Abstract
Objective:
Liraglutide has been shown to significantly improve glycemic control and reduce body weight while minimizing the risk of hypoglycemia in adult patients with type 2 diabetes (T2D). This study aimed to identify characteristics that predict clinical and economic outcomes associated with liraglutide therapy in clinical practice in the US.
Methods:
Using the Truven Health MarketScan Laboratory Database, glycemic control (A1C <7%) and diabetes-related costs were evaluated in T2D patients initiating liraglutide between January 1, 2010 and June 30, 2012. Patients were required to have ≥1 post-index claim for liraglutide and A1C values at baseline and 6 months follow-up. All valid values of baseline A1C were included. Patients previously treated with GLP-1 receptor agonist(s) or insulin, or with evidence of type 1 diabetes, pregnancy, or gestational diabetes during the study period were excluded. Multivariable regression models were used to identify predictors of glycemic control and diabetes-related costs.
Results:
Of 417 patients newly treated with liraglutide, 54.0% achieved glycemic control (A1C <7%) during follow-up. Factors associated with increased odds of glycemic control during follow-up were: being female, POS/EPO health plan type, baseline A1C, early liraglutide initiation (0–1 prior oral anti diabetics [OADs] vs ≥2), adherence to liraglutide (defined as the proportion of days covered [PDC]), and diabetic retinopathy. Being female, earlier liraglutide initiation (0–1 prior OADs), and higher patient share of liraglutide costs were associated with significantly lower diabetes-related costs during follow-up. Factors associated with significantly higher post-index diabetes-related costs were: higher baseline A1C, baseline use of sulfonylureas, and diabetic retinopathy.
Conclusions:
Within this commercially-insured population of T2D patients treated with liraglutide, gender, baseline A1C, early liraglutide initiation (0–1 prior OADs), diabetic retinopathy, better adherence, and patient share of liraglutide costs were associated with increased odds of achieving glycemic control and the odds of having higher or lower diabetes-related costs.
Introduction
Type 2 diabetes mellitus (T2D) is a chronic, progressive disease that requires lifestyle modification, medical care, and pharmacological therapy to manage glycemic control and diabetes-related complicationsCitation1,Citation2. The Centers for Disease Control and Prevention (CDC) estimated that 29.1 million people in the US had diabetes in 2012Citation3, with T2D accounting for 95% of all diabetes casesCitation4. The estimated total cost burden associated with diabetes was $245 billion in 2012; of which $176 billion were due to direct medical costs (hospital and emergency care, visits to the doctor, and medications) and $69 billion were due to indirect costs (lost productivity, absenteeism, disability and premature death)Citation3.
Self-reported data indicate that 30–50% of patients with T2D do not meet individualized targets for glycemic controlCitation1,Citation2. Furthermore, an estimated 15% of patients with T2D do not take insulin or oral antidiabetic medicationCitation4. Treatment with metformin, either alone or in combination with other hypoglycemic agents, is the recommended first-line agent for patients with T2DCitation4,Citation5. Although treatment with metformin is often initially effective, glycemic control eventually deteriorates in most patients, necessitating the addition of other antidiabetic therapiesCitation6. In patients not achieving glycemic control within 3 months of monotherapy with metformin, the addition of other anti-diabetic medications is needed, with the American Association of Clinical Endocrinologists (AACE)/American College of Endocrinology (ACE) guidelines recommending glucagon-like peptide-1 (GLP-1) receptor agonist (RA) therapy as an option for second-line therapyCitation5. A number of other classes of anti-diabetic medications (including but not limited to: thiazolidinediones, sulfonylureas, dipeptidyl peptidase-4 (DPP-4) inhibitors, meglitinides, bromocriptine, alpha-glucosidase inhibitors, sodium glucose transport proteins (SGLT-2), and insulin) are available which can be administered individually or in combination with metforminCitation6,Citation7.
