Abstract
Objective:
To assess the cost-effectiveness of insulin detemir compared with Neutral Protamine Hagedorn (NPH) insulin when initiating insulin treatment in people with type 2 diabetes mellitus (T2DM) in Denmark, Finland, Norway, and Sweden.
Methods:
Efficacy and safety data were derived from a 20-week multi-centre randomized controlled head-to-head clinical trial comparing insulin detemir and NPH insulin in insulin naïve people with T2DM, and short-term (1-year) cost effectiveness analyses were performed. As no significant differences in HbA1c were observed between the two treatment arms, the model was based on significant differences in favour of insulin detemir in frequency of hypoglycaemia (Rate-Ratio = 0.52; CI = 0.44–0.61) and weight gain (Δ = 0.9 kg). Model outcomes were measured in Quality Adjusted Life Years (QALYs) using published utility estimates. Acquisition costs for insulin and direct healthcare costs associated with non-severe hypoglycaemic events were obtained from National Health Service public sources. One-way and probabilistic sensitivity analyses were performed.
Results:
Based on lower incidence of non-severe hypoglycaemic events and less weight gain, the QALY gain from initiating treatment with insulin detemir compared with NPH insulin was 0.01 per patient per year. Incremental cost-effectiveness ratios for the individual countries were: Denmark, Danish Kroner 170,852 (€22,933); Finland, €28,349; Norway, Norwegian Kroner 169,789 (€21,768); and Sweden, Swedish Krona 226,622 (€25,097) per QALY gained. Possible limitations of the study are that data on hypoglycaemia and relative weight benefits from a clinical trial were combined with hypoglycaemia incidence data from observational studies. These populations may have slightly different patient characteristics.
Conclusions:
The lower risk of non-severe hypoglycaemia and less weight gain associated with using insulin detemir compared with NPH insulin when initiating insulin treatment in insulin naïve patients with type 2 diabetes provide economic benefits in the short-term. Based on cost/QALY threshold values, this represents good value for money in the Nordic countries. Using a short-term modelling approach may be conservative, as reduced frequency of hypoglycaemia and less weight gain may also have positive long-term health-related implications.
Introduction
Both the United Kingdom Prospective Diabetes Study (UKPDS) and the Diabetes Control and Complications Trial demonstrated that intensive glycaemic control reduces long-term complications in type 1 (T1DM) and type 2 diabetes (T2DM)Citation1–4. In the UKPDS performed in people with T2DM, the risk of any diabetes-related complication was 12% lower in the intensive therapy groupCitation3. However, the improved glycaemic control with intensive therapy also conferred an increased risk of hypoglycaemia and was associated with a significantly higher weight gain compared with less intensive therapyCitation3. The presence of non-severe hypoglycaemia is associated with lower quality-of-life and an increased prevalence of a number of diabetes-associated complicationsCitation5–7. Fear of hypoglycaemia is a major concern for many patients, it may contribute to sub-optimal insulin treatment, particularly in intensively treated patients, and it is a critical limiting factor in glucose control managementCitation8. As a group, people with diabetes fear hypoglycaemia more than they fear the long-term complications of diabetesCitation9. Furthermore, hypoglycaemia is associated with direct healthcare costs associated with the event and indirect costs due to lost work timeCitation10,Citation11.
Weight gain is also commonly associated with intensive insulin therapy, especially among patients with T2DMCitation12,Citation13, and is linked to increased risk of cardiovascular morbidity and mortalityCitation14–16. Additionally, obesity has a negative impact on patients’ health-related quality-of-lifeCitation17,Citation18, and concerns about weight gain are associated with distress, poor physical and psychological well-being, and non-adherence to glucose lowering medicationsCitation19. In a Swedish study where patients’ willingness to pay (WTP) for health improvements associated with anti-diabetes treatments was assessed, it was found that patients were willing to pay on average €23.51 per month to avoid 1 kg weight increase and €15.61 per month for each kg of weight lossCitation20.
Consequently, the main targets in diabetes management are to achieve a near-normal blood glucose level to reduce the risk of long-term complications, maintain the smallest possible risk of hypoglycaemia, and minimize weight gain in order to maintain health-related quality-of-life and improve adherence to treatment.
Long-acting insulin analogues more accurately mimic the physiological human insulin profile and provide an alternative to human insulins, such as Neutral Protamine Hagedorn (NPH) insulin. The insulin analogue insulin detemir causes fewer hypoglycaemic events and less weight gain, leading to improvements in quality adjusted life-years (QALYs) compared with NPH insulinCitation21,Citation22.
