Abstract
Objective
To assess cost-effectiveness of linezolid vs vancomycin in treating nosocomial pneumonia caused by methicillin-resistant Staphylococcus aureus (MRSA-NP) in China and the impact of renal failure on healthcare resource utilization (HCRU) and costs.
Methods
Cost-effectiveness analysis was conducted based on data from the ZEPHyR trial, with efficacy measured by treatment success and costs calculated from HCRU. Confidence intervals (CI) for cost, efficacy and incremental cost-effectiveness ratios (ICER) were calculated by non-parametric bootstrap. Chi-square test was used for renal failure rate and t-test for HCRU/cost comparisons. Impact of renal failure was assessed using regression model.
Results
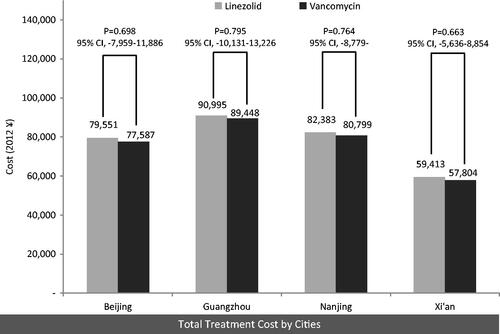
Data from 448 patients (1:1 linezolid:vancomycin) were analyzed. More patients treated with linezolid achieved success (55% [95% CI = 48–62%]) than with vancomycin (45% [38–52%]). Treatment cost were ¥79,551 (95% CI = ¥72,421–¥86,680) for linezolid vs ¥77,587 (¥70,656–¥84,519) for vancomycin in Beijing, ¥90,995 (¥82,598–¥99,393) vs ¥89,448 (¥81,295–¥97,601) in Guangzhou, ¥82,383 (¥74,956–¥89,810) vs ¥80,799 (¥73,545–¥88,054) in Nanjing and ¥59,413 (¥54,366–¥64,460) vs ¥57,804 (¥52,613–¥62,996) in Xi’an. Per successful treatment, the ICER of linezolid over vancomycin were ¥19,719
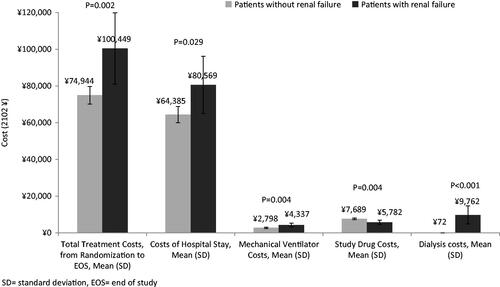
(−¥143,553 to ¥320,980) (Beijing), ¥15,532 (−¥185,411 to ¥349,693) (Guangzhou), ¥15,904 (−¥161,935 to ¥314,987) (Nanjing) and ¥16,145 (−¥100,738 to ¥234,412) (Xi’an). From simulations, the majority of linezolid cases had greater efficacy and higher costs and more than one third had greater efficacy and lower costs. More vancomycin patients developed renal failure (15% vs 4%, p < 0.001). Patients with renal failure had higher cost (Nanjng: ¥100,449 (SD = ¥65,080) vs ¥74,944 (SD = ¥49,632), p = 0.002).
Conclusion
Linezolid was more cost-effective than vancomycin in treating MRSA-NP from a Chinese payer’s perspective, and associated with less renal failure, HCRU and cost.
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) is a bacterium resistant to a number of widely used antibiotics, in effect threatening individuals worldwideCitation1,Citation2. MRSA is one of the most common pathogens identified in patients diagnosed with healthcare associated pneumonia (HAP). In China, the 2011 CHINET surveillance of bacterial resistance suggested that MRSA prevalence reached 50.6%Citation3. In a multi-centre study of HAP involving nine cities in China, HAP was found in 1.4% of all hospital admissions and 15.3% in ICU admissions, with MRSA being a commonly identified pathogen in HAPCitation4.
Vancomycin is considered a standard treatment of nosocomial pneumonia (NP), but concerns have been raised due to its nephrotoxicity and associated treatment costCitation5. Vancomycin resistance of bacterial pathogens has also become an emerging problem in China. A national study found an increase in vancomycin heteroresistance in S. aureus (Hvisa) rate (13% to 16%) in China from 2005 to 2007Citation6. Region-specific studies also echoed similar rates; a study in northeast China found a prevalence of hVISA at 10%, with figures increasing each year from 2007 to 2010Citation7.
