Abstract
Objective:
To evaluate the impact of increased access restrictions to branded oxycodone hydrochloride extended-release tablets (oxycodone HCl ER), on healthcare utilization and costs in patients using extended-release and long-acting opioids (ER/LA opioids) from the health plan perspective during the period from 1/1/2009 to 6/30/2012.
Methods:
This retrospective cohort study analyzed claims data for adult patients from US plans that increased oxycodone HCl ER access restrictions. Study groups were segmented into commercial and Medicare payers, and by prior authorization (PA) and tier change (TC) restrictions. Six-month outpatient visits and prescription utilization and costs were evaluated during the pre- and post-access restriction periods using a bootstrapped t-test and regression to test the differences.
Results:
Mean 6-month post-restriction combined pharmacy and outpatient visit costs were $1131 (p < 0.001), $660 (p = 0.009), $699 (p < 0.001), and $564 (p < 0.001) higher than pre-restriction costs in commercial PA, commercial TC, Medicare PA, and Medicare TC groups, respectively. Outpatient visits accounted for the greatest proportion of increased costs in the access restriction groups.
Conclusions:
The results of this study suggest that oxycodone HCl ER access restrictions such as PA and TC may increase medical costs without an offsetting savings in pharmacy costs.
Introduction
Chronic pain is affecting an estimated 100 million people in the US and affects more Americans than diabetes, heart disease, and cancer combinedCitation1,Citation2. Pain accounts for $635 billion annually in the US in direct and indirect costs, including lost productivityCitation2. Chronic pain is a complex condition and achieving optimal pain management is a challenge. The management of chronic pain is multifaceted and may include medication, physical therapy, and psychological treatment. Opioids are commonly used in treatment of chronic pain and treatment guidelines specify that a multi-pronged approach is essential for adequate pain management and that treatment should include both non-pharmacological and pharmacological therapyCitation3,Citation4.
The US Food and Drug Administration (FDA) recently announced class-wide safety labeling changes for all extended-release and long-acting opioid analgesics intended to treat pain. The updated indication states that extended-release and long-acting opioids (ER/LA opioids) are indicated for the management of pain severe enough to require daily, around-the-clock, long-term opioid treatment and for which alternative treatment options are inadequateCitation5. At the time of the study, ER/LA opioids were indicated for managing moderate-to-severe painCitation6. There are several FDA approved ER/LA opioid products available in the US market including morphine sulfate extended-release capsules, buprenorphine transdermal system, methadone hydrochloride tablets, fentanyl transdermal system, hydromorphone hydrochloride extended-release tablets, morphine sulfate controlled-release tablets, oxymorphone hydrochloride extended-release tablets, and oxycodone hydrochloride extended-release (oxycodone HCl ER) tablets among othersCitation7. ER/LA opioids are classified as controlled substancesCitation8. Oxycodone HCl ER is a commonly prescribed ER/LA opioid with abuse-deterrent characteristics intended to impede abuse via injection and insufflationCitation9.
In an effort to control healthcare costs, ensure appropriate utilization of specific medications, and manage potential safety issues, payers in the US have implemented various types of access restrictions on drug classes or specific productsCitation10. These techniques include requiring physicians/pharmacists to obtain authorization from patients’ health plan in order to obtain coverage for a treatment (i.e. prior authorization, PA) or classifying a product into a higher co-pay tier that has a higher out-of-pocket cost, making it more expensive to the patient (i.e., tier change, TC). Some other common access restriction techniques include step-therapy (ST), in which patients are required to try alternative drugs in a stepwise fashion before getting coverage for the targeted therapy, and non-formulary (NF), where the medication is a non-covered benefit and patients pay the full cost.
Formulary access restrictions, by their design, result in access barriers for drugs to which they are appliedCitation11,Citation12. Certain studies have reported a positive value for such access restriction programs. One study of a PA restriction on proton pump inhibitors (PPI) showed decreased use of costly PPIs among Medicaid beneficiaries in favor of increased use of a low-cost histamine 2-receptor antagonist option without measurable adverse medical consequencesCitation13. However, several studies have documented that pharmaceutical restriction policies such as increasing co-pay through tiered formularies, using step-therapy, preferred drug lists, as well as PAs may have unintended consequences such as delayed treatment and negative impact on patients’ health status, or may not have substantial savings in the total cost of careCitation14–17. For example, a study evaluating the impact on the overall utilization of pharmacologic therapy and healthcare services as a result of a PA restriction policy on access to pregabalin among fee-for-service Medicaid patients with diabetic peripheral neuropathy or postherpetic neuralgia found that, while the PA effectively reduced access to pregabalin, it resulted in increased use of opioids and alternative pain medications and increased disease-related healthcare costsCitation16.