The aim of this study was to assess the cost-effectiveness of using once-daily insulin detemir versus once-daily NPH insulin in patients initiating insulin therapy in Denmark, Finland, Norway, and Sweden. The study was based on treatment benefits related to reduction in hypoglycaemic events and less weight gain as observed in a head-to-head clinical trialCitation23.
Methods
Health economic analyses were performed reflecting the short-term treatment dilemma of balancing glycaemic control with cost of care and quality-of-life. The cost-effectiveness of initiating insulin treatment with insulin detemir compared to NPH insulin was estimated based on published differences in their hypoglycaemia and weight profiles assuming an equal effect on blood glucose control as measured by change in HbA1cCitation23. Clinical input data for the analyses were derived from a 20-week multi-centre randomized head-to-head clinical trial comparing initiation with insulin detemir (morning or evening) to NPH insulin (once daily in the evening) in 504 insulin naïve T2DM patients. Patients were randomized in a 1:1:1 ratioCitation23.
For the cost-effectiveness analyses, data from the insulin detemir evening arm and NPH insulin evening arm were utilized as these best reflect current standard treatment practice in the Nordic countries. Data from other studies comparing insulin detemir and NPH insulin were not included as these have mainly been based on twice daily treatment regimens, which are not considered to reflect current standard treatment practiceCitation24,Citation25.
A 1-year cost-effectiveness analysis (CEA) model was developed in Microsoft Excel (Microsoft Ltd, Redmond, WA, USA). Cumulative costs and utility decrements associated with hypoglycaemic events and weight gain together with the medication acquisition costs were estimated for each treatment arm to calculate an incremental cost-effectiveness ratio (ICER). The relative rate of non-severe hypoglycaemia with insulin detemir compared with NPH insulin was applied to the rate of hypoglycaemia in the NPH insulin treatment group to estimate the number of events prevented by using insulin detemir instead of NPH insulin. The weight difference between the treatment groups was assumed to affect patient’s quality-of-life throughout the year. Only direct healthcare costs were included in the analyses. This approach assumes that there would be no significant differences in long-term disease progression based on differences in HbA1c, as predicted from the treat-to-target trial designCitation23. In comparing the differences between treatment arms only parameters where statistically significant differences were observed between the two treatment arms were included. Parameters that were not statistically significantly different between the treatment arms were assumed to reflect random variation and were not included as relevant model parameters in the analyses.
Input data
Hypoglycaemia
In the clinical trialCitation23, occurrence of hypoglycaemia was based on all registered events in the intention-to-treat cohort (all randomized and treated patients regardless of compliance) and analysed as recurrent events in a Cox regression analysis. People with recurrent severe hypoglycaemia (i.e., requiring assistance) or hypoglycaemia unawareness were excluded from the trial programme (in total 166 patients failed the screening criteria for the study). Since this may have led to an under-estimation of the incidence of hypoglycaemia compared with real-world event rates, observational data from the UK Hypoglycaemia Study Group (UKHSG) were used in the analyses to better reflect the true incidence of hypoglycaemiaCitation26. As the UKHSG data were collected up until 2004, the rates of hypoglycaemia from this study were assumed to reflect the incidence rates in the NPH treatment arm. Non-severe hypoglycaemia was defined as an event with a plasma glucose level of <3.0 mmol/l or any episode where patients experienced symptoms associated with hypoglycaemia and the individual dealt with the episode aloneCitation23. Severe hypoglycaemia was defined as any hypoglycaemic episode where assistance from another person was required. The incidence of non-severe hypoglycaemia among people with T2DM on insulin for less than 2 years was 4.08 episodes per person-year, and the event rate for those on insulin for more than 5 years was 10.20 per person-yearCitation26. In the base-case analysis, the lower rate of events was used to reflect the event rate in the NPH treatment arm. This may be an overly conservative estimate since the frequency of hypoglycaemia tends to increase with longer treatment durationCitation26. Sensitivity analyses were performed using the higher rate of non-severe hypoglycaemia.
A crude rate ratio was calculated from the rate of overall confirmed hypoglycaemic events in the two treatment arms in the head-to-head randomized clinical trial. The rate ratio was used to estimate the number of non-severe hypoglycaemic events prevented by using insulin detemir compared with NPH insulin (RR = 0.52, CI = 0.44–0.61)Citation23. There were too few severe hypoglycaemic events reported in the clinical trial to estimate any rate ratios between insulin detemir and NPH for severe eventsCitation23. This may have been due to the selection criteria for the trial (see above).
