Abstract
Objectives:
Celecoxib for the treatment of pain resulting from osteoarthritis (OA) was reviewed by the Tandvårds- och läkemedelsförmånsverket–Dental and Pharmaceutical Benefits Board (TLV) in Sweden in late 2010. This study aimed to evaluate the incremental cost-effectiveness ratio (ICER) of celecoxib plus a proton pump inhibitor (PPI) compared to diclofenac plus a PPI in a Swedish setting.
Methods:
The National Institute for Health and Care Excellence (NICE) in the UK developed a health economic model as part of their 2008 assessment of treatments for OA. In this analysis, the model was reconstructed and adapted to a Swedish perspective. Drug costs were updated using the TLV database. Adverse event costs were calculated using the regional price list of Southern Sweden and the standard treatment guidelines from the county council of Stockholm. Costs for treating cardiovascular (CV) events were taken from the Swedish DRG codes and the literature.
Results:
Over a patient’s lifetime treatment with celecoxib plus a PPI was associated with a quality-adjusted life year (QALY) gain of 0.006 per patient when compared to diclofenac plus a PPI. There was an increase in discounted costs of 529kr per patient, which resulted in an incremental cost-effectiveness ratio (ICER) of 82,313kr ($12,141). Sensitivity analysis showed that treatment was more cost effective in patients with an increased risk of bleeding or gastrointestinal (GI) complications.
Conclusions:
The results suggest that celecoxib plus a PPI is a cost effective treatment for OA when compared to diclofenac plus a PPI. Treatment is shown to be more cost effective in Sweden for patients with a high risk of bleeding or GI complications. It was in this population that the TLV gave a positive recommendation. There are known limitations on efficacy in the original NICE model.
Introduction
The Tandvårds- och läkemedelsförmånsverket–Dental and Pharmaceutical Benefits Board (TLV) in Sweden is conducting systematic reviews of drugs that are included in the reimbursement system. Following the review, the reimbursement status of the drug may change. One of the first drugs to be reinvestigated by the TLV was Celebrex (Celecoxib).
Celecoxib is a COX-2 selective non-steroidal anti-inflammatory drug (NSAID) with treatment indications that include use in osteoarthritis (OA) as well as rheumatoid arthritis and ankylosing spondylitisCitation1,Citation2. OA is one of the leading causes of pain and disability worldwide. The prevalence of OA in Sweden has been estimated to be 3.8%Citation3. The most common form of arthritis, it can affect any synovial joint causing pain, functional limitation, and a reduction in quality-of-lifeCitation4. The pain is generally treated with NSAIDs or, more recently, with COX-2 selective NSAIDs. There is a known link between chronic NSAID therapy and gastrointestinal (GI) bleeding, with a reported 3200 deaths in the US as a result of complications from GI bleeding using NSAIDsCitation5.
In Sweden cost effectiveness is an important criterion for reimbursement and evidence provided by companies is carefully investigated. Although the TLV does not have explicit thresholds for cost effectiveness, interventions with an incremental cost-effectiveness ratio (ICER) below 100,000kr (14,750 US$) are ordinarily approved, whilst those between 100,000kr and 500,000kr (73,750 US$) may be approved. Approval for an intervention with an ICER above 500,000kr is unlikely. To support the cost-effectiveness case, the development of a health economic model is required.
In the UK, the National Institute for Health and Care Excellence (NICE) produces guidance on public health, health technologies, and clinical practiceCitation6. In 2008, NICE published clinical guidance for OACitation4. As part of the process, NICE assessed the cost effectiveness of current treatments from the perspective of the UK National Health Service (NHS). This included developing a model to assess COX-2 selective NSAIDs and traditional NSAIDs.
The NICE analysis demonstrated that the addition of a proton pump inhibitor (PPI) to all NSAIDs/COX-2 selective NSAIDs was cost effective; as such, for the NICE assessment, it was assumed that all NSAIDs/COX-2 selective NSAIDs would be prescribed with a PPI. In this adaptation, the cost-effectiveness of the addition of a PPI is considered. International guidelines recommend the co-prescription of a PPI with NSAIDs and COX-2 selective NSAIDs in patients with moderate–high comorbidity risksCitation7—it is assumed that this guidance is adhered to in Sweden as in the UK.
