Abstract
Objectives:
Adherence to medication is essential for optimal outcomes, especially for chronic diseases such as multiple sclerosis (MS). Studies in MS indicate that lower adherence is associated with an increased risk of relapse, hospitalization or emergency room (ER) visits, and higher medical costs. A previous investigation assessed the cost per relapse avoided for patients with MS receiving first-line disease modifying therapies (DMTs); however, the model assumed 100% adherence.
Methods:
Because real-world utilization patterns influence the actual effectiveness of medications, this analysis assessed the impact of real-world adherence from a US commercial payer perspective, using updated costs.
Results:
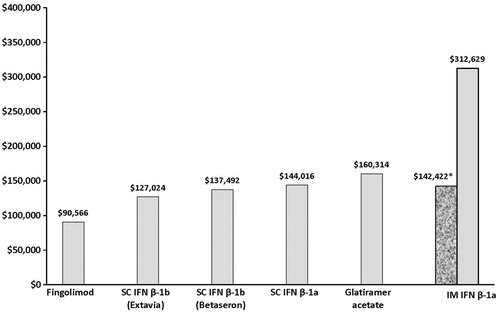
As was seen in the original study, in this revised model, fingolimod was associated with the lowest cost per relapse avoided ($90,566), followed by SC IFN β-1b (Extavia: $127,024), SC IFN β-1b (Betaseron: $137,492), SC IFN β-1a ($144,016), glatiramer acetate ($160,314), and IM IFN β-1a ($312,629). The model inputs that had the greatest impact on the results were adherence-adjusted relative relapse rate reduction (RRR) of fingolimod, the wholesale acquisition costs of fingolimod, and the average number of relapses in untreated patients with MS.
Limitations:
The estimates of DMT adherence are from a single claims database study of a large national pharmacy benefit manager that only measured adherence, not actual relapses, and the model does not incorporate manufacturer discounts and rebates, which are not publicly available.
Conclusion:
These results suggest that economic analyses of MS therapies should incorporate real-world adherence rates where available, rather than relying exclusively on trial-based efficacy estimates when considering the economic value of treatment alternatives, and that highly efficacious therapies with low adherence may yield real-world efficacy that is substantially lower than that observed in closely monitored clinical trials.
Background
Adherence to treatment increases the likelihood that the patient receives the intended benefit of a medication; however, ∼20–50% of patients with chronic diseases do not take their medications as prescribedCitation1. Depending on the condition being treated, sub-optimal medication adherence can lead to disease progression, the development of drug resistance, greater disability, acute and more intense clinical attacks, and premature death—all of which can unnecessarily increase healthcare costsCitation1,Citation2.
Multiple sclerosis (MS) is a chronic, debilitating neurological illness characterized by intermittent exacerbations of existing symptoms or onset of new symptoms, and progressive accumulation of irreversible neurological disabilityCitation3. Studies of self-injected disease modifying therapies (DMTs) in MS indicate that lower adherence is associated with an increased risk of relapseCitation4, hospitalization or emergency room (ER) visits, and medical costsCitation5. Agashivala et al.Citation6 assessed the cost per relapse avoided for patients with MS receiving first-line DMTs approved by the US Food and Drug Administration (FDA) prior to 2011 using a Microsoft® Excel-based economic model; however, the model assumed that 100% of patients were adherent to DMTs. Because real-world utilization patterns influence the actual effectiveness of medications in MS, we assessed the impact of real-world adherence on relapse rates and cost-effectiveness of first-line DMTs in MS from a US commercial payer perspective.
Methods
The model used in this analysis is an adaptation of the formerly published Microsoft Excel-based economic model developed by Agashivala et al.Citation6. The current evaluation accounted for the impact of differential adherence on effectiveness and estimated the cost-effectiveness of fingolimod compared with other first-line DMTs (subcutaneous [SC] interferon [IFN] beta [β]-1b [Extavia], SC IFN β-1b [Betaseron], SC IFN β-1a, glatiramer acetate, intramuscular [IM] IFN β-1a) in patients with relapsing forms of MSCitation7–13.