Weight
There was an increase in body weight following insulin initiation in both treatment groupsCitation23. Weight gain in the insulin detemir arm was significantly lower than in the NPH insulin group (0.7 kg vs 1.6 kg, mean difference = −0.91 kg, p = 0.005, ΔBMI = −0.32 kg/m2).
Health-related quality-of-life
Non-severe hypoglycaemic events are associated with a relatively short-term disutility from the event itself and a longer-term disutility from the fear of repeated hypoglycaemic eventsCitation27. A disutility estimate for non-severe hypoglycaemic events was derived using a postal survey including 1305 respondentsCitation28. Based on the correlation between changes in the fear of hypoglycaemia score and changes in the EQ-5D score, a disutility for hypoglycaemic events was estimated for a 3-month period following the event (−0.01418); providing a yearly utility decrement of −0.0035 per non-severe hypoglycaemic event. This estimate was used in the analyses. Severe hypoglycaemic events were not included in the analyses as there were too few observations in the clinical trial to estimate any risk reductions for severe hypoglycaemic events.
Studies have shown that at a BMI above 25 kg/m2, weight gain is associated with lower overall quality-of-life in people with diabetesCitation17,Citation18. A study performed by Lee et al.Citation29, assessed the association between increasing BMI and the impact on health-related quality-of-life among diabetes patients using the EQ-5D score. The disutility associated with increasing BMI was adjusted for age. A 1-unit increase in BMI for T2DM patients was associated with a disutility of −0.010029. This utility decrement estimate was used in the health economic analyses in the present investigation.
Healthcare costs
Acquisition costs for insulin in the four countries (Denmark, Finland, Norway and Sweden) were based on a pack price for 5 × 3 ml FlexPen (NPH insulin [Insulatard FlexPen, Novo Nordisk A/S, Bagsværd, Denmark] and insulin detemir [Levemir FlexPen, Bagsværd, Denmark]) at pharmacy selling price (PSP) excluding VAT, obtained from public sourcesCitation30–33. For comparability, all cost estimates are reported in Euros. Average yearly exchange rates from the European Central Bank have been used to convert the costs into Euros. An exchange rate of DKK 7.45Citation34, NOK 7.80Citation35, and SEK 9.0336 was used.
The daily dose was assumed to be 40 international units (IU) for both treatment regimens, based on the World Health Organization’s (WHO’s) defined daily dose (DDD)Citation37. As no significant differences in drug doses were observed in the clinical trial it was considered most appropriate to use DDD for the analyses. No discounting was applied due to the short time horizon of the analysis.
A recent survey conducted in the UK, US, Germany, and France indicated an increase in visits to healthcare professionals (HCP) following non-severe hypoglycaemic eventsCitation10. In the survey, 25% of patients reported having additional HCP contact following a non-severe hypoglycaemic event. As no country-specific data are available for Denmark, Finland, Norway, and Sweden it was assumed that the same proportion of patients would visit an HCP in the Nordic countries. Country-specific input data are shown in .
Table 1. Healthcare cost input data for each country.
Threshold values
The threshold values used to define cost-effectiveness were DKK300,000/QALY (€40,268/QALY) for DenmarkCitation38 and SEK500,000/QALY (€55,370/QALY) for SwedenCitation39. As no published values were available for Finland, a value of €40,000/QALY was used for Finland (local equivalent of the £30,000/QALY National Institute of Clinical Excellence (NICE) threshold) and a value of NOK500,000/QALY (€64,103/QALY) for Norway, based on generally accepted threshold values in NorwayCitation40.
Sensitivity analyses
One-way sensitivity analyses were performed for all four countries to assess the impact of individual parameter uncertainty. Sensitivity analyses were performed for the baseline rates of non-severe hypoglycaemic events (event rates from the clinical trialCitation23 and UKHSG event rates for T2DM patients on insulin treatment for more than 5 yearsCitation41), the impact of changes in utility decrements associated with hypoglycaemia (±50%), and the impact of using the utility decrement of 0.0052 as referenced by NICECitation42. Utility decrements associated with BMI changes were tested by using the utility estimate from the CODE-2 study of 0.0061Citation43. The impact of changes in the cost of NPH insulin (±20%) was also estimated. To assess the uncertainty associated with the trial data on the hypoglycaemia relative rates and weight gain, upper and lower confidence intervals for the RR estimate (0.44:0.61) and weight gain (−0.1; −0.53 kg/m2) were utilizedCitation23.