Safe and effective pain management using long-acting opioid therapy requires clinical skill in addition to understanding the signs of withdrawal and risks of opioid abuse, diversion, and addictionCitation4. Failure to adequately treat pain has many consequences for patients’ lives and may well increase healthcare resource utilizationCitation2. Changing pain management therapy in response to health plan access restrictions may create a need for increased medication supervision and additional patient monitoring which may add to the challenge and cost of pain management. Very limited literature exists on the impact of access restrictions to ER/LA opioids. A single study in the Medicaid population found mixed results of the impact of PA on oxycodone ER utilization and a modest but statistically significant decrease in average cost per long-acting opioid doseCitation18. Studies on access restrictions for ER/LA opioids in the commercial and Medicare population are absent in the literature.
Health plan benefit designs have varied over time in terms of patient access to oxycodone HCl ER, ranging from relatively open access with moderate out-of-pocket patient expense, to access restrictions that include a high co-pay expense, as well as requirement for PA before coverageCitation2,Citation19. The current study is intended to fill the gap in insight regarding how access restrictions imposed on branded oxycodone HCl ER impacts opioid patients in commercial and Medicare health plans. This study evaluated the impact of increased access restrictions, specifically PA and TC, for branded oxycodone HCl ER on healthcare utilization and costs of patients using ER/LA opioids, from the health plan perspective, during the period from 1/1/2009 to 6/30/2012.
Methods
Study design
This was a retrospective, observational, cohort study that utilized open-source pharmacy and medical claims data to evaluate the impact that increased oxycodone HCl ER patient access restrictions have on ER/LA opioids utilization, outpatient visits, pharmacy resource utilization, and costs. A matched case-control study was conducted as a sensitivity analysis to the primary analysis.
Data sources
Data sources for this study included the IMS Pharmacy Claims, the IMS Private Practitioner Medical Claims, and the IMS Formulary Focus databases. The IMS Pharmacy Claims database, maintained continuously from 2001, contains National Council for Prescription Drug Programs (NCPDP 5.2) claims submitted by retail, specialty, and mail-order pharmacies. This database captures ∼2 billion prescriptions per year, representing ∼50% of all US pharmacy claims. The Medical Claims database, established since 1999, captures data from the Centers for Medicare and Medicaid Services (CMS) Provider Billing Form CMS-1500 completed for patients seen in the ambulatory setting. This Medical Claims database constitutes ∼1 billion annual claims with diagnosis and visit information submitted each month by more than 870,000 physicians representing almost half of all private practice visits in the US. The IMS Formulary Focus database provides detailed formulary status such as quantity limits, step-therapy, PAs, tier position, and product restrictions on a monthly basis, and was used in this study to determine changes in the formulary status for oxycodone HCl ER. This database captures benefit design information on all formularies that were reported by a payer (both commercial and Medicare Part D), plan or pharmacy benefit manager. All databases are certified as compliant with the Health Insurance Portability, Affordability, and Accountability Act (HIPAA). A longitudinally stable synthetic identifier allows the researchers to track patients anonymously and longitudinally over time and between databases.
