Abstract
Objectives:
To use techniques of decision-analytic modeling to evaluate the effectiveness and costs of linaclotide vs lubiprostone in the treatment of adult patients with irritable bowel syndrome with constipation (IBS-C).
Methods:
Using model inputs derived from published literature, linaclotide Phase III trial data and a physician survey, a decision-tree model was constructed. Response to therapy was defined as (1) a ≥14-point increase from baseline in IBS-Quality-of-Life (IBS-QoL) questionnaire overall score at week 12 or (2) one of the top two responses (moderately/significantly relieved) on a 7-point IBS symptom relief question in ≥2 of 3 months. Patients who do not respond to therapy are assumed to fail therapy and accrue costs associated with a treatment failure. Model time horizon is aligned with clinical trial duration of 12 weeks. Model outputs include number of responders, quality-adjusted life-years (QALYs), and total costs (including direct and indirect). Both one-way and probabilistic sensitivity analyses were conducted.
Results:
Treatment for IBS-C with linaclotide produced more responders than lubiprostone for both response definitions (19.3% vs 13.0% and 61.8% vs 57.2% for IBS-QoL and symptom relief, respectively), lower per-patient costs ($803 vs $911 and $977 vs $1056), and higher QALYs (0.1921 vs 0.1917 and 0.1909 vs 0.1894) over the 12-week time horizon. Results were similar for most one-way sensitivity analyses. In probabilistic sensitivity analyses, the majority of simulations resulted in linaclotide having higher treatment response rates and lower per-patient costs.
Limitations:
There are no available head-to-head trials that compare linaclotide with lubiprostone; therefore, placebo-adjusted estimates of relative efficacy were derived for model inputs. The time horizon for this model is relatively short, as it was limited to the duration of available clinical trial data.
Conclusions:
Linaclotide was found to be a less costly option vs lubiprostone for the treatment of adult patients with IBS-C.
Introduction
Irritable bowel syndrome (IBS) is a common gastrointestinal disorder in which chronic abdominal pain or discomfort is associated with altered bowel function. IBS prevalence rates vary depending on the criteria used for diagnosis, with estimates in the US ranging from 10–15%Citation1–4. Prevalence rates are higher among women than men, with a diagnosis ratio of ∼2:1Citation1. Rome III criteria categorizes IBS into four sub-types based on a patient’s predominant stool pattern: IBS with predominant diarrhea (IBS-D), IBS with predominant constipation (IBS-C), mixed (IBS-M), and untyped (IBS-U).
Per the Rome III diagnostic criteria, patients with IBS-C must experience hard or lumpy stools in at least 25% of defecations and must have at least 3 months of recurrent abdominal pain/discomfort, with symptom onset at least 6 months prior to diagnosisCitation5. IBS-C accounts for approximately one-third of all IBS casesCitation6, and is associated with substantial healthcare costs, as well as a negative impact on patient’s quality-of-life and work productivity. An analysis among Medicaid patients with IBS-C reported annual costs in hospitalization, outpatient and emergency room visits range from $4291–$6894, with patients experiencing treatment failure incurring $1781–$2562 more than those withoutCitation7. A recent analysis of the US National Health and Wellness Survey also reported patients with IBS-C to have significantly lower levels of health-related quality-of-life, as measured by the SF-12v2, compared to matched controls, in addition to significantly higher levels of presenteeism (31.72% vs 21.43%, respectively) and overall work impairment (35.54% vs 25.29%, respectively)Citation8.
To date, there have been relatively few pharmacological treatments approved for the treatment of IBS-C. Over-the-counter laxatives, which are commonly used but not approved by the US Food and Drug Administration (FDA) to specifically treat IBS-C, are primarily directed towards managing a patient’s constipation symptoms, not the multiple symptoms that are experienced by patients with IBS-C (e.g., abdominal pain, bloating). Tegaserod was approved for the treatment of IBS-C (women only) in 2002; however, it was withdrawn from the market in 2007 due to concerns regarding an increased risk of cardiovascular events among patients receiving treatment. Lubiprostone, a chloride channel activator, was approved by the FDA for the treatment of IBS-C (women only) in 2006 and, until recently, was the only prescription drug with an IBS-C indication in the US.