Probabilistic sensitivity analysis was performed to assess the joint uncertainty of input parameters. All input parameters were defined by their probability distributions using the same ranges of uncertainty as used in the one-way sensitivity analyses. Monte Carlo simulation techniques were used to propagate the parameter uncertainty using 1000 iterations.
Results
Incremental cost-effectiveness: base case analysis
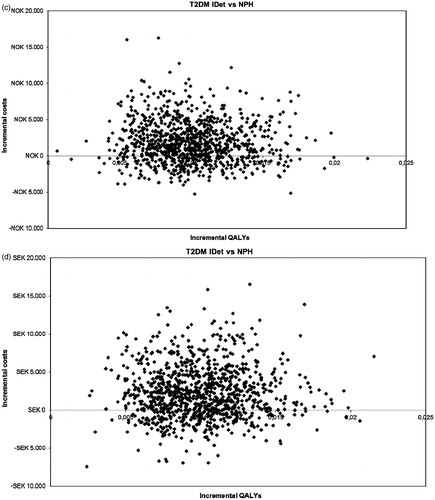
The health-related quality-of-life gain associated with initiating treatment with insulin detemir compared with NPH insulin based on the benefit of reducing the number of non-severe hypoglycaemic events and the weight benefit was 0.010 QALYs per patient per year. The incremental cost was DKK1709 (€229) in Denmark, €284 in Finland, NOK1698 (€218) in Norway, and SEK2267 (€251) in Sweden. The incremental cost-effectiveness ratio (cost/QALY) was DKK170,852 (€22,933) for Denmark, €28,349 for Finland, NOK169,789 (€21,768) for Norway, and SEK226,622 (€25,097) for Sweden (). shows the incremental costs and incremental QALYs for insulin detemir and NPH insulin for each of the four countries in the study. Insulin detemir was a cost-effective or dominating (better outcomes at a lower cost) treatment alternative in most simulations in the four countries. For Denmark, 68% of the simulations were below a cost/QALY threshold value of DKK300,000 (€40,268), in Finland 63% were below a cost/QALY threshold value of €40,000, in Norway 85% were below a cost/QALY threshold value of NOK500,000 (€64,102), and in Sweden 80% were below a cost/QALY threshold value of SEK500,000 (€55,370).
Figure 1. Incremental costs for insulin detemir vs NPH insulin in (a) Denmark, (b) Finland, (c) Norway, and (d) Sweden. (a) Scatterplot results of the probabilistic sensitivity analysis for Denmark. In 68% of the simulations, insulin detemir was a cost-effective or dominating treatment strategy (better health outcomes at a lower cost) compared with NPH insulin. (b) Scatterplot results of the probabilistic sensitivity analysis for Finland. In 63% of the simulations, insulin detemir was a cost-effective or dominating treatment strategy (better health outcomes at a lower cost) compared with NPH insulin. (c) Scatterplot results of the probabilistic sensitivity analysis for Norway. In 85% of the simulations, insulin detemir was a cost-effective or dominating treatment strategy (better health outcomes at a lower cost) compared with NPH insulin. (d) Scatterplot results of the probabilistic sensitivity analysis for Sweden. In 80% of the simulations, insulin detemir was a cost-effective or dominating treatment strategy (better health outcomes at a lower cost) compared with NPH insulin. QALYs, quality-adjusted life-years; iDET, insulin detemir; NPH, neutral protamine Hagedorn; DKK, Danish Krone; NOK, Norwegian Krone; SEK, Swedish Krona.
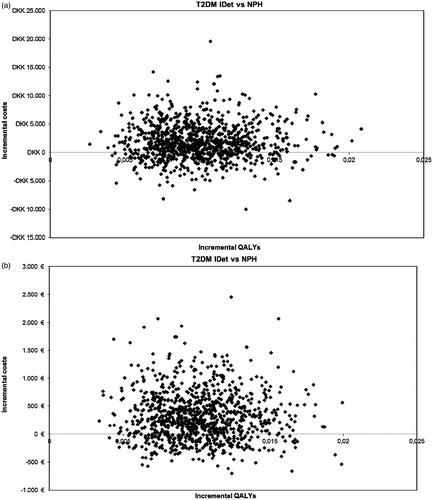
Table 2. Cost-effectiveness analysis, base case.