Sample selection
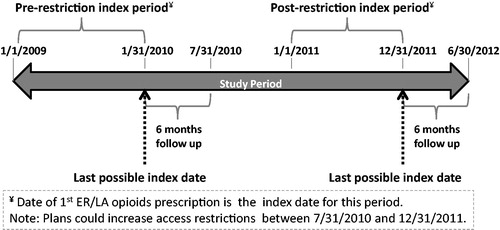
Based on the timeframe in which many health plans implemented access restriction to oxycodone HCl ER, this study designated the period of 1/1/2009 to 1/31/2010 as the pre-restriction period and the period of 1/1/2011 to 12/31/2011 as the post-restriction period. Adult patients (at least 18 years as of the index date) with at least one prescription claim for an ER/LA opioid (incident or prevalent use) during either the pre- or post-restriction periods (the date of first ER/LA opioid claim during each period was considered as the index date; patients could be indexed in both periods) and at least 6 months of continuous observation post-index, were identified from plans that increased access restrictions to branded oxycodone HCl ER during the period of 7/1/2009 to 12/31/2011. The complete study period was from 1/1/2009 to 6/30/2012, which allowed for a 6-months post-index measurement for both groups. Patients were assigned to the pre-restriction cohort if their index dates fell within the pre-restriction period (1/1/2009 to 1/31/2010) and were at least 6 months prior to imposition of an access restriction policy by the health plan. Patients whose index prescription claim fell within the post-restriction period (1/1/2011 to 12/31/2011) following the imposition of an access restriction and whose plans continued the restriction for at least 6 months after the index date were included in the post-restriction cohort. depicts a schematic representation of the study design with index and follow-up periods for pre- and post-access restriction cohorts. All patients studied received prescription coverage either through a commercial plan or Medicare Part D.
Exclusion criteria included unknown or uncertain oxycodone HCl ER formulary status, or concomitant coverage from both commercial and Medicare insurance in the 6-month post-index period. As branded oxycodone HCl ER is currently the only FDA-approved extended-release oxycodoneCitation20, any patient indexed to a generic oxycodone extended release product during the study period was excluded (a generic version of extended-release oxycodone was temporarily available until 2009). Patients receiving care through a staff model health maintenance organization were excluded from the analysis.
Two types of access restrictions were studied: (1) imposing a PA on branded oxycodone HCl ER (the PA group), and (2) moving branded oxycodone HCl ER to a higher tier co-pay (the TC group). To evaluate the potential differential impact of access restriction by payer type, patients were segmented into Medicare Part D or commercial insurance for prescription coverage, forming four study groups: commercial PA, commercial TC, Medicare PA, and Medicare TC. Patients were assigned in a hierarchal manner first to the PA groups. Additional access restrictions that were considered included non-formulary and step therapy, but the number of plans imposing these restrictions was very limited and, therefore, were not included in the study.
Measures and analysis
Demographic measures and Charlson Comorbidity Index (CCI) were reported as of index date, while comorbid diagnoses were assessed from 7 days before to 6 months following the index date. A modified Deyo method was used to calculate the CCICitation21,Citation22.
Relative utilization of oxycodone HCl ER as a portion of the entire ER/LA opioid market was calculated by dividing the total number of oxycodone HCl ER prescriptions observed during the 6-month pre- and post-restriction follow-up periods by the total number of all ER/LA opioids prescriptions observed during the 6-month pre- and post-restriction follow-up periods, respectively. Utilization change was calculated as the difference in the proportion of all prescriptions dispensed between the pre-restriction period and the post-restriction period. Pharmacy (ER/LA opioids, short-acting opioids (SAOs), and non-opioid medications) utilization, outpatient visit utilization (captured through the CMS-1500 billing form; includes physician office visits, diagnostic tests, radiology, physical therapy, etc.), and costs (billed charges; the only type of cost related data available in the databases used) were measured for 6-months following each patient’s index date and were compared between the pre- and post-restriction cohorts within each study group. All costs were adjusted to 2012 dollars using the Medical Consumer Price Index. Bootstrapping t-test and generalized linear models with gamma distribution and a log-link were utilized to test the differences in resource utilization and costs, respectivelyCitation23,Citation24.
Sensitivity analysis
As a sensitivity analysis, a matched case-control study was conducted. Patients assigned to the pre- and post-restriction cohorts in the primary analysis were considered cases, and control groups were selected patients enrolled in plans that did not increase branded oxycodone HCL ER access restrictions during the same time periods (i.e. 1/1/2009 to 1/31/2010 for the pre-restriction and 1/1/2011 to 12/31/2011 for the post-restriction period). Controls were matched to cases based on the following: gender, age ±1 year, cancer vs non-cancer diagnosis, payer type, US census region, the calendar quarter the patient was indexed, and categorical CCI index. Matching was conducted at on a 1:1 basis, except for the Medicare TC group where three cases were matched to one control due to the limited number of control patients available. Prescription and outpatient visit utilization and costs were evaluated on the matched groups in the same manner as the primary analysis.