In August 2012, linaclotide, a minimally absorbed guanylate cyclase-C (GC-C) agonist, was approved by the FDA for treatment of IBS-C in both men and women. Linaclotide and its active metabolite bind to GC-C and act locally on the luminal surface of the intestinal epithelium. This results in an increase in both intracellular and extracellular cyclic guanosine monophosphate (cGMP). Consequently, chloride and bicarbonate secretion into the intestinal lumen results, resulting in increased intestinal fluid and accelerated transit. In addition, in animal models, linaclotide-induced reduction in visceral pain is thought to be mediated by increased extracellular cGMP, which was shown to decrease the activity of pain-sensing nervesCitation9.
With the recent FDA approval of linaclotide for the treatment of IBS-C, it becomes important to evaluate the economic impact associated with its use vs alternative prescription drug treatments (i.e., lubiprostone). Therefore, the aim of this study is to use techniques of decision analysis and mathematical modeling to evaluate the effectiveness and costs of linaclotide vs lubiprostone in the treatment of adults with IBS-C.
Methods
Model overview and structure
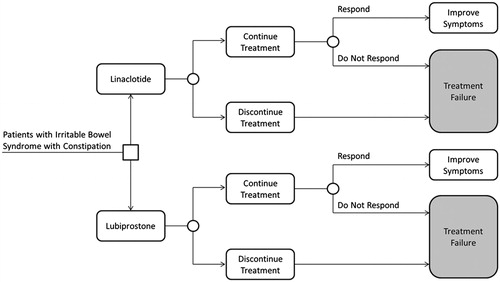
This model was developed in Microsoft Excel (version 2010) spreadsheet format using a decision–tree modeling technique, and draws upon linaclotide clinical trial data (both published and post-hoc analyses), published scientific literature, publicly available US FDA reviews of lubiprostone, and a survey of practicing physicians on resource utilization associated with treatment failure. The model population was assumed to be adults who have been diagnosed with IBS-C and are candidates for prescription treatment.
Hypothetical IBS-C patients enter the model as candidates for either linaclotide 290 mcg once daily or lubiprostone 8 mcg twice daily (). Treatment discontinuation can occur immediately after initiation. Patients who discontinue therapy are assumed to show no improvement from their baseline symptoms and are assigned clinical and economic consequences associated with treatment failure. Patients who continue drug therapy have a probability of achieving response to the assigned treatment. Patients who respond to treatment are assumed to accrue the pharmacy costs for linaclotide 290 mcg or lubiprostone 8 mcg and to have improved quality-of-life compared to those who do not respond. Patients who do not respond to treatment are assumed to accrue treatment failure costs and to have lower health utilities than patients who respond.
The model time horizon is 12 weeks, which is consistent with the publicly available lubiprostone Phase III clinical trial dataCitation10. Base-case analyses were performed from the payer’s perspective, including number of patients responding to treatment, quality-adjusted life-years (QALYs), and direct medical costs as model outputs. Indirect costs were also included in scenario analyses to provide results from the societal perspective.
Model inputs
Treatment comparators
Only prescription therapies currently approved and indicated by the US FDA for the treatment of IBS-C were included in the model; therefore, only linaclotide 290 mcg once daily and lubiprostone 8 mcg twice daily were included as treatment options. Over-the-counter remedies, such as laxatives (e.g., MiraLAX), were not considered as treatment comparators because of limited published data and lack of FDA approval for the IBS-C indication. Furthermore, it was assumed that patients entering the model were candidates for prescription treatment.
Treatment response
Economic models are often constrained by access to comparable data across treatment comparators. Therefore, available linaclotide and lubiprostone data from Phase III clinical trials were reviewed prior to model development. Measures of treatment efficacy were selected based on comparability between trial data and suitability for use as definitions of response within the model framework. Although reviewed studies sometimes used the same or similar instruments to determine treatment response, the data were generally reported differently and many end-points were not collected for both treatment typesCitation11. For instance, the primary end-point among linaclotide clinical trials included a composite measure of treatment response. A responder was a patient who, in the same week, had: (1) an improvement of ≥30% in average daily worst abdominal pain score; and (2) an increase of ≥1 complete spontaneous bowel movement (CSBM) from baseline for ≥50% of weeks evaluatedCitation12, which is consistent with current FDA regulatory guidanceCitation13. This specific end-point was not collected in clinical trials for lubiprostoneCitation10. Among trial end-points that were included in clinical trials of both treatments (e.g., quality-of-life, bloating), reported measures used different measurement scales (i.e., 0–10 vs 0–4) or did not report findings necessary for robust economic modeling (i.e., only mean values were reported without standard deviation). Therefore, post-hoc analyses of linaclotide clinical trial data were conducted to help ensure comparability with the data reported from the lubiprostone clinical trials (further details of post-hoc analyses described below and in the Appendix).