Sensitivity analyses
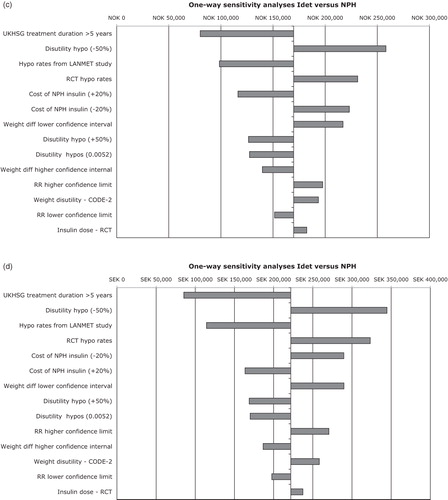
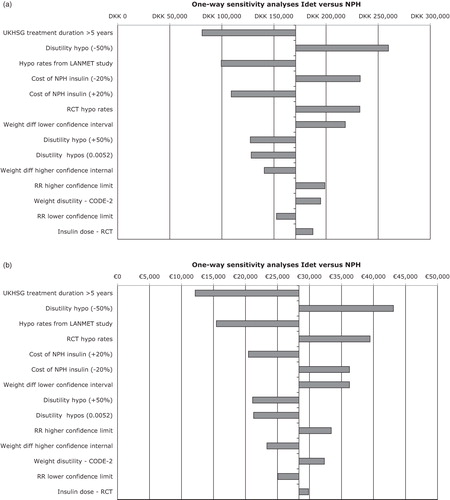
One-way sensitivity analyses were performed for key input parameters (). Results were most sensitive to the relative rate of hypoglycaemic events using insulin detemir instead of NPH insulin but they were generally robust to changes in individual input parameters. For example, if the utility decrement associated with hypoglycaemia was reduced by 50%, this resulted in the largest increases in the calculated incremental cost-effectiveness ratio, but in all four countries remained below the threshold values used to define cost-effectiveness ().
Figure 2. One-way sensitivity analyses for insulin detemir vs NPH in people with type 2 diabetes for (a) Denmark, (b) Finland, (c) Norway, and (d) Sweden. IDet, insulin detemir; NPH, neutral protamine Hagedorn; RR, rate ratio; RCT, randomized controlled trial; hypo, hypoglycaemia. Upper and lower confidence interval values were used for the sensitivity analyses. That is RR of hypoglycaemic events (0.44–0.61), weight difference (ΔBMI 0.1–0.53), hypoglycaemia rate in UKHSG group with >5 years treatment duration (10.02), LANMET hypoglycaemia rate (8).
Discussion
The health economic analysis performed in the current investigation focused on statistically significant differences in rates of non-severe hypoglycaemia and weight gain observed in a clinical trial using either insulin detemir or NPH insulin for the initiation of insulin treatment in insulin naïve people with T2DMCitation23. The results suggest that insulin detemir can be considered a cost-effective treatment alternative to NPH insulin over a 1-year period in all four countries analysed. These findings are consistent with long-term modelling studies that have compared the economic benefit of insulin analogues and NPH insulin in the treatment of T2DMCitation44–46.
Randomized clinical trials comparing the efficacy of long-acting insulin analogues generally follow the treat-to-target study designCitation47, in which the aim is to treat patients to a pre-defined target level of glycaemic control. Given this commonly used trial design it is relevant to compare the safety profile associated with achieving the HbA1c target, i.e., the rate of hypoglycaemic events and weight gain for comparable treatment regimens. A short-term modelling approach was therefore used to capture those relevant differences in outcome parameters. Basing the analyses only on differences in short-term outcomes may be a conservative approach as weight differences are also associated with differences in long-term complicationsCitation14. Similarly, the incidence of hypoglycaemia tends to increase with longer durations of insulin treatment and therefore there is a greater potential for reducing the number of hypoglycaemic events over longer time-horizons.
The analyses show that economic benefits of insulin detemir in patients with T2DM are consistent across the four Nordic countries, with very similar results between the sensitivity analysis across Denmark, Finland, Norway, and Sweden, which were robust to changes in individual input parameters. Efficacy and safety data used in the analyses were derived from a head-to-head clinical trial reflecting current clinical treatment practices using a once-daily basal insulin treatment regimen. Pooling of data from different clinical trials reflecting different treatment regimens of once- and twice-daily dosing was avoided since the heterogeneity of data would be too great.