Results
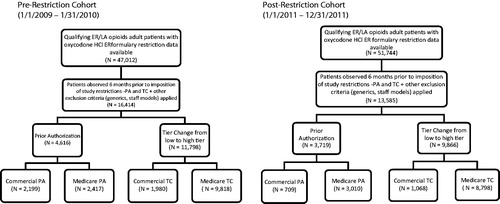
This analysis included study patients from 541 local and state-level health plans (some of these state/local level plans were part of national/regional parent plans) that imposed PA or TC restrictions at some time during the study period. The pre-restriction sample consisted of 2199, 1980, 2417, and 9818 patients in the commercial PA, commercial TC, Medicare PA, and Medicare TC groups, respectively. The post-restriction sample consisted of 709, 1068, 3010, and 8798 patients in the commercial PA, commercial TC, Medicare PA, and Medicare TC groups, respectively (). Overall, the commercial population was 55% female, with a mean age of 50.8 years; the Medicare population being older (59.5 years) and more female (62%) than the commercial population (). It is worth noting that Medicare provides coverage to the eligible disabled population regardless of age, and more than 90% of the Medicare disabled population is less than 65 years of age, which may account for the mean age of less than 65 in the Medicare population in this studyCitation25.
Table 1. Demographics and baseline characteristics by plan type.
The distribution of comorbidities and other demographic (age, gender) and clinical characteristics were reported for the pre- and post-restriction cohorts for each of the four study groups ( and ). The most common diagnoses were back, joint, and soft-tissue disorders, while ∼7–9% of patients had a cancer diagnosis, suggesting the population was composed primarily of chronic non-malignant pain patients.
Table 2. Most frequent baseline comorbidities (by ICD-9 grouping).
Oxycodone HCl ER utilization within the ER/LA opioids market was generally reduced following plans’ imposing access restrictions for oxycodone HCl ER. The largest magnitude change was observed in the commercial PA group (−8.88%), while reductions were also observed in the Medicare PA (−3.48%), and Medicare TC (−0.27%) groups, although oxycodone HCl ER utilization did increase (+2.14%) following access restrictions in the commercial TC group ().
Table 3. ER/LA opioid utilization over 6 months from pre- to post-restriction.
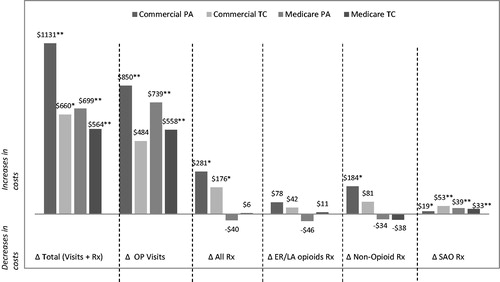
There was a significant increase in the mean number of outpatient visits in the post-restriction cohorts compared to the pre-restriction cohorts. The 6-month mean difference (post-restriction cohort – pre-restriction cohort) in outpatient visits for the study groups showed an increase of 2.49 visits (p < 0.001) for commercial PA, 1.41 visits (p = 0.008) for commercial TC, 0.90 visits (p = 0.010) for Medicare PA, and 1.03 visits (p < 0.001) for Medicare TC. The differences in the number of outpatient visits and 6-month prescription counts are shown in . The difference in healthcare costs over 6 months between the post-restriction and pre-restriction groups can be seen in . A comparison of the 6-month mean costs between the pre-restriction and post-restriction cohorts showed significantly higher medical and combined (pharmacy + medical) costs in the post-restriction cohorts. Mean combined 6-month pharmacy and medical costs were $1131 (p < 0.001) higher in the commercial PA group, $660 (p = 0.009) higher in the commercial TC group, $699 (p < 0.001) higher in the Medicare PA group, and $564 (p < 0.001) higher in the Medicare TC group. Both the commercial PA and commercial TC groups showed a significant increase in pharmacy costs (an increase of $281 (p = 0.006) and $176 (p = 0.048), respectively) during the post-restriction period. Outpatient visit costs accounted for the greatest proportion of increased costs in the post-restriction cohort across the four study groups and ranged from a 6-month cost increase of $484 (p = 0.058) in the commercial TC group to $850 (p < 0.001) in the commercial PA group. SAO pharmacy costs also increased significantly from pre- to post-restriction periods in all four groups. We note that a ∼70% reduction in sample size between the pre- and post- restriction commercial PA cohorts was observed (lack of continuous follow-up and no claim for ER/LA opioid were the main reasons for the drop in patients), which may affect the results.