Treatment response was ultimately defined by either: (1) at least a 14-point improvementCitation14 in Irritable Bowel Syndrome-Quality of Life (IBS-QoL) questionnaire overall score from baseline to week 12 (assessed the same in the linaclotide and lubiprostone trials); or (2) a ‘monthly responder’ for at least 2 out of 3 months on a 7-point assessment of global symptom relief (primary end-point in lubiprostone trial)Citation10. The IBS-QoL questionnaire consists of 34 items, each with a 5-point response scale (‘not at all’ to ‘extremely/a great deal’) and covers eight domains: dysphoria, interference with activity, body image, health worry, food avoidance, social reaction, sexual, and relationshipsCitation15. Only the overall IBS-QoL score was used for treatment response analysis. Phase III clinical trials for both treatments also included similar 7-point assessments of global symptom relief (e.g., ‘How would you rate your relief of IBS symptoms over the past week compared to how you felt before you entered the study?’). Per the lubiprostone Phase III clinical trial, a ‘monthly responder’ was defined as a response of ‘moderately relieved’ or better in 4 out of 4 weeks or a response of ‘significantly relieved’ in 2 out of 4 weeksCitation10.
Response rate data for linaclotide were derived from data on the pooled intent-to-treat (ITT) populations of the clinical trials. Since the global symptom relief definition of response was the primary end-point in the lubiprostone clinical trial, response rates were calculated as the pooled average from publically available trial-specific results of lubiprostone for IBS-CCitation10. The clinical trial data for linaclotide included a similar 7-point assessment of global symptom relief; however, the primary end-point to evaluate treatment response in the trial was not defined in the same way as the end-point for lubiprostone, given that linaclotide trial end-points (e.g., overall CSBM/abdominal pain responder) were constructed using the FDA guidelines for treatment trials in IBSCitation13. Therefore, post-hoc analyses of linaclotide trial data using the same definition as lubiprostone were conducted to ensure comparability between response rates.
For the IBS-QoL measure of response, only the mean IBS-QoL scores (i.e., no standard deviations) were reported from the publically available FDA reviews of lubiprostone. Therefore, the ratio of the standard deviation to the mean of IBS-QoL for linaclotide was used to estimate the standard deviation for lubiprostone. Curve-fitting techniques using a normal distribution were then used to impute the percentages of subjects who achieved the defined threshold of response (i.e., ≥14 points) for both linaclotide and lubiprostone. For linaclotide, the percentage of patients with response (i.e., ≥14 points) was obtained from a post-hoc analysis of the mean IBS-QoL score change from baseline collected for the ITT population from linaclotide trials 302 and 31 and fitted to a normal distribution.
For both definitions of response defined above, placebo-adjusted odds ratios (OR) of response for linaclotide vs lubiprostone were constructed (full details regarding the techniques involved in constructing response rates and the placebo-adjusted ORs are provided in the Appendix).
Treatment discontinuation
In the base case analysis, the discontinuation rate for any reason (e.g., due to adverse events or lost-to-follow-up) was assumed to be zero for both treatment options.
Economic inputs
Drug costs were included in the model as daily costs. Daily cost for lubiprostone 8 mcg was calculated as twice the wholesale acquisition cost (WAC) price per tablet ($9.16/day), based on the 2013 Truven Health Analytics Redbook database (updated as of October 2, 2013)Citation16. The WAC daily price for linaclotide is $7.70/day for linaclotide 290 mcgCitation17. Non-responders and those who discontinue therapy incurred pharmacy costs associated with a 1-month supply of treatment (30 days); responders incurred pharmacy costs associated with treatment for the full timeframe of the model (12 weeks).
Direct costs associated with treatment failure were derived from a web-based survey study conducted among a sample of 20 primary care physicians (PCPs) and 21 gastroenterologists (GEs) across geographically representative regions of the US. The survey captured data on referral patterns (to/from gastroenterologists), test/procedure ordering and follow-up physician visits for typical patients who did (e.g., ‘response’) and did not (e.g., ‘failure’) achieve a satisfactory relief of symptoms to a recent treatment for IBS-C. Survey items included questions regarding the proportion of patients who would receive tests/procedures and follow-up physician visits. Healthcare costs were estimated by applying associated unit costs (derived from the 2012 Medicare physician payment schedule) to the corresponding utilization. All patients were assumed to begin treatment with PCPs. The cost of treatment failure was defined as the cost difference between physician-deemed non-responders and responders, incorporating both PCP work-up costs and the costs of referrals to GEs (see the Appendix for full details of the physician survey)Citation18.