The structure and development of the NICE model has been well describedCitation8. It drew upon data from three randomized controlled trials (RCTs): CLASSCitation9 (celecoxib, diclofenac, and ibuprofen), MEDALCitation10 (etoricoxib and diclofenac), and TARGETCitation11,Citation12 (lumiracoxib, naproxen, and ibuprofen). The focus of these trials was to demonstrate the rates of adverse events (AEs) associated with treatment rather than to demonstrate efficacy and, thus, they were performed at high-end to supratherapeutic doses. However, the results of CLASS did demonstrate that celecoxib was as effective as NSAIDs in treating OA. This was confirmed in the subsequent SUCCESS trial that compared celecoxib with diclofenac and naproxenCitation13.
The NICE model analysed the cost effectiveness of non-selective NSAIDs plus PPI (diclofenac 100 mg/day, naproxen 750 mg/day, ibuprofen 1200 mg/day) and COX-2 selective NSAIDs plus PPI (celecoxib 200 mg/day, etoricoxib 60 mg/day), and paracetamol 3000 mg/day. Omeprazole was selected as the PPI in the NICE model, as it was the cheapest at the timeCitation8 and has been demonstrated to be an effective and safe gastroprotective agentCitation14. It was assumed in the model that all treatments were equally effective at controlling OA symptoms, but were differentiated by their individual GI and cardiovascular (CV) risks.
The NICE analysis established that treatment with celecoxib plus PPI was cost effective compared to treatment with diclofenac plus PPI in patients with an increased GI risk in the UKCitation4. Sensitivity analysis demonstrated that these results were not sensitive to either the assumed duration of treatment or to the baseline risk of GI events in the population and were relatively insensitive to the baseline risk of CV eventsCitation4. However, there were considerable uncertainties over the relative rates of AEs from the RCTs. The NICE analysis also included a report based on observational data that suggested a less attractive cost-effectiveness ratio for celecoxib plus PPI compared with diclofenac plus PPICitation15.
At the request of the TLV, the UK NICE model was reconstructed from the published model and adapted to examine the cost effectiveness of celecoxib compared to diclofenac from an economic and epidemiological perspective of the Swedish healthcare system. The cost effectiveness of the addition of a PPI was also investigated and, if appropriate, celecoxib plus PPI would be compared to diclofenac plus PPI.
Methods
Overview of NICE OA model structure
The NICE OA model was a lifetime Markov model, divided into 3-month cycles. Patients could be treated with an active therapy for one cycle in the base case, but could be varied up to a lifetime of treatment in sensitivity analysesCitation15. The model examined the AEs associated with treatment with NSAIDs and COX-2 selective NSAIDs, and the reduction in these when a PPI is added. AEs included in the model were: GI symptoms/dyspepsia; serious GI events (symptomatic ulcer, complicated GI bleed); and CV events (myocardial infarction (MI), stroke, heart failure (HF)). Patients ceased treatment in the model when they suffered a serious GI or CV event. NSAIDs and COX-2 selective NSAIDs are used to control the symptoms of OA, but they are not disease modifying, and treatment with these drugs will not influence timing of surgery. Therefore, surgery was excluded from the model.
The model simulated the dynamic AE path of a patient receiving medication for treatment of OA. There were 14 possible health states consisting of no complications, GI symptoms/dyspepsia, symptomatic ulcer, post-symptomatic ulcer, complicated GI bleed, post-complicated GI bleed, MI, post-MI, stroke, post-stroke, HF, post-HF, post-treatment with no complications, and death. Each health state had associated costs and quality-adjusted life year (QALY) gains and a probability of transitioning to other states. Patients were not able to cross-over to other treatments in the model, except following a serious AE, when the active treatment was replaced by paracetamol.