Figure 3. Change in healthcare resource utilization over 6 months (post-restriction – pre-restriction). Resource counts are measured in terms of number of outpatient (OP) visits (all outpatient encounters captured through the CMS-1500 billing form) or number of prescriptions filled. All bars represent average 6-month difference in resource counts from pre-restriction to post-restriction periods. Bootstrapping t-test was utilized to test the differences in resource utilization. *0.001 < p ≤ 0.05; **p ≤ 0.001.
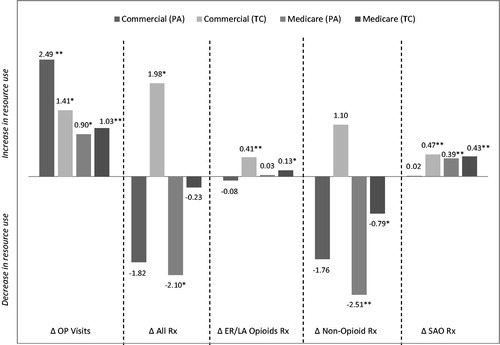
Figure 4. Change in healthcare costs over 6 months (post-restriction – pre-restriction). Outpatient (OP) visits include all outpatient encounters captured through the CMS-1500 billing form. All bars represent average 6-month difference in charge amounts from pre-restriction to post-restriction periods (normalized to 2012 dollars). Generalized linear model with gamma distribution and a log-link was utilized to test the differences in costs. *0.001 < p ≤ 0.05; **p ≤ 0.001.
A matched case-control sensitivity analysis was conducted, where patients included in the primary analyses were considered cases (i.e., patients enrolled in plans that increased branded oxycodone HCl ER access restrictions), and controls were selected patients enrolled in plans that did not increase branded oxycodone HCl ER access restrictions. Not all patients in the primary analyses could be matched to controls; therefore, sample sizes in the sensitivity analysis were smaller than in the primary analysis. In the case-control sensitivity analysis, we observed that all four case groups demonstrated an increase in the number of OP visits in the post-restriction period compared to the pre-restriction period. There were 2.13 (p < 0.001) additional outpatient visits among commercial PA cases, a 1.29 (p < 0.017) visit increase among commercial TC cases, a 0.81 (p < 0.021) increase in outpatient visits among Medicare PA cases, and one extra outpatient visit (p < 0.001) among Medicare TC cases from the pre- to post-restriction period. Among the matched control groups, three of the four groups showed no significant change in the number of outpatient visits in the post-restriction period compared to the pre-restriction period: commercial TC = 0.06 (p = 0.902), Medicare PA = 0.26 (p = 0.499), and Medicare TC = 0.35 (p = 0.340). Among controls, commercial PA was the only group which showed a significant increase in outpatient visits in the post-restriction period over the pre-restriction period (2.01, p = 0.001). Overall, the sensitivity analysis confirmed the direction of the findings from the primary analysis, that outpatient visits increased more in the plans imposing access restrictions than in plans that did not. As in the primary analysis, the interpretation of the results from the commercial PA case-control comparison is also hindered due to a ∼70% reduction in sample size between the pre- and post-restriction cohorts. Therefore, results of this group should be interpreted with caution.
Discussion
Health plans implement access restrictions on pharmaceutical products for a variety of reasons including cost control, safety, and utilization management. The increase in FDA approval for a wide variety of specialized medicines has put additional demands on health plans to insure that the right patients receive the right therapyCitation11. While access restrictions have been shown to be effective in reducing targeted drug spend, many studies have demonstrated that there can be unintended consequences of imposing such restrictions, or the savings may not be substantialCitation14–17. Results of this study suggest that imposing access restrictions such as PA and TC on oxycodone HCl ER may not result in cost savings. This study specifically examined healthcare utilization and cost and was not designed to determine the appropriateness of therapy, safety issues, or other non-financial benefits of access restrictions.