Indirect cost for treatment failure was derived from data collected from the Work Productivity and Activity Impairment (WPAI) questionnaire in the Phase III clinical trial for linaclotide and was only applied to non-respondersCitation19. The average number of reported hours missed from work due to IBS-C was multiplied by average wage data from the Bureau of Labor StatisticsCitation20 to calculate the indirect costs due to lost work productivity. This value was then multiplied by the incremental proportion of non-responders missing work (i.e., proportion of non-responders missing work minus proportion of responders missing work) in order to calculate the incremental indirect cost associated with treatment failure.
Utility data
To the best of our knowledge, there are no published health utility estimates for patients with IBS-C who respond or do not respond to a recent course of treatment. We, therefore, calculated base-case utility values using the difference in EQ-5D scores from the Phase III clinical trials for linaclotide for all patients (pooling both linaclotide and placebo) who did and did not meet the response criteriaCitation19. Data were pooled for the linaclotide and placebo arms in order to construct utility values that were independent of treatment type (given that utility values were not provided for lubiprostone in publically-available data). Utility values were estimated separately for each definition of treatment response.
Model outcomes
Model outcomes are primarily from the payer’s perspective and include the percentage of patients responding to each treatment as well as the direct medical costs and QALYs associated with response/non-response. Indirect work productivity costs were not included in the base-case analysis; however, they were incorporated in a separate analysis to test the robustness of model results and include results from a societal perspective.
Sensitivity analyses
Sensitivity analyses to assess uncertainty in the results based on potential variation in base-case model inputs were performed. Low and high estimates for the following parameters were included in the one-way analysis: direct and indirect costs for treatment failure, discontinuation rate (high estimates include the discontinuation rate for ‘any reason’ from each treatment’s Phase III clinical trial; 30.1% and 23.1% for linaclotide (post-hoc analyses) and lubiprostoneCitation10, respectively), odds ratio for treatment response, and health utility values. Low estimates for the direct cost of treatment failure were based on the lower bound of the estimated base case costs derived from the web-based survey of physicians. High estimates for the direct cost of treatment failure were based on the findings from a retrospective database analysis of Medicaid administrative claims dataCitation7. High and low estimates for the odds ratios of treatment response for linaclotide vs lubiprostone were derived in the following manner: One thousand samples were bootstrapped from the linaclotide trial data, using sampling with replacement. Odds ratios for each of the 1000 replicates were computed for linaclotide vs placebo. Each of these replicates was then used to compute 1000 odds ratios for linaclotide vs the single available lubiprostone response rate. The standard deviation for the linaclotide vs placebo odds ratio obtained from the bootstrapping was considered as the standard error (SE) for the odds ratio and was then applied to the base-case odds ratio to calculate high and low estimates (i.e., base case ±1.96*SE). Sensitivity estimates for health utilities were derived from the upper and lower 95% confidence limits of EQ-5D data from the Phase III linaclotide clinical trial (low estimates correspond to the smallest difference between responder and non-responder utility values; high estimates correspond to the greatest difference between responder and non-responder utility values).
Probabilistic sensitivity analyses were undertaken using 1000 second-order Monte Carlo simulations. The odds ratio for treatment response (using a log-normal distribution with SEs obtained from the bootstrapping technique described above), drug cost for linaclotide (uniform distribution between low and high estimates; assumed to be ±10% of the actual drug cost), the cost of treatment failure (using gamma distribution), and health utilities (using a uniform distribution) were varied within the model.
Model assumptions
The demographic and clinical characteristics of the modeled patient populations are assumed to be consistent with the patient populations in the linaclotide and lubiprostone Phase III clinical trials. It is assumed that all efficacy reported in the clinical trials is attributable to the study treatment effect of linaclotide and lubiprostone (i.e., the improvement in response rate vs placebo observed in the trials is attributable to linaclotide or lubiprostone, not due to other reasons such as difference in demographics). Patients who respond to treatment are assumed to respond immediately upon treatment initiation, and all responders have the same health utility, regardless of treatment (same assumption for non-responders).