Within states decision trees were used to calculate the probability of a patient moving between states and to determine which type of AE (if any) was experienced. Two sets of transition probabilities were used: one set from RCT data and another set from observational data. The probability of incurring an AE within the trees differed depending on the treatment option and whether the patient was in a post-event state where there was an increased risk of a repeat event. The relative risks of AEs for celecoxib vs diclofenac, and for NSAIDs and COX-2 selective NSAIDs with and without PPI, are presented in .
Table 1. Relative risks for adverse events.
The NICE reference case required that the model outputs were expressed in terms of costs and QALYs (primary outcomes) and incremental cost per QALY gained (secondary outcome). Utility values, therefore, were required for each health state and were elicited from the literature. The scores for GI symptoms/dyspepsia, symptomatic ulcer, and complicated bleed states were 3-month scores from the Canadian Coordinating Office for Health Technology Assessment (CCOHTA) surveyCitation16. The utility data for stroke came from Pickard et al.Citation17, but short-term utility data for other CV events was unavailable, so longer term utility scores were adjustedCitation15. The 3-month utility used for HF came from the Harvard CE RegistryCitation15 and, following advice from the guideline development group, the model assumed that after 3 months the patient reverts to the no-complications utility score.
The model was reconstructed from the published data and methods and the reported results from the NICE model replicated.
Update to Swedish costs
The model was then adapted to reflect Swedish costs and decision perspectives. Costs included in the model consisted of drug acquisition cost and treatment of AEs. Wider societal costs, which were not considered in the NICE base case calculation of cost/QALY such as cost of unemployment because of ill health, are considered by the TLV in Sweden. However, given that OA is generally a disease of the elderly, these were not included in the adapted model. A summary of the costs included and their values is presented in . (Costs are additionally presented in US dollars, converted using XE, November 13, 2012.)
Table 2. Swedish costs applied in the updated model.
Acquisition prices for relevant drugs were taken from PM-Läkemedel Dnr761/2010Citation18. In this the TLV sets out the lowest pharmacy price for a normal dose in a package of 100 tablets or fewerCitation18. The costs for treating dyspepsia, symptomatic ulcer, and GI bleeds were estimated based on standard treatment episodes. These estimates were based on treatment guidelines from the county council of StockholmCitation19. The standardized treatment episodes were reviewed by two specialists and deemed appropriate. The interventions associated with each standardized treatment episode were assigned costs according to the regional price list of Southern SwedenCitation20.
The costs of treating CV events for the first 3 months were based on Swedish DRG code 122 (without complications) for MI, DRG code 014B and 015 for stroke, and DRG 127 for HF. Following Latimer et al.Citation8, the costs for stroke were a weighted average of the code for stroke and transient ischemic attacks. The weighting was based on the number of inpatient admissions for the two events in Sweden according to the Swedish inpatient registryCitation21. The costs of treating long-term CV events after 3 months were taken from Zethraeus et al.Citation22 and inflated to 2009 prices using the consumer price indexCitation23. Costs estimated for patients that had established heart disease or previous stroke were used as an approximation.
Costs and benefits were discounted annually at a rate of 3% in line with TLV guidelines. A deterministic approach was used to assess the cost effectiveness at the mean parameter values.
Sensitivity analysis
One of the key concerns of the TLV was the provision of robust sensitivity analysis. Therefore, scenarios were run to examine the effect of age (varying age from 55 to 65), treatment length (extending treatment from 3 months to 24 months), varying the relative risk of CV events between celecoxib and diclofenac, varying the relative risk of GI events between celecoxib and diclofenac, and varying AE treatment costs. Additionally, a probabilistic sensitivity analysis (PSA) was performed, sampling 1000 parameter sets from the distributions around parameters in the model to generate a probability distribution of calculated cost-effectiveness ratios, reflecting the combined uncertainty in the underlying parameters of the model.