A reduction in oxycodone HCl ER utilization was observed following the implementation of access restriction in three study groups (Medicare TC, Medicare PA, commercial PA) but not in the commercial TC group. These changes in utilization were accompanied by an increase in outpatient visits and an increase in short-acting opioid prescriptions across all groups, as well as a net increase in combined medical and pharmacy costs among all groups.
Quality management of chronic pain is challenging as it requires individualized treatmentCitation26–29. In addition to managing patients’ therapy, physicians are burdened with additional regulations which put additional scrutiny on prescribers of narcotic analgesicsCitation30,Citation31. The management of chronic pain with opioids is a unique therapeutic challenge that may not represent an optimal target for cost savings by formulary management using access restrictions. Switching opioids is a complex task that requires dose adjustments and careful monitoringCitation32. Additional outpatient visits may be needed since patients may experience a change in effectiveness of the pain medication or side-effects, and physicians have to balance the risk of toxicity and withdrawal while maintaining adequate pain control. Given the challenges of opioid management, switching stable patients by limiting therapeutic options as a result of access restrictions may create a burden for the patient and provider without a resulting net decrease in costs to the health plan.
In addition to the increase in outpatient visits observed in this study, there are administrative costs associated with managing access restrictions (the cost of implementing a PA has been estimated to range from $10–$75 per event evaluationCitation33–35), which were not evaluated in this study. Consequently, the net financial impact to the health plans may have been under-estimated.
While increased outpatient visits may be due to the interruption of patients’ pain management resulting from the oxycodone HCl ER access restriction, other explanations may include the natural evolution of the patient’s underlying disease and pain severity, or other non-pain-related health issues that necessitate additional outpatient visits. In addition to that, external environmental factors such as changes in state regulations can also affect prescribing patterns and utilization.
Formulary access changes for other ER/LA opioids may have had an impact on oxycodone hydrochloride extended-release tablets utilization, and this may partially explain the observed increase of oxycodone HCl ER share in commercial TC plans. The effect of changes in access to other ER/LA opioids was not considered in this analysis, but it was observed that there was an overall increase in the number of prescriptions and costs for SAOs.
The mixed oxycodone HCl ER utilization changes observed in this study are consistent with the findings of an earlier study of the impact of PA restrictions on controlled-release (CR) oxycodone utilization in state Medicaid programs which showed mixed resultsCitation18. While a few states achieved a significant decrease in CR oxycodone use, most did not. Although that study showed an aggregate 8% decrease in CR oxycodone utilization among the ER/LA opioids following imposition of a PA, the impact on other healthcare utilization (e.g. OP visits) were not studied. In the same study, a more rigorous PA was found to result in a non-significant decrease in CR oxycodone use along with a limited (−$0.31 per long-acting opiate dose) but statistically significant reduction in average cost per long-acting dose. The researchers determined CR oxycodone is comparatively refractory to PA requirements when compared to other reports of the impact of PA programs on other Medicaid drug utilization and costs. Morden et al.Citation18 further concluded that these restrictions can actually result in significant increases in underlying costs to Medicaid programs and the US healthcare system overall, which is consistent with the increase in costs observed in the current study.
Unintended consequences or unsubstantial savings of patient pharmacy access restrictions have been reported repeatedly in other therapeutic areas such as cardiovascular diseases, GI disorders, schizophrenia/bipolar disorder, and diabetesCitation14–17. However, opioids may present a unique level of complexity in terms of therapeutic management and we have found no studies for commercial or Medicare patients specific to health plan costs and utilization of ER/LA opioids. Consequently, this study presents novel information and provides policymakers with additional real-world insight regarding the impact that patient access restrictions for ER/LA opioids may have.
While there may be unmeasured confounders among the study groups, key patient clinical characteristics (comorbidity and percentage of cancer patients), and age and gender demographic characteristics were similar between the pre- and post-restriction cohorts so as to have little prognostic value on outcomes. Additionally, a case-control sensitivity analyses was conducted where patients were matched on age, gender, US census region, CCI, and cancer diagnosis, among other variables. Various degrees of sample size differences between the pre- and post-restriction cohorts were observed among the study groups (commercial PA group had the highest difference in the sample size between pre and post-restricted cohorts); hence, those results should be interpreted with caution.