Linezolid offers an alternative and new choice for treating MRSA caused NPCitation8. Its efficacy in the treatment of serious Gram-positive complicated infections, including MRSA, has been described in several recent clinical trialsCitation9–11. Heightened efficacy and lower mortality has been noted comparatively to vancomycin and, hence, has promise as a valuable option for patients within the present China landscape.
Few studies have compared the healthcare resource utilization (HCRU) and cost-effectiveness of linezolid vs vancomycin therapy in the treatment of MRSA-NP in the published literature, in particular with respect to China. This lack of evaluation presents a challenge for decision-makers when adopting new technologies under increasing pressure to contain healthcare costs. Although linezolid had much higher drug acquisition costs compared to treatment of vancomycin, several clinical trials have shown an improved clinical efficacy in linezolid vs vancomycin. Therefore, we hypothesized that linezolid is a cost-effective alternative to vancomycin in the treatment of MRSA caused by NP from a Chinese payer’s perspective.
The first objective of the study was to assess the incremental cost-effectiveness of linezolid vs vancomycin in the treatment of MRSA-NP in four major cities (Beijing, Guangzhou, Nanjing, and Xi’an) throughout China. These four large cities are economically and geographically diverse, representing the North (Beijing), South (Guangzhou), East (Nanjing), and West (Xi’an) of China. The second objective was to assess the impact of renal failure on costs between treatments in the city of Nanjing.
Methods
Study design
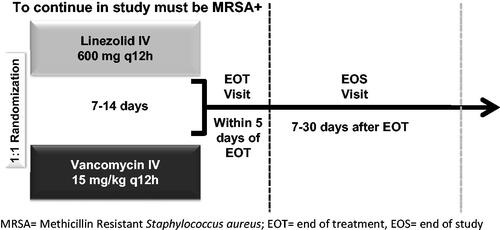
This is a post-hoc analysis using data of the modified intent-to-treat cohort (mITT) from a Phase IV, randomized, double-blind, multi-center study among MRSA-NP patients (Linezolid in the Treatment of Subjects with Nosocomial Pneumonia Proven to Be Due to Methicillin-Resistant Staphylococcus aureus [ZEPHyR] study)Citation12. shows the study design of the clinical trial. Briefly, patients were randomly assigned at a 1:1 ratio via a centralized randomization system to either vancomycin or linezolid treatment. Efficacy was measured as treatment success (defined as cure + improvement) at the end of study (EOS), i.e. 7–30 days after the end of treatment. More details of the ZEPHyR study design are described in the original clinical trial paper by Wunderink et al.Citation12. The renal failure sub-group included only patients who developed renal failure during the study period from randomization to the end of study (EOS), and excluded patients receiving dialysis prior to randomization. Patients were considered to have renal failure if they had at least one of the following: (1) investigator-reported renal failure as an adverse event; (2) acute kidney injury as defined by RIFLE criteria (a consensus definition/classification system for acute kidney injury published by the Acute Dialysis Quality Initiative Group)Citation13; or (3) the initiation of dialysis after study drug started.
This is a secondary, post-hoc clinical trial database analysis, which does not include any individually identifiable data. The clinical trialCitation12 is fully compliant with the letter and spirit of the Health Insurance Portability and Accountability Act of 1996 and the Institutional Review Board review was waived.
HCRU and costs
Mechanical ventilation (MV) days, intensive care unit (ICU) days, and hospital length of stay (LOS) were measured from treatment initiation through the EOS. Unit costs reflecting Chinese costs of treatments were collected from each of the four cities (Beijing, Guangzhou, Nanjing, and Xi’an) in 2012. Given the fact that most physicians in China use branded vancomycin for patients admitted in the ICU for fear of the insufficient efficacy of generic vancomycin, the acquisition cost of branded vancomycin was applied in this study. Chinese Yuan (¥) were applied to counts of resource uses to calculate total costs and costs by resource use type ().
Table 1. China unit costs (Yuan, ¥) of four cities.
Comparison of outcomes between treatment groups
Between-group differences were tested using chi-square tests for categorical variables (e.g. rates of patient who developed renal failure between treatments) and t-tests for continuous variables (e.g. costs/HCRU) unless otherwise stated. To account for small sample sizes, Fisher exact tests were used for comparisons, where one or more cells had expected counts of less than 5; non-parametric Wilcoxon-Mann-Whitney tests were used when at least one comparison group had less than 30 patients.