Results
Using a lifetime time horizon, the model demonstrated that treatment with celecoxib (without PPI) resulted in costs of 43,583kr and benefits of 11.923 QALYs. Diclofenac resulted in costs of 43,120kr and 11.915 QALYs. This demonstrated that, over a patient’s lifetime, treatment with celecoxib (without PPI) when compared to treatment with diclofenac (without PPI) was associated with an increase in discounted costs of 463kr per patient and a gain in QALYs of 0.008 per patient, resulting in an ICER of 59,769kr (8816 US$) (). The comparison against no-treatment showed an increase in costs of 1022kr and a 0.003 gain in QALYs, resulting in an ICER of 299,564kr (44,186 US$) ().
Table 3. Swedish base case results without PPI.
The addition of a PPI to both diclofenac and celecoxib decreased the costs (by 151kr for celecoxib, by 216kr for diclofenac), and increased the QALYs (by 0.01 for each) ( and ). Since the addition of a PPI led to a gain in QALYs and a cost saving, the addition of a PPI to both diclofenac and celecoxib met the criteria for being more cost-effective than each agent alone, and, therefore, all further analyses in this article compare celecoxib plus PPI to diclofenac plus PPI.
Table 4. Addition of PPI to celecobxib—results.
Table 5. Addition of PPI to diclofenac—results.
The results demonstrated that, over a patient’s lifetime, treatment with celecoxib plus PPI when compared to treatment with diclofenac plus PPI was associated with an increase in discounted costs of 529kr per patient and a gain in QALYs of 0.006 per patient, resulting in an ICER of 82,313kr (12,141 US$) (). The comparison against no-treatment was included in the base case results and showed an increase in costs of 871kr and a 0.01 gain in QALYs, resulting in an ICER of 86,615kr (12,776 US$) ().
Table 6. Swedish base case results with PPI.
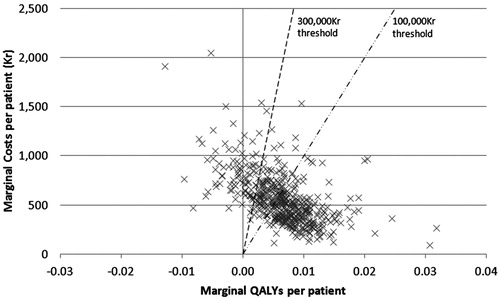
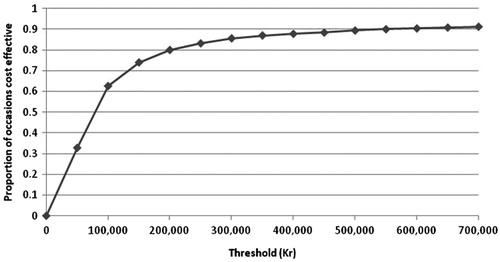
PSA was performed and the results plotted on a cost-effectiveness plane with lines representing thresholds of 100,000kr and 300,000kr (). It can be seen that many of the points are clustered around the centre of the thresholds, with some results present on either side. Only 10% of points show celecoxib plus PPI to be dominated by diclofenac plus PPI; most of the points show celecoxib plus PPI to be cost effective compared to diclofenac plus PPI. The results were then plotted on a cost-effectiveness acceptability curve (). This analysis showed that there was a 58% chance of celecoxib plus PPI being cost effective at a willingness-to-pay threshold of 100,000kr (14,750 US$) per QALY, and an 82% chance of celecoxib being cost effective at a threshold of 300,000kr (44,250 US$) per QALY.
Sensitivity analyses found that the ICER was most sensitive to the relative risk and cost of dyspepsia/GI symptoms, but in all scenarios the ICER was well below the 500,000kr threshold, and below 100,000kr in most cases.
A range of sensitivity and scenario analyses were performed, firstly varying the treatment period from 3 months to 24 months and age from 55 to 65 and then combining these with an additional scenario where the risk of CV event for celecoxib and diclofenac were set as equal ().
Table 7. Sensitivity analysis results.