Effectiveness
Annualized relapse rates (ARR) and relative relapse rate reduction (RRR) for individual comparators were identified from the respective placebo-controlled clinical trials. Patients were considered adherent if they had a medication possession ratio (MPR) ≥80% over a 1-year period. The proportion of patients who were adherent to each DMT was identified from a previous retrospective claims studyCitation14. Assumptions in this model included: adherent patients would have an ARR similar to those observed in clinical trials and non-adherence would have the same effect on trial-based ARR across all comparators. The impact of non-adherence (MPR < 80%) on real-world relapse rates was calculated based on published estimates from a retrospective claims database studyCitation4, which demonstrated that adherent patients (MPR ≥ 80%) had ∼21% lower risk of having MS relapses than non-adherent patients (relative risk [RR] = 0.787, 95% confidence interval [CI] = 0.698–0.887). Thus, non-adherent patients are at ∼27% higher risk of having relapses than adherent patients.
The estimated relapse rate for non-adherent patients was calculated as the trial-based ARR divided by the RR of adherent patients having a relapse. After weighting for the proportion of patients who were adherent and non-adherent, the adherence-adjusted RRR was calculated. The proportion of patients adherent to first-line DMTs has previously been reported to be 89.2% (adherence adjusted RRR of 53%) for fingolimod, 72.4% (adherence-adjusted RRR of 29%) for SC IFN β-1b (includes Betaseron and Extavia), 77.8% (adherence-adjusted RRR of 28%) for SC IFN β-1a, 82.1% (adherence-adjusted RRR of 26%) for glatiramer acetate, and 79.2% (adherence-adjusted RRR of 13%) for IM IFN β-1aCitation14.
Costs
Costs were derived similarly to Agashivala et al.Citation6, where 2012 wholesale acquisition costs (WAC) for all DMTs were included in the analysis and obtained using AnalySource® (Syracuse, NY), a web-based pricing toolCitation15. Monitoring requirements differed between treatments, and were based on each agent’s prescribing information, and unit monitoring costs were obtained from the 2010 Physician’s Fee and Coding Guide as previously shown in Agashivala et al.Citation6 and othersCitation7–13,Citation16. In addition, two ophthalmologic tests in the first year and two electrocardiograms (EKGs) during the first-dose observation (FDO) period for fingolimod were included, based on the new version of the fingolimod label. The cost of relapse was calculated similarly to Agashivala et al.Citation6. Relapse costs were inflated to 2011 US dollars using the consumer price indexCitation17.
Statistical analyses
Based on the aforementioned inputs, the cost per relapse avoided was calculated for each of the products over a 2-year time horizon. Univariate sensitivity analyses were performed to assess the impact of differential hypothetical adherence (range: 0–100%) for fingolimod and other key inputs, including direct cost of a relapse, WAC cost of fingolimod, and average number of untreated relapses. In the base-case scenario, this economic model evaluated the IM IFN β-1a intent to treat (ITT) population (ARR: 18%); however, the data on IM IFN β-1a were derived from a trial that was terminated once the initial patients reached the 104-week follow-up timepointCitation18–20. Therefore, all patients did not receive treatment for a full 2 years. It is possible that an analysis using the completer population might yield different results; therefore, a sensitivity analysis was performed using the data from patients who completed 2 years of treatment during the trial (ARR: 32%)Citation18–20.
Results
In the original model that assumed 100% adherence, fingolimod was associated with the lowest cost per relapse avoided ($74,843), followed by SC IFN β-1b (Extavia: $94,423), SC IFN β-1b (Betaseron: $102,530), SC IFN β-1a ($108,940), glatiramer acetate ($124,512), and IM IFN β-1a ITT ($197,073)Citation6. As seen in , the adapted model showed that fingolimod remained the most cost-effective option ($90,566 vs $127,024-$312,629) among the DMTs. However, for all DMTs, higher costs per relapse avoided and wider differences between costs per relapse avoided for each of the drugs were found in the present analysis.
Figure 1. Cost per relapse avoided. $, US dollars; SC, subcutaneous; IFN, interferon; β, beta; IM, intramuscular. * Using IM IFN completers data, ARR = 32%.