Cost-effectiveness analysis (CEA)
A cost-effectiveness analysis was conducted to evaluate both direct costs and clinical outcomes (i.e. treatment success). Treatment success was defined as clinical cure or clinical improvement at the end of study (i.e. 7–30 days after end of treatment). Details regarding these criteria can be found in the Appendix of the original clinical trial paper by Wunderink et al.Citation12. Incremental cost-effectiveness ratio (ICER) was calculated to compare the cost-effectiveness of linezolid vs vancomycin. The ICER measures the additional cost of linezolid over vancomycin per unit of the difference in treatment success between the study groups. Ninety-five per cent CIs of the ICERs were computed using a bootstrap approach, which estimates the distribution of the ICER through a large number of simulations. Bootstrap is a non-parametric method without prior assumption on the distributions of the estimated statisticsCitation14. Acceptance curves were also created, informing whether the study treatment is considered to be cost-effective under different willingness-to-pay thresholdsCitation15.
In order to understand the sensitivity of the results to key model parameters, we conducted a one-way sensitivity analysis via Tornado chart and varied the unit costs and utilization observed within linezolid and vancomycin cohorts by 25% and recalculated ICERs.
Additionally, in year 2012, the National Development and Reform Commission (NDRC) began removing the up-to-15% margin on drug price which had been allowed since 2006 in ChinaCitation16. The goal is to roll out the policy of removing the this up-to-15% margin on drug price by 2015 throughout ChinaCitation15. Given this trend of the change in drug prices, we performed a scenario analysis by reducing the study drug price back to its ‘raw’ price (i.e. without its current price margin).
Renal failure analysis (for Nanjing)
We evaluated the impact of renal failure on cost/HCRU in the city of Nanjing. Nanjing was selected for this renal failure analysis because its unit price was in the middle among the four cities, representing an average price level in China. HCRU and costs were compared (1) between patients who developed renal failure vs those did not among the mITT cohort, and (2) between treatments among the sub-group of patients who developed renal failure using appropriate tests. To adjust for the mortality difference between treatments, cost per person-day was calculated and compared between treatments within the renal failure sub-group.
Finally, we performed a generalized linear model (GLM) with log link and gamma distribution to examine the impact of developing renal failure on the total cost in Nanjing.
Results
Patient cohorts
Detailed data of patient disposition and characteristics, clinical and microbiologic response, and safety outcomes for the ZEPHyR trial study have been described elsewhereCitation12. A total of 1184 intent to treat patients (diagnosed with healthcare associated pneumonia or hospital acquired pneumonia) were randomized to either linezolid or vancomycin treatment and 448 patients comprised of mITT population (linezolid, n = 224; vancomycin, n = 224) who had confirmed MRSA pneumonia and received ≥1 dose of the study drug. After excluding 57 patients who had missing clinical outcome data, 391 patients (186 linezolid/205 vancomycin) were left for the CEA. Forty-three patients (10%) were identified as patients who developed renal failure during the trial based on our criteria for renal failure.
Patients had a mean age of 61.8 years. A total of 65.6% of patients were male and 68.6% were white. No significant difference was found in co-morbid conditions between linezolid (n = 224)/vancomycin (n = 224) groups, except that fewer vancomycin patients (vs linezolid) had co-morbid atrial fibrillation at baseline (18% vs 28%, p = 0.01) (). Although not significant, it is worthwhile to notify that vancomycin-treated patients had more with renal impairment at baseline vs the linezolid group (34% vs 28%, p = 0.12). Similarly, most of the demographic and clinical characteristics were similar between the patients who developed renal failure (n = 43) and those who did not (n = 405), except for co-morbid renal impairment (58% vs 28%, p < 0.001), sepsis/septic shock (30% vs 12%, p < 0.001), diabetes (56% vs 40%, p = 0.04) and follow-up duration from randomization until EOS (20.3 [SD 9.9] vs 23.6 [SD 10.1] days, p = 0.04). Linezolid treated patients had a lower rate of renal failure vs vancomycin-treated patients (9 (4%) vs 34 (15%), p < 0.001). At EOS, clinical success was significantly greater in linezolid- compared to vancomycin-treated patients in the mITT population (54.8% vs 44.9%, respectively; p = 0.049).