One-way sensitivity analysis demonstrated that results were most sensitive to changes in the relative rate of hypoglycaemia between treatment strategies, and the utility decrement associated with non-severe hypoglycaemic events and baseline risk of hypoglycaemic events. The crude rate ratio of 0.52 was derived from the head-to-head trialCitation23. A similar risk reduction has been observed in another head-to-head trial comparing twice daily basal insulin detemir treatment with NPH insulinCitation24. Hypoglycaemia rates from an observational study were used for the analysis as this was considered to be a better approximation of the actual event rate in ‘real-world’ treatment settingsCitation48 compared with the rates observed in clinical trials. Similar or higher rates of non-severe hypoglycaemia have been observed in other observational studiesCitation49–51. The applied baseline rate of hypoglycaemic events reflects people that have been on insulin therapy for less than 2 years. As the frequency of hypoglycaemia increases with longer disease duration and longer use of insulin therapy, the applied baseline rate of non-severe hypoglycaemia is likely to be a conservative estimate for a T2DM population that has been receiving insulin for more than 2 yearsCitation41. Furthermore, we used overall rates of hypoglycaemia and did not separate into daytime and nocturnal hypoglycaemia rates. However, nocturnal hypoglycaemia is a major issue and concern for people with diabetes and is feared more than any other type of hypoglycaemiaCitation52. Future analysis should also include the cost-effectiveness of insulin analogue regimens on the reduction of nocturnal hypoglycaemia.
In this study, a utility decrement of 0.0035 per non-severe hypoglycaemic event was used. In comparison, a utility decrement of 0.0052 per hypoglycaemic event avoided was applied in a health technology assessment performed for NICECitation42, and in a study from Sweden a disutility of 0.07 was reported in a group of T2DM patients experiencing symptoms of hypoglycaemia during the last month in comparison to a patient group not experiencing these symptomsCitation51. Hence, the utility decrement estimate used in these analyses was lower than utility decrement estimates used elsewhere and may also be considered a conservative estimate. Non-severe hypoglycaemic events are associated with considerable direct and indirect costs due to additional healthcare contacts and absence from workCitation10. Indirect costs related to work time lost were not included in these analyses. However, with the reduced number of hypoglycaemic events in the insulin detemir treatment arm, it is expected that insulin detemir would be even more cost-effective if indirect costs were also included in the analyses.
Possible limitations of the study are that data on hypoglycaemia and relative weight benefits from a clinical trial were combined with hypoglycaemia incidence data from observational studies. These populations may not be readily comparable. We also cannot rule out the possibility that the risk reduction in real world clinical practice may be higher or lower than observed in the trial population. Furthermore, the rate of hypoglycaemia was derived from the UKHSG study and applied as the baseline risk in the NPH insulin treatment group. As data in the UKHSG study were not presented by treatment group, we cannot be certain that all the patients were treated with NPH insulin. However, as the incidence rates used reflected a T2DM patient group on insulin for less than 2 years, it may be assumed that the majority of patients were on a basal (e.g., once daily long acting) insulin plus oral anti-diabetes drug treatment regimen. This assumption is supported when comparing with data from other observational studies in people with T2DM where hypoglycaemia incidence rates were higherCitation49–51.
Conclusions
This cost-effectiveness analysis shows that the reduction in non-severe hypoglycaemic events and relative weight benefits associated with the use of the long-acting insulin analogue insulin detemir in the initiation of insulin treatment of T2DM provide economic benefits over a 1-year period compared with using NPH insulin. Even in the short-term, the intervention represents good value for money in the countries analysed. This analysis highlights the importance of considering short-term treatment differences from a health economic perspective.
Transparency
This survey was sponsored by Novo Nordisk A/S (Bagsværd, Denmark). MR was supported by grants from the Swedish Science Council, Region Skåne, ALF, The Novo Nordisk Foundation, the Craaford Foundation, and Påhlsson Foundation. This publication was supported by Novo Nordisk A/S (Bagsværd, Denmark) with editorial support from John Clarke, ESP Bioscience (Crowthorne, UK).