Liraglutide 1.2 and 1.8 mg (Victoza) is a once daily injectable GLP-1 RA approved by the US Food and Drug Administration (FDA) as a second-line adjunct therapy for glycemic control in adults with T2DCitation8. Clinical trials and retrospective database studies have found that patients treated with liraglutide achieve greater improvements in glycemic control while minimizing the risk of weight gain or hypoglycemiaCitation9–13. Data from head-to-head randomized clinical trials and observational studies have shown liraglutide to be a cost-effective regimen for improving glycemic control in T2DCitation14–16.
The efficacy of GLP-1 RAs has resulted in the recommendation of this class as an option for second-line therapy in recent ADA and AACE/ACE treatment guidelines for T2DCitation4,Citation5,Citation17. Current treatment guidelines also emphasize selection of appropriate therapy or a patient-centered approach to managing patients with T2DCitation4,Citation5,Citation17. As such, it is important to understand whether there are factors associated with improved response to recommended therapies. Accordingly, the present retrospective study sought to identify factors that predict clinical and economic outcomes associated with use of liraglutide in clinical practice. Specifically, we aimed to identify factors associated with the achievement of glycemic control as well as diabetes-related costs among patients with T2D initiating therapy with liraglutide.
Methods
Data source
Administrative medical and pharmacy claims data for this study were drawn from the Truven Health Analytics’ MarketScan Lab Database for the period July 1, 2009 to December 31, 2012. The Lab Database adds laboratory results to the data elements contained in the MarketScan private payer claims databases (i.e., the Commercial Claims and Encounters and Medicare Supplemental Databases) and, therefore, contains inpatient and outpatient medical, outpatient pharmacy, enrollment, and outpatient laboratory test results data for patients with private insurance. Data contributors to the MarketScan private payer claims databases are primarily large, self-insured, US employers. All database records were de-identified and fully complied with US patient confidentiality requirements (HIPAA) of 1996 and, therefore, were exempted from Institutional Review Board (IRB) approval.
Patient selection
Adults aged 18 years and older with at least one outpatient pharmacy claim for liraglutide between January 1, 2010 and June 31, 2012 and a subsequent liraglutide claim appearing during follow-up were identified for initial inclusion. The date of the first liraglutide prescription was defined as the index date. Patients were required to have at least one medical claim with a diagnosis of T2D (International Classification of Diseases, Ninth Edition, Clinical Modification [ICD-9-CM] 250.x0, 250.x2) in the 6 months prior to the first claim for liraglutide or on the date of initial therapy. Continuous health plan coverage for a minimum of 180 days before (baseline period) and 225 days after (follow-up period) the index date was also required. Finally, an A1C result at baseline (90 days before to 14 days after the index date) and an A1C result at 6 months follow-up (within ±45 days) were required. Patients with pharmacy claims for liraglutide (once daily), exenatide (twice daily), exenatide (once weekly), or insulin during the baseline period and those with evidence of type 1 diabetes, pregnancy, or gestational diabetes at any time during the study period were excluded (see the Appendix for codes used to identify exclusions). Patients with baseline insulin use were excluded to eliminate any potential benefit of the additive or synergistic glucose-lowering effect of an insulin plus liraglutide regimen.
Patient characteristics
Patient demographic characteristics at baseline were captured and included age, gender, health plan type (comprehensive, exclusive provider organization [EPO], health maintenance organizations [HMO], preferred provider organizations [PPO], point-of-service plan [POS] with and without capitation, and other), geographic region (Northeast, North Central, South, West, unknown), primary payer (Commercial or Medicare Supplemental), and urban or rural residence. Clinical characteristics measured during the baseline period included baseline A1C, the Deyo-Charlson Comorbidity Index (CCI) score (an aggregate measure of comorbidity using select diagnoses associated with chronic disease)Citation18, number of unique outpatient medications prescribed, number of unique ICD-9-CM codes at the 3-digit level, a series of diabetes-related comorbid conditions (diabetic neuropathy, diabetic retinopathy, disorders of lipid metabolism, hypertension, and ischemic heart disease), anti-diabetic therapies (by class), a summary measure of OAD use prior to liraglutide initiation (0–1 OADs vs ≥2 as a proxy measure for early vs late initiation of liraglutide), and concomitant medication use. Total all-cause healthcare costs incurred during the baseline period were also assessed. Finally, post-index adherence to liraglutide (defined as the proportion of days covered [PDC]) and the proportion of liraglutide costs paid for out-of-pocket by the patient were evaluated.