Table 2. Patients characteristics by comparison groups within mITT population.
Patients were followed up for a duration of 23.3 (SD = 10.1) days from drug initiation (randomization) through EOS. A total of 175 patients (39%) remained in the hospital and 56 patients (13%) were in the ICU at EOS. Fifty-one (11%) patients were receiving mechanical ventilation (MV) at EOS; out of these patients, 10 (2%) were receiving MV outside of ICU and 41 (9%) were receiving MV in the ICU.
Healthcare costs
For both treatments, Guangzhou had the highest total treatment costs, which was almost double of the total costs in Xi’an (lowest total cost). The total treatment costs of linezolid vs vancomycin were similar across the four cities studied ().
Incremental cost-effectiveness ratio (ICER)
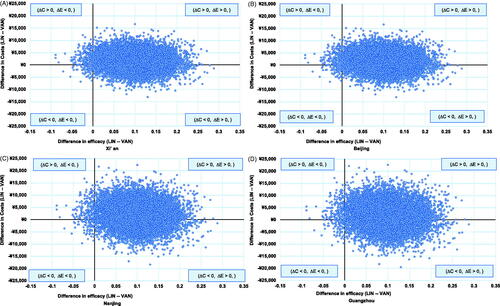
The ICER of linezolid vs vancomycin were ¥19,719 (95% CI: −¥143,553 to ¥320,980), ¥15,532 (−¥185,411 to ¥349,693), ¥15,904 (−¥161,935 to ¥314,987), and ¥16,145 (−¥100,737 to ¥234,412) per treatment success for Beijing, Guangzhou, Nanjing, and Xi’an, respectively. Note that the negative ICER may not be readily interpretable (i.e. the negative sign may be due to a negative difference in efficacy or costs). The bootstrap simulation showed that the majority of cases had greater efficacies and higher costs for linezolid (i.e. quadrant I plane): Beijing (64%), Guangzhou (59%), Nanjing (61%), Xi’an (66%) (). More than one third of cases had greater efficacies and lower costs for linezolid (i.e. linezolid dominated vancomycin): Beijing (33%), Guangzhou (38%), Nanjing (37%), Xi’an (32%), and only <2% had greater efficacies and lower costs for vancomycin in all cities (vancomycin dominated linezolid) ().
The acceptance curves suggested that 50% of bootstrap samples had an ICER less than ¥19,846, ¥16,129, ¥16,183, and ¥16,125 per treatment success and 95% of bootstrap samples had an ICER less than ¥186,666, ¥201,674, ¥182,529, and ¥140,743 per treatment success for Beijing, Guangzhou, Nanjing, and Xi’an, respectively.
Sensitivity analysis of CEA
The one-way sensitivity analysis via Tornado chart showed that the ICER was most sensitive to the number of ICU days with linezolid or vancomycin across all four cities. However, initiating treatment with linezolid maintained cost-savings in most of the sensitivity analyses. Varying the parameters by 25% did not change the overall conclusions.
Additionally, after removing the ∼15% margin of drug prices, the unit drug price dropped to ¥400.06 per 600 mg for linezolid and ¥250.44 per 1000 mg for vancomycin in all four cities. The ICER of linezolid vs vancomycin for the scenario analysis became ¥16,174 (95% CI: −¥150,605 to ¥305,775), ¥11,997 (−¥192,963 to ¥331,824), ¥12,360 (−¥168,765 to ¥299,471), and ¥12,611(−¥107,322 to ¥220,189) per treatment success for Beijing, Guangzhou, Nanjing, and Xi’an, respectively. Compared to the base case ICER estimates from the main analysis, it appeared that the treatment of linezolid vs vancomycin is further cost-effective after removing the current margin on drug prices.
Renal failure analysis (for Nanjing)
Patients with renal failure (vs no renal failure) had significantly longer HCRU (MV days: 12.0 [SD 9.9] vs 7.8 [SD 9.0], p = 0.004) and ICU days (13.5 [SD 9.9] vs 10.0 [SD 8.5], p = 0.013), except for LOS (18.8 [SD 9.8] vs 18.2 [SD 9.6], p = 0.74). Patients who developed renal failure also incurred higher total costs, bed-day, MV, and dialysis costs vs those who did not within the mITT cohort (). After adjusting for survival, duration of follow-up time, ventilator-associated pneumonia (VAP) status, co-morbid multi-organ failure, chronic obstructive pulmonary disease (COPD) and region using the GLM, the cost remained significantly higher among patients who developed renal failure vs those who did not ().