References
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977-86
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854-65
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837-53
- Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643-53
- Alvarez-Guisasola F, Yin DD, Nocea G, et al. Association of hypoglycemic symptoms with patients' rating of their health-related quality of life state: a cross sectional study. Health Qual Life Outcomes 2010;8:86
- Johnston SS, Conner C, Aagren M, et al. Evidence linking hypoglycemic events to an increased risk of acute cardiovascular events in patients with type 2 diabetes. Diabetes Care 2011;34:1164-70
- Sommerfield AJ, Deary IJ, McAulay V, et al. Moderate hypoglycemia impairs multiple memory functions in healthy adults. Neuropsychology 2003;17:125-32
- Cryer PE. Hypoglycemia: still the limiting factor in the glycemic management of diabetes. Endocr Pract 2008;14:750-6
- Diabetes Association Workgroup on Hypoglycemia, American Diabetes Association. Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care 2005;28:1245-9
- Brod M, Christensen T, Thomsen TL, et al. The impact of non-severe hypoglycemic events on work productivity and diabetes management. Value Health 2011;14:665-71
- Hammer M, Lammert M, Mejias SM, et al. Costs of managing severe hypoglycaemia in three European countries. J Med Econ 2009;12:281-90
- Pontiroli AE, Miele L, Morabito A. Increase of body weight during the first year of intensive insulin treatment in type 2 diabetes: systematic review and meta-analysis. Diabetes Obes Metab 2011;13:1008-19
- van Dieren S, Czernichow S, Chalmers J, et al. Weight changes and their predictors amongst 11 140 patients with type 2 diabetes in the ADVANCE trial. Diabetes Obes Metab 2012;14:464-9
- Eeg-Olofsson K, Cederholm J, Nilsson PM, et al. Risk of cardiovascular disease and mortality in overweight and obese patients with type 2 diabetes: an observational study in 13,087 patients. Diabetologia 2009;52:65-73
- Johnson WD, Brashear MM, Gupta AK, et al. Incremental weight loss improves cardiometabolic risk in extremely obese adults. Am J Med 2011;124:931-8
- Ridderstråle M, Gudbjornsdottir S, Eliasson B, et al. Obesity and cardiovascular risk factors in type 2 diabetes: results from the Swedish National Diabetes Register. J Intern Med 2006;259:314-22
- Gough SC, Kragh N, Ploug UJ, et al. Impact of obesity and type 2 diabetes on health-related quality of life in the general population in England. Diabetes Metab Syndr Obes 2009;2:179-84
- Hlatky MA, Chung SC, Escobedo J, et al. The effect of obesity on quality of life in patients with diabetes and coronary artery disease. Am Heart J 2010;159:292-300
- Peyrot M, Skovlund SE, Landgraf R. Epidemiology and correlates of weight worry in the multinational Diabetes Attitudes, Wishes and Needs study. Curr Med Res Opin 2009;25:1985-93
- Jendle J, Torffvit O, Ridderstråle M, et al. Willingness to pay for health improvements associated with anti-diabetes treatments for people with type 2 diabetes. Curr Med Res Opin 2010;26:917-23
- Meneghini LF, Rosenberg KH, Koenen C, et al. Insulin detemir improves glycaemic control with less hypoglycaemia and no weight gain in patients with type 2 diabetes who were insulin naive or treated with NPH or insulin glargine: clinical practice experience from a German subgroup of the PREDICTIVE study. Diabetes Obes Metab 2007;9:418-27
- Valentine WJ, Aagren M, Haglund M, et al. Evaluation of the long-term cost-effectiveness of insulin detemir compared with neutral protamine hagedorn insulin in patients with type 1 diabetes using a basal-bolus regimen in Sweden. Scand J Public Health 2011;39:79-87
- Philis-Tsimikas A, Charpentier G, Clauson P, et al. Comparison of once-daily insulin detemir with NPH insulin added to a regimen of oral antidiabetic drugs in poorly controlled type 2 diabetes. Clin Ther 2006;28:1569-81
- Hermansen K, Davies M, Derezinski T, et al. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care 2006;29:1269-74
- Pieber TR, Draeger E, Kristensen A, et al. Comparison of three multiple injection regimens for Type 1 diabetes: morning plus dinner or bedtime administration of insulin detemir vs. morning plus bedtime NPH insulin. Diabet Med 2005;22:850-7
- UK Hypoglycaemia Study Group. Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia 2007;50:1140-7