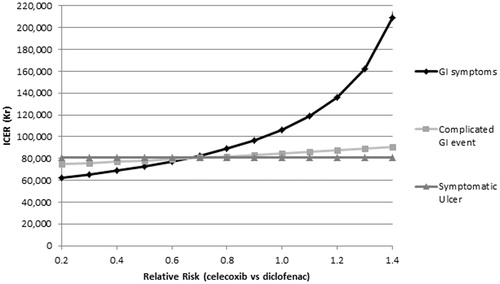
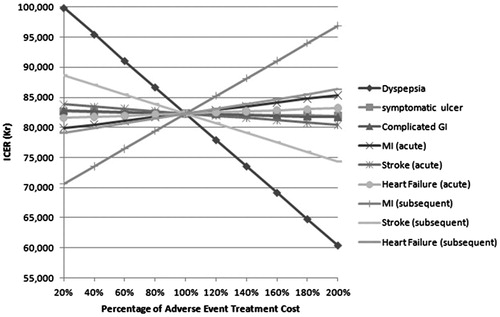
These were further investigated by examining a range of relative risk values for GI and CV events, which show that the model is particularly sensitive to changes in the risk of GI symptoms and stroke ( and ). A final sensitivity analysis was performed varying the cost of AEs. These analyses demonstrated that the model is particularly sensitive to changes in the cost of treating dyspepsia and the long-term treatment costs of MI.
Discussion
The limitations of the original NICE model were discussed in the published report of the model. In particular these focussed on issues with amalgamating and pooling RCT and observational evidence, leading to large amounts of observational data being excluded and assumptions that had to be made regarding the relationship between dose and efficacy.
There were additional limitations when adapting the model to a Swedish perspective, as we were unable to validate if the data used in the NICE model are the same as in the Swedish population. GI complications were based on typical events in the average patient, but the GI events caused by COX-2 selective NSAIDs used in these types of patients may be less or more complicated on average. Costing of long-term disease states for CV events was based on historic published literature. As the treatment patterns and, therefore, costs may have changed, more recent information would be preferable. In its final decision the TLV stated that the model has large uncertainties and mentions that small changes in the relative risks in GI events had large effects on ICERs. However, their conclusion was that these uncertainties are smaller for patients with increased GI risks, hence their positive decision for use of the drug in that populationCitation18.
Conclusion
The results from the analysis show that, over a lifetime model, celecoxib plus PPI is cost effective from the perspective of the Swedish healthcare system when compared to treatment with diclofenac plus PPI. The key drivers of the model were found to be the celecoxib relative risk of stroke and GI symptoms as well as the costs of treating dyspepsia and subsequent MI.
Celecoxib plus PPI has been shown to be as effective as NSAIDs in the management of OA symptoms and as such provides a valuable treatment optionCitation9,Citation13. In Swedish patients it has been shown to have a favourable ICER compared with diclofenac plus PPI. Although some uncertainties in the model exist, the TLV has accepted its use in patients with high-risks of bleeding or GI complications.
Transparency
Declaration of funding
This study was sponsored by Pfizer Inc.; however, the publication of study results was not contingent on the sponsor’s approval or censorship of the manuscript.
Declaration of financial/other relationships
NB and BP have disclosed that they received funding from Pfizer to conduct this study. RA has disclosed that he received funding from Pfizer as a consultant. ME has disclosed that he is a current employee of Pfizer and holds stock in the company. NB and BP are employees of BresMed, ME is an employee of Pfizer, and RA is an employee of ScHARR, University of Sheffield, Sheffield, UK, who were paid consultants to Pfizer in connection with the development of this manuscript. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors would like to thank Pfizer for giving full permission to use their clinical data and the University of Sheffield for giving full permission to use their economic model.
Notes
*Celebrex is a registered trademark of Pfizer Inc.