Outcome measures
Achievement of glycemic control (A1C <7%)Citation19 and total diabetes-related healthcare costs during the 6-month follow-up were evaluated. Glycemic control was assessed using A1C values appearing during follow-up, categorized as A1C <7% vs A1C ≥7%. Diabetes-related healthcare costs were identified from claims carrying an ICD-9-CM diagnosis code indicating T2D, diabetic nephropathy, diabetic neuropathy, diabetic retinopathy, hyperglycemia, or hypoglycemia. Additionally, claims with Healthcare Common Procedure Coding System (HCPCS) codes or National Drug Codes (NDC) indicating diabetes testing (e.g., testing for impaired fasting glucose, impaired glucose tolerance) and supplies were considered diabetes-related. Finally, diabetes-related prescription costs were those associated with prescription claims for anti-diabetic medications. All expenditures were inflation-adjusted to 2013 constant dollars using the Medical Care component of the Bureau of Labor Statistics Consumer Price Index (http://www.bls.gov/cpi/).
Statistical analysis
All study measures were summarized using descriptive statistics. Bivariate statistics were used to compare the demographic and clinical characteristics, and healthcare costs of patients who did and did not achieve glycemic control during follow-up. Chi-square tests were used to evaluate the statistical significance of differences for categorical variables and student’s t-tests were used for continuous variables. Cross-tabulations and correlations of study variables were examined to identify potential interactions and multicollinearity. Multivariable logistic regression was used to identify predictors associated with achieving glycemic control. Assessed as potential predictors of glycemic control were age, gender, health plan type and payer, and the following baseline clinical characteristics: baseline A1C, number of unique outpatient medications prescribed, number of unique diagnoses, diabetes-related co-morbid conditions (diabetic neuropathy, diabetic retinopathy, disorders of lipid metabolism, hypertension, and ischemic heart disease), anti-diabetic therapies (by class, including metformin, sulfonylureas, and DPP-4 inhibitors), OAD use prior to liraglutide initiation (0–1 OADs vs ≥2), adherence to liraglutide (PDC ≥80%), and the proportion of liraglutide costs paid for out-of-pocket by the patient. Due to the non-normal distribution and skewed nature of healthcare costs, generalized linear modeling (GLM) with the log link and gamma variance functions was used to identify predictors of diabetes-related healthcare costs. The same factors assessed as potential predictors of glycemic control were included in the model of diabetes-related healthcare costs, with the exception of adherence to liraglutide. The rationale for this exclusion was the hypothesized correlation of adherence to—and pharmacy costs associated with—liraglutide. The nominal level of statistical significance for all tests was set at 0.05.
Results
Patient selection
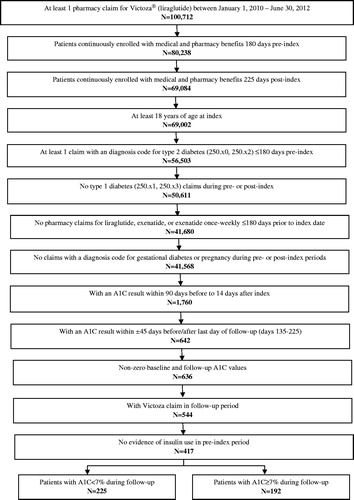
depicts the patient identification process resulting in the final study population of 417 patients newly treated with liraglutide. Of these, 54.0% achieved glycemic control (A1C <7%) after the 6-month follow-up.