Treatment costs are based on recommended dosing and frequency of administration in each product’s prescribing information. The results from the physician survey on patient management (diagnostic tests, procedures, and physician visits) for a hypothetical patient reporting no satisfactory symptom relief during treatment are assumed to be representative of all patients who experience treatment failure. In addition, the Medicare fee schedule used to estimate costs of diagnostic test, procedures, and physician visits is assumed to be applicable to private payers.
Results
Base-case results
For both response definitions, linaclotide 290 mcg once daily was less expensive and had higher rates of response and more QALYs gained when compared to lubiprostone 8 mcg twice daily (). When response was based on improvement in IBS-QoL score, the percentage of responders was 61.8% and 57.2% for patients receiving linaclotide 290 mcg once daily and lubiprostone 8 mcg twice daily, respectively. Total direct costs were estimated to be $803 per linaclotide treated patient and $911 per patient treated with lubiprostone. When response was based on global assessment of symptom relief, the percentage of responders was 19.3% and 13.0% for patients receiving linaclotide 290 mcg once daily and lubiprostone 8 mcg twice daily, respectively. Total costs were estimated to be $977 per linaclotide treated patient and $1056 per patient treated with lubiprostone. Incremental benefits in QALYs were also seen for linaclotide over the 12 week analysis period (). When indirect costs were included in the analysis (i.e., cost of lost work productivity), results were similar for both response definitions (data not shown).
Table 1. Base-case and sensitivity analysis model inputs.
Table 2. Model results for 1000 patients with irritable bowel syndrome with constipation (direct costs only).
Sensitivity analysis results
When treatment response was based on a global assessment of symptom relief, linaclotide 290 mcg once daily had lower costs and higher response rates and QALYs vs lubiprostone 8 mcg twice daily for the treatment of IBS-C in all one-way sensitivity analyses. For the IBS-QoL definition of response, linaclotide had lower costs and higher response rates for all one-way sensitivity scenarios except when the discontinuation rate from each treatment’s Phase III clinical trial was used (23.1% for lubiprostone and 30.1% for linaclotide). This resulted in lower direct costs for linaclotide vs lubiprostone ($879 vs $954 per patient), and lower response rates (43.2% vs 44.0%).
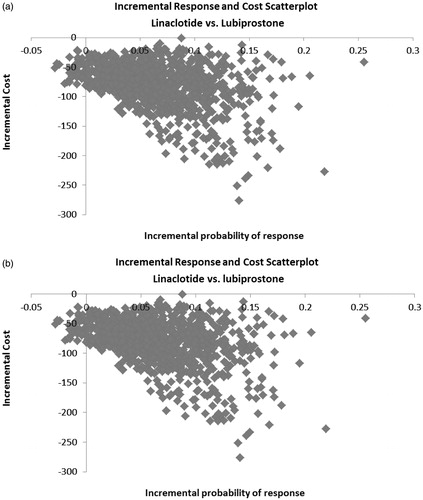
For both definitions of treatment response, the distribution of 1000 Monte Carlo simulations showed that the majority (i.e., >90%) of model iterations resulted in linaclotide being in the lower right quadrant of the incremental cost vs response plane (i.e., lower cost, higher response) ().
Discussion
Results from our economic model show that linaclotide is associated with better response rates and lower costs than lubiprostone when used for the treatment of IBS-C among adult patients. Treatment for IBS-C with linaclotide produced more responders than lubiprostone for both response definitions (19.3% vs 13.0% and 61.8% vs 57.2% for IBS-QoL and global assessment of symptom relief, respectively), lower per-patient costs ($803 vs $911 and $977 vs $1056), and slightly higher QALYs (0.1921 vs 0.1917 and 0.1909 vs 0.1894) over the 12-week time horizon. These results are robust in one-way sensitivity analyses and consistent for both definitions of treatment response analyzed in the model (improvement in IBS-QoL score and global satisfaction of IBS-C symptom relief). Results were similar when indirect costs were also included in the analysis, for both definitions of response. Therefore, under most scenarios and assumptions, linaclotide is a lower cost treatment option for patients with IBS-C; providing similar or improved treatment response rates and QALYs compared to lubiprostone.