References
- Celebrex® Product Information. Kansas: Pfizer, Inc., 2010. http://www.celebrex.com. Accessed September 19, 2012
- Pfizer AB 191 90 Sollentuna. Swedish Celebrex Summary of Product Characteristics. http://www.fass.se/LIF/produktfakta/artikel_produkt.jsp?NplID=20101030000078&DocTypeID=6. Accessed September 19, 2012
- Jonsson D, Husberg M. (1995). Samhallsekonomiska kostnader for reumatiska sjukdomar. Linköping, Sweden: Linköping University; CMT Rapport; 5
- National Collaborating Centre for Chronic Conditions. Osteoarthritis: national clinical guidelines for care and management in adults. London: Royal College of Physicians, 2008. http://www.nice.org.uk/nicemedia/live/11926/39720/39720.pdf, Accessed September 19, 2012
- Tarone RE, Blot WJ, McLaughlin JK. Nonselective nonaspirin nonsteroidal anti-inflammatory drugs and gastrointestinal bleeding: relative and absolute risk estimates from recent epidemiologic studies. Am J Ther 2004;11:17-25
- National Institute for Health and Clinical Excellence. http://www.nice.org.uk. Accessed January 27, 2011
- McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr Cart 2014;22:363-88
- Latimer N, Lord J, Grant RL, et al. National Institute for Health and Clinical Excellence Osteoarthritis Guideline Development Group. Cost effectiveness of COX 2 selective inhibitors and traditional NSAIDs alone or in combination with a proton pump inhibitor for people with osteoarthritis. BMJ 2009;339:b2538
- Silverstein FE, Faich G, Goldstein JL, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA 2000;284:1247-55
- Cannon CP, Curtis SP, FitzGerald GA, et al. Cardiovascular outcomes with etoricoxib and diclofenac in patients with osteoarthritis and rheumatoid arthritis in the Multinational Etoricoxib and Diclofenac Arthritis Long-term (MEDAL) programme: a randomised comparison. Lancet 2006;368:1771-81
- Farkouh ME, Kirshner H, Harrington RA, et al. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), cardiovascular outcomes: randomised controlled trial. Lancet 2004;364:675-84
- Schnitzer TJ, Burmester GR, Mysler E, et al. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), reduction in ulcer complications: randomised controlled trial. Lancet 2004;364:665-74
- Singh G, Fort JG, Goldstein JL, et al. Celecoxib versus naproxen and diclofenac in osteoarthritis patients: SUCCESS-I study. Am J Med 2006;119:255-66
- Wilson I, Langstrom G, Wahlqvist P, et al. Management of gastroduodenal ulcers and gastrointestinal symptoms associated with nonsteroidal anti-inflammatory drug therapy: a summary of four comparative trials with omeprazole, ranitidine, misoprostol, and placebo. Curr Ther Res 2001;62:835-50
- Appendix D, National Collaborating Centre for Chronic Conditions. Osteoarthritis: national clinical guidelines for care and management in adults. London: Royal College of Physicians, 2008. http://www.nice.org.uk/nicemedia/live/11926/39720/39720.pdf. Accessed January 27, 2011
- Maetzel A, Krahn M, Naglie G. The cost effectiveness of rofecoxib and celecoxib in patients with osteoarthritis or rheumatoid arthritis. Arthritis Rheumatis 2003;49:283-92
- Pickard A, Johnson JA, Feeny DH. Responsiveness of generic health-related quality of life measures in stroke. Quality Life Res 2005;14:207-19
- Tandvårds- ochläkemedelsförmånsverket – Dental and Pharmaceutical Benefits Board. Price Database. Stockhold: TLV. http://www.tlv.se/in-english-old/price-database. Accessed June 24, 2010
- Stockholm County Council. Treatment guidelines. Stockholm: Vårdinformation StorStockholm, 2010. http://www.viss.nu. Accessed August 30, 2010
- Region Skånes primärvård. Region price list. Lund: Södra Regionvårdsnämnden, 2010. http://www.skane.se. Accessed August 30, 2010
- The Swedish National Inpatient Register (Slutenvårdsregistret). http://www.sos.se. Accessed August 30, 2010
- Zethraeus N, Molin T, Henriksson P, et al. Cost of coronary heart disease and stroke: the case of Sweden. J Intern Med 1999;246:151-9
- Statistiska centralbyrå. Consumer price index Stockholm: Statistiska centralbyrå, 2008. http://www.scb.se. Accessed January 27, 2011