:1447-58
- Brüggenjürgen B, Rupprecht HJ, Willich SN, et al. Cost of atherothrombotic diseases – myocardial infarction, ischaemic stroke and peripheral arterial occlusive disease – in Germany. J Public Health 2005;13:216-24
- Dorman P, Dennis M, Sandercock P. Are the modified ‘’simple questions’’ a valid and reliable measure of health related quality of life after stroke? United Kingdom Collaborators in the International Stroke Trial. J Neurol Neurosurg Psychiatry 2000;69:487-93
- Haacke C, Althaus A, Spottke A, et al. Long-term outcome after stroke: evaluating health-related quality of life using utility measurements. Stroke 2006;37:193-8
- Bakhai A, Ferrieres J Iñiguez A, et al. Clinical outcomes, resource use, and costs at 1 year in patients with acute coronary syndrome undergoing PCI: results from the multinational APTOR registry. J Interv Cardiol 2012;25:19-27
- Calvert MJ, Freemantle N, Cleland JG. The impact of chronic heart failure on health-related quality of life data acquired in the baseline phase of the CARE-HF study. Eur J Heart Fail 2005;7:243-51
- Sullivan PW, Slejko JF, Sculpher MJ, et al. Catalogue of EQ-5D scores for the United Kingdom. Med Decis Making 2011;31:800-4
- Consumer Price Index. Federal Statistics Office; 2012. http://www.destatis.de/EN/FactsFigures/NationalEconomyEnvironment/Prices/ConsumerPriceIndices/Tables_/ConsumerPricesCategories.html?cms_gtp=151228_list%253D1%2526151226_slot%253D2&https=1. Accessed November 24, 2012
- Institute for Quality and Efficiency in Health Care. General methods for the assessment of the relation of benefits to cost, 2009. Version 1.0-19/11/2009. http://www.ispor.org/PEguidelines/source/Germany_AssessmentoftheRelationofBenefitstoCosts_En.pdf. Accessed December 10, 2012
- Maini BS, O’Neill W, Palacios I, et al. Cost-effectiveness and quality of life improvements: Impella® hemodynamic support compared with intra-aortic balloon pump in high-risk patients receiving PCI. J Am Coll Cardiol 2012;59(Suppl S):E68
- Maini BS, Buyantseva L, Popma J, et al. Cost-effectiveness of Impella® hemodynamic support in the emergent care patient group. J Am Coll Cardiol 2012;59(Suppl S):E477
- Song F, Loke YK, Walsh T, et al. Methodological problems in the use of indirect comparisons for evaluating healthcare interventions: survey of published systematic reviews. BMJ 2009;338:b1147
- Glenny AM, Altman DG, Song F, et al. Indirect comparisons of competing interventions. Health Technol Assess 2005;9:1-134, iii–iv
- Neumann T, Biermann J, Erbel R, et al. Heart failure: the commonest reason for hospital admission in Germany: medical and economic perspectives. Dtsch Arztebl Int 2009;106:269-75
- Lauten A, Engström AE, Jung C, et al. Percutaneous left ventricular support with the Impella 2.5 assist device in acute cardiogenic shock - Results of the Impella EUROSHOCK-Registry. Circ Heart Fail [Epub ahead of time]. doi:10.1161/CIRCHEARTFAILURE.112.967224
- Seyfarth M, Sibbing D, Bauer I, et al. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol 2008;52:1584-8