Our study has various limitations, including a short time horizon and the need for additional analyses for several parameter estimates where available data were lacking. The time horizon for this model is relatively short (i.e., 12 weeks) given the chronic nature of IBS-C. However, there is some suggestion that the benefits of treatment may extend over time. For example, in the 4-week randomized withdrawal period after completion of the 12-week Phase III linaclotide clinical trial in IBS-C, it is observed that treatment efficacy outcomes (e.g., abdominal pain, complete spontaneous bowel movement) are sustained for the patients who remain on treatmentCitation12. For patients who discontinued treatment after the 12-week trial duration, symptoms returned and were similar to baseline. Additionally, a 26-week Phase III clinical trial for linaclotide demonstrated sustained improvement in abdominal pain and bowel symptoms among patients with IBS-C over the full study durationCitation21. Further long-term clinical results would help extend the findings from our economic model.
The direct costs associated with treatment failure were derived from the findings from a one-time, web-based survey of 20 primary-care physicians and 21 gastroenterologists that asked about treatment patterns and resource use among patients with IBS-C who were not responding to recent treatment. The classification of patient response in the survey differed from the response definition used for the clinical inputs in the model. Additionally, several assumptions were required in calculating the cost estimate, including the assumption that all patients were seen by a PCP prior to being referred to a GI specialist. Therefore, there may be some bias in the estimates of direct medical costs. This survey was undertaken because there are no published studies that present data on this issue. Resource use estimates from this survey of 41 physicians may be reasonably generalizable given that the surveyed physicians were heterogeneous in age, sex, patient volume, years of practice, and US geographic region. In addition, costs are based on Medicare fee schedules, which are likely lower compared with those of a private payer and, therefore, provide conservative cost estimatesCitation22.
There are no available head-to-head trials that compare linaclotide with lubiprostone; therefore, placebo-adjusted estimates of relative efficacy were derived. For the definition of response based on global assessment of symptom relief (the primary end-point in lubiprostone Phase III clinical trial), post-hoc analyses of linaclotide clinical trial data were conducted using the same definition as lubiprostone to ensure comparability of response rates, given the inconsistent definition between the linaclotide and lubiprostone trials. However, due to a lack of sufficient data from the publically available FDA review of lubiprostone clinical trials, the IBS-QoL measure of response required curve-fitting techniques to impute the percentages of subjects who achieved the defined threshold of response for both linaclotide and lubiprostone, which may not exactly reflect the clinical trial results. The results from the model should be conservative because the response rate derived for linaclotide from the curve-fitting technique produced a lower odds ratio of response (see the Appendix) compared to the odds ratio calculated if response rates from the actual linaclotide clinical trial data were used.
The model assumes that those patients who discontinue therapy incur costs for 1 month (30 days) of drug treatment, and that patients who respond take the medication once daily for the full model time horizon. In actual clinical practice, initial prescription days supplied could be shorter. Among patients that do not discontinue, the actual use of the treatments may also be less frequent than daily and may not be required for the full model duration.
Finally, the model is based on an homogenous clinical trial population and treatment protocol, which may not be representative of real world clinical practice. Studies of the comparative effectiveness of linaclotide and lubiprostone through analysis of real-world data would be valuable. Due to limited availability of comparable data and lack of FDA approval for the IBS-C indication, other over-the-counter treatments and prescription laxatives were not included in this model.
Conclusions
In this model, linaclotide was found to be a less costly treatment option compared to lubiprostone for patients with IBS-C, for two patient relevant definitions of response (improvement in IBS-QoL score and global symptom relief). The introduction of linaclotide into the limited pharmacologic treatment landscape for IBS-C will provide an additional treatment option with similar effectiveness and lower average per patient costs.
Transparency
Declaration of funding
Funding for this study was provided by Ironwood Pharmaceuticals and Forest Research Institute.