Table 3. Unadjusted and adjusted cost (¥) between patients who developed renal failure and those who did not in the city of Nanjing.
Within the sub-group of patients who developed renal failure during the trial (n = 43), patients had similar demographic and clinical characteristics between treatments (vancomycin [n = 34]/linezolid [n = 9]). Linezolid-treated (vs vancomycin-treated) patients who developed renal failure had numerically lower HCRU (MV days: 7.6 [SD 3.6] vs 13.2 [SD 10.7], p = 0.21; ICU days: 9.9 [SD 6.6] vs 14.4 [SD 10.5], p = 0.30; LOS: 16.1 [SD 11.0] vs 19.5 [SD 9.5], p = 0.26) within the sub-group of patients who developed renal failure. When correcting for mortality differences using a per-person day approach, linezolid tended to incur a lower per person-day total cost (¥4805 [SD ¥1930] vs ¥5347 [SD ¥2395], p = 0.32).
Discussion
Our study is the first study which evaluated the cost-effectiveness of linezolid vs vancomycin for treating NP caused by MRSA from a Chinese payer’s perspective. The ICERs calculated for the four major cities revealed that linezolid was a cost-effective alternative treatment in NP caused by MRSA compared to a current standard treatment of vancomycin. The point estimate of ICER for linezolid vs vancomycin ranged from ¥15,532 (Guangzhou) to ¥19,719 (Beijing). The 95th percentile of ICER obtained from bootstrap samples ranged from ¥140,743 (Xi’an) to ¥201,674 (Guangzhou). In the majority of bootstrapping samples, there was a trade-off in costs and effectiveness between linezolid and vancomycin. Linezolid dominated vancomycin in approximately one third of total cases and only less than 2% of the cases showed vancomycin dominated linezolid across all the four cities.
The sensitivity analysis of changing the parameters up to 25% (e.g. ICU days and efficacy by treatment arms) confirmed the robustness of our results. Additionally, our scenario analysis further adds supportive evidence to our findings. By 2015, a new policy is expected to be implemented through the National Development and Reform Committee (NDRC), National Health and Family Planning Commission of the People’s Republic of China (NHFPC), and the Ministry of Human Resources and Social Security of the People’s Republic of China (MoHRSS) to decrease drug price by ∼15% on average nationwideCitation17. Our scenario analysis suggested that linezolid appeared even more cost-effective with the adjusted drug prices under the new policy.
Our study results are consistent with previous studies about CEA of linezolid vs vancomycin from different countries’ perspectives. A similar study, which used the same clinical trial data as our study and applied US unit costs, suggested that linezolid vs vancomycin was cost effective at a ICER of $16,516 per treatment successCitation18. Another US study found a $30,000 per QALY gain by using linezolid vs vancomycin in treating ventilator-associated pneumoniaCitation19. A study in Germany found that the cost per patient cured was 4813 in 2006 EurosCitation20. These studies, from their own country’s perspective, revealed that linezolid was a cost-effective treatment vs comparators, because the estimated cost per QALY was less than the acceptable threshold.
In the ZEPHyR trial, vancomycin-treated patients were at a higher risk of developing renal failure compared to linezolid. In the analysis of the renal failure sub-group for the city of Nanjing, we found that renal failure was a significant and independent cost driver (i.e. increased the total treatment costs by 26.0%) after adjusting for covariates. The relative higher drug acquisition costs of linezolid were partially offset by longer ICU stay and renal replacement treatments found amongst vancomycin-treated patients.
This finding is particularly relevant and important in China, where antibiotics (mainly aminoglycosides, β-lactams, glycopeptides [including vancomycin] and quinolones) are the most common causes of drug-induced acute kidney injury (AKI)Citation21,Citation22. A recent Chinese study involving 84 patients with Gram-positive infections found nephrotoxicity associated with vancomycin specifically occurred from 11–14%, depending on the criteria usedCitation23. Another study highlighted the clinical and economic burden associated with AKI, which was prevalent in hospitalized patients in China: in-hospital mortality among patients with AKI was estimated to be more than 5-times higher than those without AKI and resulted in substantially increased LOS and hospital costsCitation24. It is worthwhile to note that only direct costs were assessed in our study and the literature, and that these costs were likely to be under-estimated because renal failure is also known to be associated with significant indirect costCitation25,Citation26.