Using billed charges as a proxy for healthcare cost is likely to over-estimate the actual cost of care to the health plan. This cost over-estimation should be taken into consideration while interpreting the absolute measures in the study results. However, as this study examined the difference in charges between pre- and post-restriction periods in an equal manner, this limitation does not change the direction of the study conclusions. Medical encounters not billed via a CMS-1500, in particular hospital encounters, were not available in the open source databases used in this study. This study examined all cause healthcare resource utilization in the outpatient setting. As the validity of coding for pain-specific clinical encounters could not be ascertained from the available data, this study did not distinguish between outpatient visits for pain and visits for other disease states. Although this might be considered as a limitation of the current study, some studies have concluded that the attribution of cost to encounter with a specific set of diagnoses may in fact substantially under-estimate the true cost associated with the conditionCitation36,Citation37. While under-reporting of resource use and charges is possible due to out-of-network, unrecorded observations and similar effects, there is no reason to believe that such reporting is biased toward any of the study groups.
The results of this study should be considered preliminary, and further research using longer follow-up periods, different populations, and types of formulary restrictions such as non-formulary and step therapy are warranted. This study was conducted prior to the FDA announcement on class-wide safety labeling changes; revised indications for ER/LA opioids may have an impact on the outcomes studied. In addition, the observation period of 6 months may be inadequate to capture any potential long-term effects on the cost of care following the imposition of access restrictions. Further studies are called for to determine if long-term savings can be realized from such policies while evaluating impact on access to care and other outcomes.
The results of this retrospective study suggest that oxycodone HCl ER access restrictions including PA and TC are not associated with decreased billable costs in commercial and Medicare health plans. Implementing access restrictions on oxycodone HCl ER may increase the number of outpatient visits and costs for patients receiving ER/LA opioids, without an offsetting decrease in pharmacy costs. Along with the cost of implementing such restrictions, such restrictive strategies may impose a net cost increase to payers. While cost saving might not be the primary goal of implementing access restrictions by a health plan, health plans should consider such unintended consequences of access restrictions on oxycodone HCl ER when identifying therapeutic areas to be considered for formulary restrictions.
Transparency
Declaration of funding
This study was sponsored by Purdue Pharma L.P. (Stamford, CT).
Declaration of financial/other relationships
From Health Outcomes & Pharmacoeconomics (RBJ, DS), Purdue Pharma L.P., Stamford, CT; Health Economics and Outcomes Research (CCC, APD, RW) IMS Health, Plymouth Meeting, PA.
Acknowledgments
The authors wish to thank Katharine Coyle for her medical writing assistance on this manuscript.
References
- American Academy of Pain Medicine. http://www.painmed.org/patientcenter/facts_on_pain.aspx#refer. Accessed August 13, 2013
- Relieving pain in America, a Blueprint for Transforming, Prevention, Care, Education and Research. Institute of Medicine Report from the Committee on Advancing Pain Research, The National Academies Press, Washington, DC. 2011
- Argoff C, Silvershein D. A comparison of long- and short-acting opioids for the treatment of chronic noncancer pain: tailoring therapy to meet patient needs. Mayo Clinic Proc 2009;84:602-12
- Chou R, Fanciullo G, Fine P, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain 2009;10:113-30
- The United States Food and Drugs Administration News Release. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm367726.htm. Accessed April 29, 2014
- The United States Food and Drugs Administration. Goal of label changes: better prescribing, safer use of opioids. 2013. Washington DC, USA. http://www.fda.gov/forconsumers/consumerupdates/ucm367660.htm. Accessed April 30, 2014
- The United States Food and Drugs Administration. List of extended-release and long-acting opioid products required to have an opioid REMS. Washington DC, USA. http://www.fda.gov/drugs/drugsafety/Informationbydrugclass/ucm251735.htm. Accessed April 20, 2014