Patient demographics and clinical characteristics
and present the demographic and clinical characteristics, respectively, of the study population by glycemic control status. Patients had an average age of 53.7 years. Slightly more than half of the sample were female (52.8%), enrolled in a PPO health plan (52.8%), and lived in the South region of the US (54.7%). Most patients resided in urban areas (89.0%) and were insured with commercial insurance only (93.5%). The most prevalent comorbidities in each cohort were: disorders of lipid metabolism (45.1%) and hypertension (35.7%), followed by diabetic neuropathy (7.0%), diabetic retinopathy (6.5%), and ischemic heart disease (6.2%). More than half of the study population (55.6%) was prescribed two or more unique OADs at baseline. Metformin (54.0%), sulfonylureas (35.5%), and monotherapy/fixed-dosed combination (FDC) DPP-4 inhibitors (34.5%), thiazolidinediones (24.2%), and other than FDC products combination (29.7%) were the most common anti-diabetic drugs used during the baseline period. As shown in , 47.5% of patients were adherent (PDC ≥80%) with liraglutide during follow-up.
Table 1. Demographic characteristics of patients initiating therapy with liraglutide by glycemic control statusa.
Table 2. Pre-index clinical characteristics of patients initiating therapy with liraglutide by glycemic control statusa.
Table 3. Adherence and diabetes-related costsa during follow-up of patients initiating therapy with liraglutide by glycemic control statusb.
Characteristics by glycemic control status at follow-up
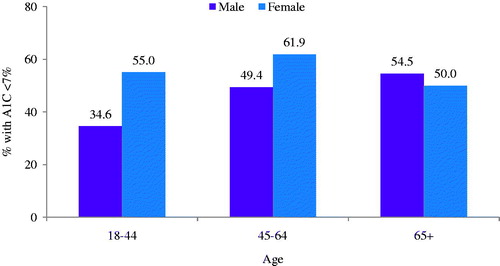
As indicated in , 54.0% of the study population achieved glycemic control during follow-up. Compared to those achieving glycemic control, those not achieving control were slightly older (mean age = 54.3 vs 53.1 years; p = 0.18) and less likely to be female (58.2% vs 46.4%; p = 0.02) (). This pattern, however, was not consistent across age-groups (). As expected, the mean A1C at baseline was significantly lower in patients who achieved glycemic control than those who did not achieve control (7.3% vs 8.6%; p < 0.05); among patients uncontrolled at baseline, 39.0% achieved A1C < 7% during follow-up (). Patients achieving glycemic target during follow-up also had higher average baseline CCI scores (1.5 vs 1.3; p < 0.05), fewer baseline OAD claims (1.5 vs 2.0; p < 0.05), as well as lower baseline use of sulfonylureas (25.8% vs 46.9%; p < 0.05), DPP-4 inhibitors (28.0% vs 42.2%; p < 0.05), and combination product therapies (23.1% vs 37.5%; p < 0.05). During follow-up, unadjusted total liraglutide expenditures were higher among patients who achieved glycemic control ($2244 vs 2002; p < 0.05) compared to those who did not achieve control (see and ).
Figure 3. Post-index glycemic control (A1C value closest to last day of follow-up, occurring within ±45 days before/after last day of follow-up) by baseline glycemic control (A1C result within 90 days before to 14 days after index).
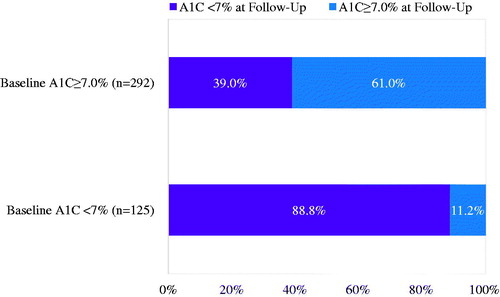
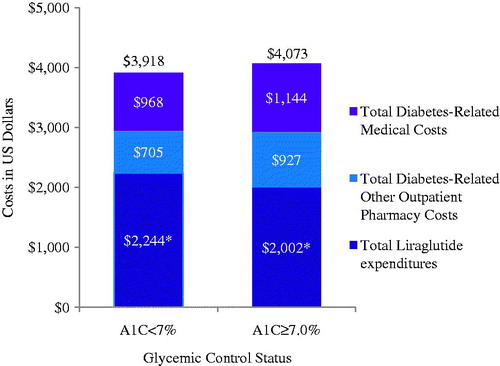
Figure 4. Diabetes-related healthcare expenditures among patients initiating therapy with liraglutide by glycemic control status. *p < 0.05. Diabetes-related costs included claims with an ICD-9-CM diagnosis code for T2D, diabetic nephropathy, diabetic neuropathy, diabetic retinopathy, hyperglycemia, or hypoglycemia, plus claims indicating diabetes testing (e.g., testing for impaired fasting glucose, impaired glucose tolerance) and supplies, as well as prescription claims for anti-diabetic medications.