Declaration of financial/other relationships
DT is an employee of Ironwood Pharmaceuticals and owns stock/stock options in Ironwood Pharmaceuticals. PS is a former employee of Ironwood Pharmaceuticals and owns stock/stock options in Ironwood Pharmaceuticals. RC is an employee of Forest Research Institute, LLC a subsidiary of Actavis plc and owns stock/stock options in Actavis. SB is a former employee of Forest Research Institute and owns stock in Actavis. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- Mertz HR. Irritable bowel syndrome. N Engl J Med 2003;349:2136-46
- Drossman DA, Li Z, Andruzzi E, et al. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci 1993;38:1569-80
- About irritable bowel syndrome (IBS). International Foundation for Functional Gastroinestinal Disorders (IFFGD), 2010. www.aboutibs.org/#rates. Accessed February 15, 2013
- Hillila MT, Farkkila MA. Prevalence of irritable bowel syndrome according to different diagnostic criteria in a non-selected adult population. Aliment Pharmacol Ther 2004;20:339-45
- Longstreth G. Rome III diagnostic criteria: functional constipation and IBS-C. Gastroenterology 2006; http://ww3.peerviewpress.com/gpimgs/fastcast/n165/pdf/table2.pdf. Accessed May 3, 2011
- Brandt LJ, Chey WD, Foxx-Orenstein AE, et al. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol 2009;104(1 Suppl):S1-35
- Guerin A, Carson R, Kaminsky M, et al. Medical costs associated with treatment failure with over-the-counter or prescription constipation treatments in patients with IBS-C in a Medicaid population. J Manag Care Pharm 2013;19:179
- Dibonaventura M, Sun SX, Bolge SC, et al. Health-related quality of life, work productivity and health care resource use associated with constipation predominant irritable bowel syndrome. Curr Med Res Opin 2011;27:2213-22
- Ironwood Pharmaceuticals I. LINZESS (linaclotide) Prescribing Information. United States Food and Drug Administration, 2013. http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202811s000lbl.pdf. Accessed June 17, 2013
- Center for Drug Evaluation and Research. Medical review: Lubiprostone for the treatment of irritable bowel syndrome with constipation (Application 21-908s005). United States Food and Drug Administration, 2008
- Vieira MC, Ellis A, Lembo T, et al. Randomized controlled trials of treatments for irritable bowel syndrome with constipation and chronic constipation: evaluation of measurement and reporting of efficacy and identification of evidence gaps. Presented at the ACG Annual Scientific Meeting: October 19-24 2012 Las Vegas, NV, 2012
- Rao S, Lembo AJ, Shiff SJ, et al. A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am J Gastroenterol 2012;107:1714-24
- U.S Food and Drug Administration CfDEaR. Guidance for industry: Irritable Bowel Syndrome - Clinical evaluation of drugs for treatment. U.S Department of Health and Human Services, 2012
- Drossman D, Morris CB, Hu Y, et al. Characterization of health related quality of life (HRQOL) for patients with functional bowel disorder (FBD) and its response to treatment. Am J Gastroenterol 2007;102:1442-53
- Patrick DL, Drossman DA, Frederick IO, et al. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci 1998;43:400-11
- Red Book Online: Lubiprostone WAC Price. Thomson Reuters, 2012. Accessed August 31, 2012
- Red Book Online: LINZESS (linaclotide) WAC Price. Truven Health Analytics 2013. Accessed March 22, 2013
- Menzin J, Huang H, Carson RT, et al. Assessment of practice patterns and treatment-failure costs among patients with irritable bowel syndrome with constipation: findings from a physician survey study. Presented at the ISPOR 15th Annual European Congress, 3–7 November 2012, Berlin, Germany, 2012
- Huang H, Taylor D, Carson RT, et al. Impact of treatment response on health utilities and work productivity among patients with irritable bowel syndrome with constipation: pooled results from Phase III Clinical Trials. Presented at the ISPOR 15th Annual European Congress, 3–7 November 2012, Berlin, Germany, 2012
- Bureau of Labor Statistics. May 2010 National occupational employment and wage estimates United States. United States Department of Labor, 2011. http://www.bls.gov/oes/current/oes_nat.htm#00-0000. Accessed March 6, 2012
- Chey WD, Lembo AJ, Lavins BJ, et al. Linaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol 2012;107:1702-12
- Miller ME, Zuckerman S, Gates M. How do Medicare physician fees compare with private payers? Health Care Financ Rev 1993;14:25-39
- Drossman DA, Chey WD, Johanson JF, et al. Clinical trial: lubiprostone in patients with constipation-associated irritable bowel syndrome–results of two randomized, placebo-controlled studies. Aliment Pharmacol Ther 2009;29:329-41
Appendix
Calculation of placebo-adjusted odds ratios
Given that there are no available head-to-head trials that compare linaclotide with lubiprostone, this economic model uses placebo-adjusted odds ratios of response to determine the relative response of linaclotide vs lubiprostone. This methodology was an acceptable approach given the similarities in study design (e.g., inclusion based on Rome criteria; patients with previous gastrointestinal surgery excluded) and patient demographics for each clinical trial (e.g., linaclotide sample: mean age 43.3; 90.6% female; 77.5% white; mean abdominal pain 5.7 on 0–10 scale; mean constipation severity 3.8 on 1–5 scale; lubiprostone sample: mean age 46.1; 90.8% female; 77.4% white; mean abdominal pain 2.07 on 0–4 scale; mean constipation severity 2.22 on 0–4 scale)Citation12,Citation23. The odds ratio was defined as the ratio of the odds of response in the treatment group to the odds of it occurring in the placebo group (represented by the formula below):
Table A1. Odds of response by definition of irritable bowel syndrome with constipation response to treatment.