MRSA is a worldwide recognized nosocomial pathogen, which significantly increase the mortality and morbidity and, therefore, imposes a high burden on healthcare resource. According to a review study of epidemiological data for HAP in Asian countries, MRSA was one of the most common pathogens causing HAP (16%), which significantly contributed to the mortality and morbidity in hospitalized patients in ChinaCitation27. A recent Chinese national surveillance program for antimicrobial resistance among gram-positive bacteria found that the prevalence of MRSA was over 50%Citation3. The high prevalence of MRSA and potentially significant economic burden associated with the infection emphasized the importance of taking measures to minimize the spread of MRSA with appropriate antibiotic treatments.
For over 40 years, vancomycin was the treatment of choice for MRSA infections. However, recent research suggested that a tendency towards decreasing susceptibility to vancomycin in MRSA has emerged in ChinaCitation28,Citation29. In addition, the nephrotoxicity of vancomycin is increasingly recognized in the literature, which adds burden to the healthcare systemCitation5,Citation21,Citation22. Given the increasing healthcare costs and limited resource/budget in healthcare, it is important for decision-makers to be aware of the economic impact of the clinical decisions. Our study results showed that linezolid was a cost-effective alternative of vancomycin treatment from the Chinese payer’s perspective.
In 2012, Chinese MOH implemented a new regulation for clinical use of antibacterial agents, which incorporated an internationally accepted stewardship of classifying antibiotics in Non-Restricted, Restricted, and Special categories. Mostly, both vancomycin and linezolid are classified in the Special category; however, linezolid is imposed by additional use restriction in many districts given its high drug price, such as only allowing tertiary hospitals to use the drug, or being classified as second-line treatment to be for reimbursement. According to our CEA results, linezolid’s high drug costs are offset by its superior clinical outcome in treatment success over vancomycin, less hospital resource use, and lower rate of renal failure. Our study provides decision-makers with valuable evidence to allocate limited health budget between interventions in a more efficient way as to maximize health in the Chinese society.
Our study has several limitations. First, HCRU/cost data in the clinical trial were only measured through the EOS visit for all mITT patients. Therefore, LOS was likely under-estimated, given that 39% of patients were still inpatient at EOS for both linezolid and vancomycin. This limitation is often observed in clinical trial studies where HCRU data after the EOS visit are usually not collected and they are truncated at the clinical evaluation. In our study, the proportion of patients who remained hospitalized at EOS was balanced between treatment groups, as was time to EOS visit, thus we expect that the effect of this limitation on the differences in HCRU/costs between treatment groups may be minimal. Second, Chinese unit costs were applied to the ZEPHyR trial, which is a randomized clinical trial recruiting patients from multiple sites globally. The majority of the patients were from the US (63%), followed by Asia (13%). Therefore, the HCRUs may not accurately reflect the real clinical practice in China. However, region did not appear as a significant covariate in the multiple regression analysis of HCRU in the trial data. Third, this analysis was conducted in the MRSA-confirmed mITT population. Consequently, these data did not include the HCRUs or costs of empiric treatment. Fourth, the unit cost of branded vancomycin was applied to the economic model, given that most physicians prescribe branded vancomycin in China, especially in the ICU where the patient condition is usually severe. Therefore, the study results may not be generalized to patients who received generic vancomycin. Last, but not least, the study was conducted based on the data from a clinical trial in which linezolid was administrated intravenously only. However, this may be different from the real world clinical practice in which oral linezolid is allowed. It has been previously noted that the oral formulation conferred an advantage to linezolid over vancomycin, given its facilitation of outpatient useCitation30,Citation31. Additional research is needed in this regard to explore the potential impact of oral linezolid and optimally dosed vancomycin on HCRU, including the risk of renal events, and the associated economic costs (including direct and indirect costs) in real world settings from a Chinese payer perspective.