Predictors of glycemic control
Results from the logistic regression model used to identify predictors of achieving glycemic control are presented in . As shown, females were more likely to achieve glycemic control than males (OR = 1.75; p < 0.05). Other factors associated with increased odds of achieving glycemic control were earlier initiation of liraglutide (0–1 OAD vs ≥2 during baseline) (OR = 2.11; p < 0.05), diabetic retinopathy (OR = 3.55; p < 0.05), and adherence to liraglutide (OR = 1.83; p < 0.05). As expected, higher baseline A1C was associated with reduced odds of achieving glycemic control during follow-up (OR = 0.48; p < 0.05).
Table 4. Odds ratios and 95% confidence intervals from logistic regression model estimating the probability of achieving glycemic control among patients initiating therapy with liraglutide.
Predictors of diabetes-related costs
shows the results of the GLM estimating diabetes-related costs among patients initiating therapy with liraglutide. Results from the model indicate that significant predictors of higher costs include higher baseline A1C (OR = 1.05; p < 0.05), baseline use of sulfonylureas (OR = 1.18, p < 0.05), and diagnosed diabetic retinopathy (OR = 1.41, p < 0.05). Early initiation of liraglutide (0–1 OADs vs ≥2 during baseline) (OR = 0.85, p < 0.05), being female (OR = 0.82, p < 0.05), and higher patient out-of-pocket share of liraglutide costs (OR = 0.93, p < 0.05) were associated with significantly lower total diabetes-related costs at follow-up. Results of additional models estimating pharmacy and, separately, medical costs, showed no significant association between glycemic control and each particular cost outcome.
Table 5. Cost ratios and 95% confidence intervals from generalized linear model (GLM) predicting higher diabetes-related costsa among patients initiating therapy with liraglutide.
Discussion
To the best of our knowledge, this is the first retrospective study to investigate factors associated with clinical and economic outcomes of T2D patients newly treated with liraglutide in clinical practice. Current treatment guidelines for optimal management of T2D advocate selection of anti-diabetic drugs based on their efficacy, along with patient characteristics and preferencesCitation5,Citation17. Identification of demographic and clinical characteristics associated with glycemic control in T2D may be helpful to optimize treatment, improve patient adherence, and ultimately reduce the burden of diabetes. In the present study, lower baseline A1C, early liraglutide initiation (i.e., fewer OADs used prior to initiation), diabetic retinopathy, and better adherence to liraglutide were associated with higher likelihood of achieving A1C goal of <7%. Additionally, an age-dependent effect of gender on glycemic control was observed in this cohort. In particular, younger women achieved higher rates of glycemic control relative to males and older age groups.
Optimal glycemic control is crucial for minimizing the diabetes-related complications in patients with T2D, and data indicate that early initiation of treatment in T2D can significantly delay the onset or progression of micro- and macrovascular complicationsCitation20. Early initiation of liraglutide has been associated with improved glycemic control in both randomized clinical trials and observational studiesCitation21–24. Furthermore, initiation of liraglutide in treatment-naïve patients or patients previously treated with one OAD has been associated with better responses compared to those initiating liraglutide after two OADsCitation23,Citation24. Consistent with these findings, patients initiating liraglutide following treatment with one or no OADs were more likely to achieve glycemic control than patients treated with two or more OADs prior to liraglutide initiation in the present study.