Sensitivity analysis
The model inputs that had the greatest impact on the results were adherence-adjusted RRR of fingolimod, the WAC of fingolimod, and the average number of relapses in untreated patients with MS (). Assuming that all patients on fingolimod had an MPR < 80%, the total cost per relapse avoided on fingolimod ($131,931; ) increased, but it was still lower than for most DMTs, except Extavia-branded SC IFN β-1b ($127,024) (). When the effectiveness data from IM IFN β-1a completers (ARR: 32%) were applied, the cost per relapse avoided was $142,422—similar to the other injectable drugs used to treat MS but lower than that achieved in the ITT scenario (ARR: 18%). As the proportion of adherent patients decreased, the total cost per relapse avoided increased. However, only in the scenario where all patients on fingolimod were non-adherent and the relapse rates for untreated patients was low, was Extavia the more cost-effective DMT. In all of the other scenarios, fingolimod remained the more cost-effectiveness option among current DMTs. Increasing the other parameters assessed in the sensitivity analysis also resulted in costs per relapse for fingolimod that were less than the other DMTs (). For example, increasing the number of relapses for untreated patients by 10% increased the cost per relapse to $100,127, increasing the WAC of fingolimod by 10% led to a total cost per relapse of $98,982, and increasing the direct cost of relapse by 10% increased the total cost per relapse to $91,017. In addition, the cost per relapse avoided for fingolimod remained lower among all the DMTs, even when the rest of the DMTs were assumed to have optimal adherence (results not shown).
Discussion
The purpose of this adaptation of a previously published economic modelCitation6 was to assess the impact of real-world adherence on cost-effectiveness of fingolimod compared with other first-line DMTs (SC IFN β-1b [Extavia], SC IFN β-1b [Betaseron], SC IFN β-1a, glatiramer acetate, IM IFN β-1a)Citation7–13 in patients with relapsing forms of MS. Because this analysis took into consideration adherence, it is more applicable to naturalistic clinical settings where patients are generally less adherent than in clinical trialsCitation21.
In two Phase 3 randomized trials, FREEDOMS and TRANSFORMS, fingolimod resulted in a 54% reduction in ARR vs placebo over 24 months and a 52% reduction in ARR vs IM IFN β-1a over 12 monthsCitation22,Citation23. This high efficacy in reducing relapse rates substantially contributed to the cost-effectiveness of fingolimod. Furthermore, real-world data have shown that patients are highly adherent to fingolimod and are more adherent compared with self-injected DMTsCitation14. The high efficacy and high adherence of fingolimod contributed to the lower cost per relapse avoided relative to the other DMTs. In this case the real-world efficacy of the comparators, already lower than that of fingolimod, was further discounted due to lower observed real-world adherence relative to fingolimod. If fingolimod had demonstrated lower adherence than the other DMTs, its cost per relapse avoided would have been higher, as shown in the sensitivity analysis, reducing its cost effectiveness.
Adherence to MS drugs has been shown to have an impact on clinical and economic outcomesCitation4,Citation24. Patients that have an adherence rate lower than 80% are more likely to have more relapses, hospitalizations, and ER visits in 1 year in comparison to adherent patients. A variety of factors have been shown to contribute to non-adherence, including tolerability and the complexity of the regimen, which takes into account delivery route and dosing frequencyCitation24. Thus, the oral administration route of fingolimod and absence of tolerability factors like injection-site reactions, flu-like symptoms, etc., may have contributed to the observed higher adherence ratesCitation4.
In addition to adjusting for differences in DMT adherence, this analysis differed from the original economic modelCitation6 by using updated WAC (2012) and healthcare costs (2011) to revise the results to more current conditions and to account for recent changes in the fingolimod label, such as the monitoring required during the FDO period. This analysis has similar limitations to the original modelCitation6. The model did not include the costs associated with treating adverse events because it has been found previously that the cost of treating common adverse events is low and severe adverse events are rare for all of the DMTsCitation6. The model time horizon was only 2 years and, because MS is a progressive disease, the cost-effectiveness of the various treatments may change with time. However, a 2-year time horizon is consistent with the perspective of commercial health plans in the US. This economic model evaluated the IM IFN β-1a ITT population. The data related to IM IFN β-1a were derived from a trial that was terminated once the initial patients reached 104 weeks of follow-upCitation18–20. As part of the sensitivity analysis of the present investigation, the ARR for patients who completed a full two years of treatment or placebo were used. When the effectiveness data from IM IFN β-1a 2-year completers were used, the cost per relapse avoided was similar to the other injectable drugs to treat MS. This model also did not incorporate discounts or rebates to health plans since that information often varies by health plan and is not publicly available. A final limitation is that we did not incorporate the reduced drug acquisition costs associated with non-adherence and reduced refill rates.