Table A2. Actual and fitted distribution of irritable bowel syndrome with constipation quality-of-life responders.
Placebo-adjusted odds ratios were calculated individually for lubiprostone and linaclotide for each definition of response. Odds ratios of response for linaclotide vs lubiprostone were then calculated by dividing the placebo-adjusted odds ratio for linaclotide by the placebo-adjusted odds ratio for lubiprostone (Table A1).
Calculation of response rates
Response rate data for linaclotide were derived from data on the pooled intent-to-treat populations of its Phase III clinical trials, while response rate data for lubiprostone was derived from publically available FDA reviews. For the global symptom relief definition of response, only post-hoc analyses of linaclotide were required in order to ensure the calculation of response was the same between the two treatments. For the IBS-QoL measure of response, publically available data for lubiprostone only included the mean change from baseline for IBS-QoL score; however, the model’s response variable requires the percentage of patients with at least a 14-point increase from baseline. Additionally, the standard deviations were not reported for lubiprostone in the FDA review.
To address these issues, the ratio of the standard deviation to the mean of IBS-QoL for linaclotide was used to estimate a standard deviation for lubiprostone. A normal distribution was then fitted to the reported lubiprostone mean and the estimated standard deviation, using the properties of the normal cumulative distribution function to estimate the percentage of patients with a score of 14 or greater. To be conservative, the fitted response rate was also used for linaclotide, which produces a lower odds ratio than the actual response rate (calculated OR linaclotide vs lubiprostone = 1.21; actual OR linaclotide vs lubiprostone = 1.41).
Physician survey of treatment failure
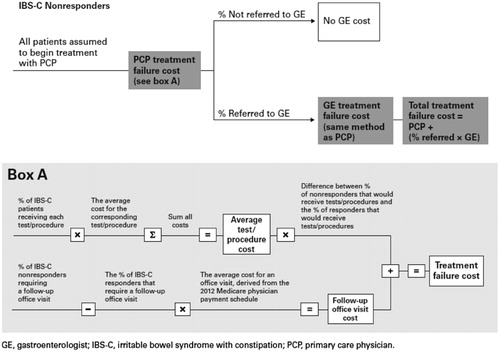
Treatment patterns and resource use findings were estimated from a web-based survey of 20 primary care physicians (PCPs) and 21 gastroenterologists (GEs), focusing on IBS-C patients without response to recent treatment (see Table A3 for physician responses). Separate cost estimates were derived for primary care physicians and gastroenterologists. The average costs for tests, procedures, and visits were obtained from the 2012 Medicare physician payment schedule. Costs for procedures were the sum of national average payments for facility and non-facility services. Laboratory tests were based on the average payment corresponding to Medicare’s 60% National Limitation Amount. The 60% National Limitation Amount is the maximum amount that Medicare will reimburse for clinical laboratory tests services, calculated from historical payment rate data (see Table A4 for testing and procedure costs). The median physician response rate for each resource use type was multiplied by the corresponding unit cost. The summation of all costs was then multiplied by the incremental percentage of non-responders that would receive a test/procedure or office visit (e.g., percentage ordering test for non-response minus percentage ordering test for response).
Table A3. Physician survey results for the practice patterns of patients with irritable bowel syndrome with constipation.
Table A4. Costs of procedures and tests for irritable bowel syndrome with constipation.
Total cost of treatment failure associated with care under a PCP was included in the estimate because all patients were assumed to begin treatment under the care of a PCP. In addition, the total cost of treatment failure associated with care under a GE was applied for a proportion of patients referred to a GE. Thus, direct cost for treatment failure = (PCP treatment failure cost) + (proportion referred * GE treatment failure cost) (see Figure A1).