- United States Department of Justice. Drug Enforcement Agency. Drug scheduling. Washington DC, USA. http://www.justice.gov/dea/druginfo/ds.shtml. Accessed August 13, 2013
- Purdue Pharma. Oxycontin Full Prescribing Information 2014. Stamford, CT, USA. http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/022272s022lbl.pdf. Accessed April 20, 2014
- Academy of Managed Care Pharmacy, Common practice in formulary management systems. Academy of Managed Care Pharmacy, 2000. Alexandria, VA, USA. http://www.amcp.org/WorkArea/DownloadAsset.aspx?id=9274. Accessed April 28, 2014
- Leung M, Halpern M, West N. Pharmaceutical technology assessment: perspectives from payers. JMCP 2012;18:256-64
- Seigfried R, Corbo T, Saltzberg M, et al. Deciding which drugs get onto the formulary: a value-based approach. Value Health 2013;16:901-6
- Delate T, Mager, DE, Sheth J. Clinical and financial outcomes associated with a proton pump inhibitor prior-authorization program in a Medicaid population. AJMC 2005;11:29-36
- Fischer M, Polinski J, Servi A, et al. Prior authorization for biologic disease-modifying antirheumatic drugs: a description of US Medicaid programs. Arthritis Rheum 2008;59:1611-7
- Soumeria S, Zhang F, Ross-Degnan D, et al. Use of atypical antipsychotic drugs for schizophrenia in Maine Medicaid following a policy change. Health Affairs 2008;27:185-95
- Bazalo G, Weiss R, Joshi A. Impact of prior authorization for pregabalin on health plan drug expenditures. AJMC 2010;16:S154-9
- Margolis J, Cao Z, Onukwugha W, et al. Healthcare utilization and cost effects of prior authorization for pregabalin in commercial health plans. AJMC 2010;16:447-56
- Morden N, Zerzan J, Rue T, et al. Medicaid prior authorization and controlled-release oxycodone. Med Care 2008;46:573-80
- Merchant S, Noe L, Howe A, et al. Budget impact analysis of tapentadol extended release for the treatment of moderate to severe chronic noncancer pain. Clin Therapeut 2013;35:359-72
- US Department of Health and Human Services. Food and Drug Adminstration. FDA actions on OxyContin Products 4/16/2013. FDA news release 2013. Washinton DC. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm348252.htm. Accessed September 26, 2013
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613-9
- Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130-9
- Davison AC, Hinkley DV. Bootstrap methods and their applications. Cambridge, England: Cambridge University Press, 1997
- McCullagh P, Nelder JA. Generalized Linear Models. 2nd edn. London: Chapman & Hall, 1989
- Table 2. Annual Statistical Report on the Social Security Disability Insurance Program, 2011. http://www.ssa.gov/policy/docs/statcomps/di_asr/2011/sect01a.html#table2. Accessed April 28, 2014
- Cantrill S, Brown M, Carlisle R, et al. Clinical policy: critical issues in the prescribing of opioids for adult patients in the emergency department. Ann Emerg Med 2012;60:499-525
- Schultz D. Opioid use and abuse: a pain clinic perspective. Minn Med 2013;96:42-4
- Nelsen D. Opioids for the management of non-cancer pain. J Ark Med Soc 2013;109:252-3
- Noble M, Treadwell J, Tregear S, et al. Long-term opioid management for chronic noncancer pain. Cochrane Database Sytem Rev 2010;(1):CD006605
- Milhofer J. Finding a feasible solution. States employ different legislative strategies to curb misuse. Minn Med 2013;96:52-4
- Nosyk B, Anglin M, Brissette S, et al. A call for evidence-based medical treatment of opioid dependence in the United States and Canada. Health Aff 2013;32:1462-9
- McPherson ML. Demystifying opioid conversion calculations: a guide for effective dosing. Bethesda, MD: American Society of Health-System Pharmacists, 2009
- Reissman D. What is the real cost of prior authorization? Drug Benefit Trends 2000;12:22-4
- Moeller D. Manage medical advances with automated prior authorization. Managed Executive 2009. http://managedhealthcareexecutive.modernmedicine.com/mhe/article/articleDetail.jsp?id=615491. Accessed November 20, 2013
- Balkrishnan R, Joish V, Bhosle MJ, et al. Prior authorization of newer insomnia medications in managed care: is it cost saving? J Clin Sleep Med 2007;3:393-8
- Frost FJ, Hurley JS, Petersen HV, et al. A comparison of two methods for estimating the health care costs of epilepsy. Epilepsia 2000;41:1020-6
- Tunceli O, Wade R, Gu T, et al. Cost of diabetes: comparison of disease-attributable and matched cohort cost estimation methods. CMRO 2010;26:1827-34