Previous research has demonstrated the cost-effectiveness of liraglutide among T2D patientsCitation14,Citation15; however, to date, the correlates and predictors of healthcare costs of T2D patients treated with liraglutide are largely unknown. Results of the present study show that higher baseline A1C together with baseline use of sulfonylureas and diagnosed diabetic retinopathy were the strongest predictors of higher diabetes-related costs among T2D patients treated with liraglutide. In a recent retrospective study, T2D patients initiating therapy with sitagliptin as compared to liraglutide had greater use of sulfonylureas at baseline, and significantly higher total diabetes-related costs over 6 months of follow-upCitation16. In the present study, we find that use of fewer OADs (0–1 OAD vs ≥2 during baseline) prior to initiating liraglutide, being female, and higher patient out-of-pocket share of liraglutide costs are associated with lower total diabetes-related costs.
While a number of variables were considered in these analyses, it is important to note that there is a high degree of overlap between the patient characteristics associated with a higher likelihood of achieving glycemic control and those that predict higher/lower diabetes-related costs. Interestingly, while some characteristics, like early initiation of liraglutide, are predictors of desirable outcomes on both metrics (i.e., increased likelihood of achieving glycemic control and increased likelihood of lower diabetes-related costs), others, like diabetic retinopathy, are inversely related to one of the desirable outcome (i.e., increased likelihood of achieving glycemic control but increased likelihood of higher diabetes-related costs). Similarly, it may be expected that a predictor of lower costs, such as increased patient out of pocket share for liraglutide, might adversely impact a predictor of better glycemic control, namely, adherence to liraglutide.
Limitations
Several limitations inherent to administrative claims data apply to the present studyCitation25. First, administrative claims data are collected for billing rather than research purposes, and, therefore, diagnostic and procedural coding may be driven by reimbursement rather than clinical factors. Furthermore, administrative claims data may be subject to data coding limitations and data entry error. Also, this study was limited to patients with commercial health coverage and to those with private Medicare supplemental coverage. Consequently, results of this analysis may not be generalizable to T2D patients with other types of health insurance or to the uninsured.
There are a few additional caveats specific to the present study that merit consideration. First, compared to those identified exclusively from administrative claims data, our study population is relatively small due to the linked laboratory results requirements (i.e., A1C values). Nevertheless, the combination of laboratory and administrative claims data sources represents a distinct strength of our study. Second, because of our minimum period of required follow-up of 225 days, patients who died or transitioned to long-term disability due to serious health conditions during the study period were excluded from the study population. This may have introduced survivor and/or selection bias toward potentially healthier T2D patients. Also, the length of follow-up of the present study may be too short to observe differences in healthcare costs that may emerge over time between patients who do and do not achieve glycemic control. Our study assessed glycemic control using a binary measure representing A1C <7%. Future research may investigate factors associated with achieving control at different levels and also absolute change in A1C. Additionally, the patient population included in this current study is limited to patients who did not use insulin at baseline. This was done to eliminate any potential benefit of the additive or synergistic glucose-lowering effect of an insulin plus liraglutide regimen. Therefore, it is not known whether the results of the current study can be extrapolated to T2D patients taking insulin. Finally, while a variety of potentially important predictors of glycemic control and diabetes-related healthcare costs were included in the multivariable analyses, the array of potential predictors examined was limited to those characteristics that could be measured using claims or laboratory data. As such, important factors such as body weight or BMI were not captured in this analysis. Similarly, because the data used in this study was derived from a commercial insurance plan in the US, it is not known to what extent results, particularly related to cost, are generalizable to healthcare systems in other countries.
Conclusions
T2D patients treated with liraglutide have been found to achieve clinical goals at lower diabetes-related pharmacy costs per successfully treated patient than with exenatide twice-dailyCitation15 or lower drug costs per patient per year as compared to sitagliptinCitation26. Within this commercially-insured population of adult T2D patients newly treated with liraglutide, achievement of glycemic control was associated with early liraglutide initiation (i.e., fewer prior OADs), baseline A1C, adherence to liraglutide, being female, enrollment in a POS/EPO health plan, and diagnosed diabetic retinopathy. Higher total diabetes-related costs were associated with higher baseline A1C, baseline use of sulfonylureas, and diabetic retinopathy. Earlier initiation of liraglutide, being female, and higher out-of-pocket pharmacy costs were associated with lower diabetes-related costs. Research investigating patient demographic and clinical characteristics associated with successful outcomes of specific medications may serve as a first step in personalizing therapy in patients with diabetes.