- Fidler C, Elmelund Christensen T, Gillard S. Hypoglycemia: an overview of fear of hypoglycemia, quality-of-life, and impact on costs. J Med Econ 2011;14:646-55
- Currie CJ, Poole CD, Woehl A, et al. The health-related utility and health-related quality of life of hospital-treated subjects with type 1 or type 2 diabetes with particular reference to differing severity of peripheral neuropathy. Diabetologia 2006;49:2272-80
- Lee AJ, Morgan CL, Morrissey M, et al. Evaluation of the association between the EQ-5D (health-related utility) and body mass index (obesity) in hospital-treated people with type 1 diabetes, type 2 diabetes and with no diagnosed diabetes. Diabet Med 2005;22:1482-6
- Regional Pay and Practitioners Tariff Board Organization Agreement. Agreement about general practice. 2010. http://www.medicinpriser.dk. Accessed March 21, 2012
- Hujanen T, Kapiainen S, Tuominen U, et al. The unit cost of healthcare in Finland. 2006. http://asiakas.kela.fi/laakekys_app/LaakekysApplication?kieli=en. Accessed March 21, 2012
- The Norwegian Medical Association. Normal tariff for private specialist practice 2011-2012. 2012. www.legemiddelverket.no. Acccessed March 21, 2012
- Medicine prices in Sweden. 2012. http://www.tlv.se/beslut/sok-i-databasen. Accessed March 21, 2012
- European Central bank. Danish krone (DKK). 2011. http://www.ecb.int/stats/exchange/eurofxref/html/eurofxref-graph-dkk.en.html. Accessed December 6, 2011
- European Central Bank. Norwegian krone (NOK). 2011. http://www.ecb.int/stats/exchange/eurofxref/html/eurofxref-graph-nok.en.html. Accessed December 6, 2011
- European Central Bank. Swedish krona (SEK). 2011. http://www.ecb.int/stats/exchange/eurofxref/html/eurofxref-graph-sek.en.html. Accessed December 6, 2011
- WHO Collaborating Centre for Drug Statistics Methodology. ATCC/DDD Index 2011. Norwegian Institute for Public Health, 2011. http://www.whocc.no/atc_ddd_index/. Accessed July 27, 2011
- Keiding H, Færgeman O. Økonomiske konsekvensberegninger og tilskudsregler – belyst ved et eksempel. Ugeskrift for læger 2008;170:651-4
- Lundin D. Kostnadseffektivitet som kriterium för subvention av läkemedel – en bra idé? Ekonomiskdebatt 2004;6:32-40
- Dagens Medicin. 2012. http://www.dagensmedisin.no/nyheter/kostnader-for-ekstra-levear-forskjell-pa-eldre-og-yngre-/. Accessed May 8, 2012
- Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia 2007;50:1140-7
- National Institute for Clinical Excellence. Technology Appraisal Guidance No 53. Guidance on the use of long-acting insulin analogues for the treatment of diabetes - insulin glargine. London: National Institute for Clinical Excellence, 2002
- Bagust A, Beale S. Modelling EuroQol health-related utility values for diabetic complications from CODE-2 data. Health Econ 2005;14:217-30
- Grima DT, Thompson MF, Sauriol L. Modelling cost effectiveness of insulin glargine for the treatment of type 1 and 2 diabetes in Canada. Pharmacoeconomics 2007;25:253-66
- McEwan P, Poole C, Tetlow T, et al. Evaluation of the cost-effectiveness of insulin glargine versus NPH insulin for the treatment of type 2 diabetes in the UK. Curr Med Res Opin 2007;23:S21-S31
- Valentine WJ, Erny-Albrecht KM, Ray JA, et al. Therapy conversion to insulin detemir among patients with type 2 diabetes treated with oral agents: a modeling study of cost-effectiveness in the United States. Adv Ther 2007;24:273-90
- US Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research. Guidance for industry diabetes mellitus: developing drugs and therapeutic biologies for treatment and prevention. 2008. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071624.pdf. Accessed September 1, 2011
- Matza LS, Boye KS, Yurgin N, et al. Utilities and disutilities for type 2 diabetes treatment-related attributes. Qual Life Res 2007;16:1251-65
- Donnelly LA, Morris AD, Frier BM, et al. Frequency and predictors of hypoglycaemia in type 1 and insulin-treated type 2 diabetes: a population-based study. Diabet Med 2005;22:749-55
- Leiter LA, Yale JF, Chiasson JL, et al. Assessment of the impact of fear of hypoglycemic episodes on glycemic and hypoglycemia management. Can J Diabetes 2005;29:186-92
- Lundkvist J, Berne C, Bolinder B, et al. The economic and quality of life impact of hypoglycemia. Eur J Health Econ 2005;6:197-202
- Heller S. Nocturnal hypoglycaemia. In: Frier BM, Fisher M, eds. Hypoglycaemia in diabetes. 2nd ed. Chichester, UK: John Wiley & Sons Ltd, 2007. p 84-99