Conclusions
Using data from a multi-national randomized trial and applying Chinese unit costs, linezolid was shown to be cost-effective compared to vancomycin in treating patients with nosocomial pneumonia caused by MRSA from a Chinese payer’s perspective. Our study provides evidence informing clinical and economic optimization in the management of MRSA-NP for clinicians and decision-makers in China. Linezolid was associated with a significantly lower rate of renal failure than vancomycin. Using Nanjing city as an example, patients who developed renal failure incurred more HCRU and greater cost vs those who did not, indicating a potential clinical and cost advantage of linezolid over vancomycin.
| Abbreviations | ||
| HCRU | = | Healthcare resource utilization |
| mITT | = | Modified intent to treat |
| ZEPHyR | = | Linezolid in the Treatment of Subjects with Nosocomial Pneumonia Proven to Be Due to Methicillin-Resistant Staphylococcus aureus |
| ICER | = | Incremental cost-effectiveness ratio |
| CI | = | Confidence interval |
| MRSA | = | Methicillin Resistant Staphylococcus aureus |
| NP | = | Nosocomial pneumonia |
| hVISA | = | Vancomycin heteroresistance in S. aureus |
| HAP | = | Healthcare associated pneumonia |
| RIFLE | = | Risk (R), injury (I), failure (F), sustained loss (L) and end-stage kidney disease (E) |
| MV | = | Mechanical ventilation |
| ICU | = | Intensive care unit |
| NDRC | = | National Development and Reform Commission |
| GLM | = | Generalized linear model |
| SD | = | Standard deviation |
| EOS | = | End of study |
| VAP | = | Ventilator-Associated Pneumonia |
| COPD | = | Chronic obstructive pulmonary disease |
| MoHRSS | = | Ministry of Human Resources and Social Security of the People’s Republic of China |
| NHFPC | = | National Health and Family Planning Commission of the People’s Republic of China |
| CEA | = | Cost-effectiveness analysis |
| QALY | = | Quality-adjusted life year |
| AKI | = | Acute kidney injury |
Transparency
Declaration of funding
This study was funded by Pfizer Investment Co. Ltd.
Declaration of financial/other relationships
YW and XG are employees of Pharmerit International. SL is a former employee of Pharmerit. International. Pharmerit International was paid by Pfizer Investment Co. Ltd. in connection with this study. QL is a physician who received an honorarium from Pfizer in connection with their work on this study. SH and YXC are employees of Pfizer Inc. and Pfizer Investment Co. Ltd., respectively
Notice of correction
Please note that Figure 4 has been corrected since the article was first published online (22nd October 2015).
Acknowledgments
The authors would like to thank Jennifer Stephens and Caitlyn Solem from Pharmerit International for their contribution in the study design, analysis plan and results interpretation. We appreciate the work from the following team members of our original US study using the same clinical trial data: Dr M. Niederman from the Winthrop-University Hospital, Richard Chambers, DE Myers, S. Haider, and J. Z. Li from Pfizer Inc., and Professor Ben van Hout from the University of Sheffield.
References
- Goetghebeur M, Landry PA, Han D, et al. Methicillin-resistant Staphylococcus aureus: a public health issue with economic consequences. Can J Infect Dis Med Microbiol 2007;18:27-34
- Richards MJ, Edwards JR, Culver DH, et al. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect Contr & Hosp Epidemiol 2000;21:510-15
- Hu F, Zhu D, Wang F. CHINET surveillance of bacterial resistance in China. China J Infect Chemother 2011;12:321-9
- Liu YN, Cao B, Wang H, et al. [Adult hospital acquired pneumonia: a multicenter study on microbiology and clinical characteristics of patients from 9 Chinese cities]. Zhonghua jie he he hu xi za zhi = Zhonghua jiehe he huxi zazhi = Chinese J Tuberculosis Respir Dis 2012;35:739-46
- Pannu N, Nadim MK. An overview of drug-induced acute kidney injury. Crit Care Med 2008;36(4 Suppl):S216-23
- Sun W, Chen H, Liu Y, et al. Prevalence and characterization of heterogeneous vancomycin-intermediate Staphylococcus aureus isolates from 14 cities in China. Antimicrobial Agents Chemother 2009;53:3642-9