Conclusions
Adherence has a substantial impact on real-world clinical effectiveness in MS, which in turn influences cost-effectiveness. Higher rates of adherence among patients treated with fingolimod translated into higher estimated real-world cost-effectiveness in this model. Fingolimod had the lowest cost per relapse avoided in most of the scenarios ranging from 0–100% of patients adherent (MPR ≥ 80%) to treatment. Highly efficacious drugs with high adherence rates provide the greatest real-world effectiveness and may provide the best value for the financial investment. In contrast, highly efficacious therapies with low adherence may yield real-world efficacy that is substantially lower than that observed in closely monitored clinical trials. In conclusion, economic analyses of MS therapies should incorporate real-world adherence rates where available, rather than relying exclusively on trial-based efficacy estimates when considering the economic value of treatment alternatives. This approach may be applicable to other disease areas where medication non-adherence has a substantial effect on clinical events and healthcare costs.
Transparency
Declaration of funding
The research was funded by Novartis Pharmaceuticals Corporation, which also provided funding for the development of this manuscript.
Declaration of financial/other relationships
DB received financial support from Novartis Pharmaceuticals Corporation for his participation in the project. NA and EK are employees of Novartis Pharmaceuticals Corporation. At the time research was conductions and this manuscript was submitted, KR was a fellow of Novartis Pharmaceuticals Corporation.
Acknowledgments
Meredith Rogers, MS, and Michelle Adams, BSJ, MAIA, provided editorial and written assistance, which was funded by Novartis Pharmaceuticals Corporation.
References
- Kripalani S, Yao X, Haynes RB. Interventions to enhance medication adherence in chronic medical conditions. Arch Intern Med 2007;167:540-50
- Sabate E, editor. Adherence to long-term therapies: evidence for action. Geneva: World Health Organization. 2003. whqlibdoc.who.int/publications/2003/9241545992.pdf. Accessed February 2012
- Kurtzke JF, Beebe GW, Nagler B, et al. Studies on the natural history of multiple sclerosis. 7. Correlates of clinical change in an early bout. Acta Neurol Scand 1973;49:379-95
- Tan H, Cai Q, Agarwal S, et al. Impact of adherence to disease-modifying therapies on clinical and economic outcomes among patients with multiple sclerosis. Adv Ther 2011;28:51-61
- Steinberg SC, Faris RJ, Chang CF, et al. Impact of adherence to interferons in the treatment of multiple sclerosis: a non-experimental, retrospective, cohort study. Clin Drug Investig 2010;30:89-100
- Agashivala N, Dastani HB, Carlton R, et al. Cost-effectiveness of fingolimod in treating patients with relapsing-remitting multiple sclerosis. Am J Pharm Benefits 2011;3:320-8
- Avonex [package insert]. Cambridge, MA: Biogen Idec, Inc.; 2011
- Rebif [package insert]. Rockland, MA: EMD Serono, Inc.; 2011
- Betaseron [package insert]. Montville, NJ; Bayer HealthCare Pharmaceuticals, Inc.; 2010
- Extavia [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2009
- Copaxone [package insert]. Tel Aviv, Israel; Teva Pharmaceutical Industries Ltd.; 1998
- Tysabri [package insert]. Cambridge, MA: Biogen Idec, Inc.; 2012
- Gilenya [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2012
- Abouzaid S, Wu N, Kim E, et al. Comparison of compliance to fingolimod and other first-line disease modifying treatments among patients with multiple sclerosis. Poster presented at the AMCP’s 2012 Educational Conference, October 3–5, 2012, Cincinnati, OH
- AnalySource® Web site. https://www1.analysource.com/qry/as_products.taf?_purgefilter=Y&_nc=ee630cd74f711729196667e52744f70c. Accessed August 2011
- Physician’s Fee and Coding Guide. Duluth, GA: Mag Mutual; 2010
- Consumer price index. http://www.bls.gov/cpi/. Accessed November 27, 2012
- Jacobs LD, Cookfair DL, Rudick RA, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Ann Neurol 1996;39:285-94
- Bell CF. The pursuit of transparency and quality improvement in cost-effectiveness analysis—a case study in disease-modifying drugs for the treatment of multiple sclerosis. J Man Care Pharm 2011;17:463-8
- Becker RV, Dembek C. Effects of cohort selection on the results of cost-effectiveness analysis of disease-modifying drugs for relapsing-remitting multiple sclerosis. J Man Care Pharm 2011;17:377-81
- Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clin Ther 1999;21:1074-90
- Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010;362:402-15
- Khatri B, Barkhof F, Comi G, et al. Comparison of fingolimod with interferon beta-1a in relapsing-remitting multiple sclerosis: a randomized extension of the TRANSFORMS study. Lancet Neurol 2011;10:520-9
- Treadway K, Cutter G, Salter A, et al. Factors that influence adherence with disease modifying therapy in MS. J Neurol 2009;256:568-76