- Hu J, Ma XX, Tian Y, et al. Reduced vancomycin susceptibility found in methicillin-resistant and methicillin-sensitive staphylococcus aureus clinical isolates in Northeast China. PloS One 2013;8:e73300
- Zyvox® [package insert]. New York, NY: Pfizer Inc. 2012
- Itani KM, Dryden MS, Bhattacharyya H, et al. Efficacy and safety of linezolid versus vancomycin for the treatment of complicated skin and soft-tissue infections proven to be caused by methicillin-resistant Staphylococcus aureus. Am J Surg 2010;199:804-16
- Kohno S, Yamaguchi K, Aikawa N, et al. Linezolid versus vancomycin for the treatment of infections caused by methicillin-resistant Staphylococcus aureus in Japan. J Antimicrobial Chemother 2007;60:1361-9
- Sharpe JN, Shively EH, Polk HC Jr. Clinical and economic outcomes of oral linezolid versus intravenous vancomycin in the treatment of MRSA-complicated, lower-extremity skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. Am J Surg 2005;189:425-8
- Wunderink RG, Niederman MS, Kollef MH, et al. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis Offl Publ Infect Dis Soc Am 2012;54:621-9
- Bellomo R, Ronco C, Kellum JA, et al. Acute Dialysis Quality Initiative w: acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care (London, England) 2004;8:R204-12
- Efron B. An introduction to the Bootstrap. New York: Chapman and Hall, 1993
- Fenwick E, O'Brien BJ, Briggs A. Cost-effectiveness acceptability curves–facts, fallacies and frequently asked questions. Health Econ 2004;13:405-15
- Notice of medicine and pharmaceutical market order rectification. Available from: http://www.sdpc.gov.cn/zfdj/jggg/zyfw/t20060602_128839.htm. Accessed October 3 2015
- A notification regarding the promoting of the reform in drug price at country level public hospital. Available from: http://www.ndrc.gov.cn/zcfb/zcfbtz/2012tz/t20120919_505765.htm. Accessed October 3 2015
- Niederman MS CJ SC, Wan Y, Gao X, et al. Incidence of renal failure and associated economic burden among patients with nosocomial pneumonia caused by methicillin resistant staphylococcus aureus (MRSA-NP) treated with linezolid or vancomycin: a secondary analysis of a multi-center randomized double-blind clinical trial. San Deigo, CA: IDWeek, 2012
- Shorr AF, Susla GM, Kollef MH. Linezolid for treatment of ventilator-associated pneumonia: a cost-effective alternative to vancomycin*. Crit Care Med 2004;32:137-43
- De Cock E, Krueger WA, Sorensen S, et al. Cost-effectiveness of linezolid vs vancomycin in suspected methicillin-resistant Staphylococcus aureus nosocomial pneumonia in Germany. Infection 2009;37:123-32
- Cao Ja, Su Tb, Li Xb. Acute kidney injury caused by anti-infective drugs. Adverse Drug React 2010;12:325-8
- Che M, Yan Y, Zhang Y, et al. [Analysis of drug-induced acute renal failure in Shanghai]. Zhonghua Yi Xue Za Zhi 2009;89:744-9
- Du B, Chen DC, Liu DW. [Efficacy and nephrotoxicity of vancomycin in the treatment of Gram positive infections]. Chin Critical Care Med 2003;15:32-4
- Fang Y, Ding X, Zhong Y, et al. Acute kidney injury in a Chinese hospitalized population. Blood Purif 2010;30:120-6
- Julián-Mauro JC, Cuervo J, Rebollo P, et al. Employment status and indirect costs in patients with renal failure: differences between different modalities of renal replacement therapy. Off Publ Spanish Soc Nephrol 2013;33:333-41
- Saigal CS, Joyce G, Timilsina AR. Direct and indirect costs of nephrolithiasis in an employed population: opportunity for disease management & quest. Kid Int 2005;68:1808-14
- Chawla R. Epidemiology, etiology, and diagnosis of hospital-acquired pneumonia and ventilator-associated pneumonia in Asian countries. Am J Infect Contr 2008;36(4 Suppl):S93-100
- Zhuo C, Xu YC, Xiao SN, et al. Glycopeptide minimum inhibitory concentration creep among meticillin-resistant Staphylococcus aureus from 2006–2011 in China. Int J Antimicrob Agents 2013;41:578-81
- Zhang X, Hu Q, Yuan W, et al. First report of a sequence type 239 vancomycin-intermediate Staphylococcus aureus isolate in Mainland China. Diagn Microbiol Infect Dis 2013;77:64-8
- Grau S, Rubio-Terres C. Pharmacoeconomics of linezolid. Exp Opin Pharmacother 2008;9:987-1000
- Vinken AG, Li JZ, Balan DA, et al. Comparison of linezolid with oxacillin or vancomycin in the empiric treatment of cellulitis in US hospitals. Am J Therapeut 2003;10:264-74