Transparency
Declaration of funding
This study was funded by Novo Nordisk Inc. and conducted by Truven Health Analytics, Bethesda, MD, USA.
Declaration of financial/other relationships
ED, LLG, and GL are employees of Truven Health Analytics. Truven Health Analytics was paid by Novo Nordisk Inc. in connection with the conduct of the study and development of this manuscript. JL and MH are employees and shareholders of Novo Nordisk A/S. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
Editorial support for this manuscript was provided by Santosh Tiwari, who was compensated by Truven Health Analytics.
References
- Stark Casagrande S, Fradkin JE, Saydah SH, et al. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988-2010. Diabetes Care 2013;36:2271-9
- Ali MK, Bullard KM, Gregg EW. Achievement of goals in U.S. Diabetes Care, 1999–2010. N Engl J Med 2013;369:287-8
- Centers for Disease Control and Prevention. National diabetes statistics report: estimates of diabetes and its burden in the United States, 2014. Atlanta, GA: US Department of Health and Human Services, 2014
- American Diabetes Association. Standards of medical care in diabetes-2015. Diabetes Care 2015;38(1 Suppl):S1-93
- American Association of Clinical Endocrinologists and American College of Endocrinology. Comprehensive diabetes management algorithm. Endocr Pract 2015;21(1 Suppl):S1-87
- Rojas LB, Gomes MB. Metformin: an old but still the best treatment for type 2 diabetes. Diabetol Metab Syndr 2013;5:6
- Bennett WL, Wilson LM, Bolen S, et al. Oral diabetes medications for adults with type 2 diabetes: an update. Comparative Effectiveness Review No. 27. (Prepared by Johns Hopkins University Evidence-based Practice Center under Contract No. 290-02-0018.) AHRQ Publication No. 11-EHC038-EF. Rockville, MD: Agency for Healthcare Research and Quality. March 2011. Available at: www.effectivehealthcare.ahrq.gov/reports/final.cfm.
- Victoza® [package insert]. Plainsboro, NJ: Novo Nordisk; 2010.
- Perry CM. Liraglutide: a review of its use in the management of type 2 diabetes mellitus. Drugs 2011;71:2347-73
- Zinman B, Schmidt WE, Moses A, et al. Achieving a clinically relevant composite outcome of an HbA1c of <7% without weight gain or hypoglycaemia in type 2 diabetes: a meta–analysis of the liraglutide clinical trial programme. Diabetes Obes Metab 2012;14:77-82
- Lee WC, Dekoven M, Bouchard J, et al. Improved real-world glycaemic outcomes with liraglutide versus other incretin-based therapies in type 2 diabetes. Diabetes Obes Metab 2014;16:819-26
- Chitnis AS, Ganz ML, Benjamin N, et al. Clinical effectiveness of liraglutide across body mass index in patients with type 2 diabetes in the United States: a retrospective cohort study. Adv Ther 2014;31:986-99
- Chitnis AS, Ganz ML, Hammer M, et al. Real-world clinical effectiveness of liraglutide in individuals 65 years and older with type 2 diabetes in the United States. J Diabetes Metab 2014;5:1-6
- Langer J, Hunt B, Valentine WJ. Evaluating the short-term cost-effectiveness of liraglutide versus sitagliptin in patients with type 2 diabetes failing metformin monotherapy in the United States. J Manag Care Pharm 2013;19:237-46
- DeKoven M, Lee WC, Bouchard J, et al. Real-world cost-effectiveness: lower cost of treating patients to glycemic goal with liraglutide versus exenatide. Adv Ther 2014;31:202-16
- Li Q, Chitnis A, Hammer M, et al. Real-world clinical and economic outcomes of liraglutide versus sitagliptin in patients with type 2 diabetes mellitus in the United States. Diabetes Ther 2014;5:579-90
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015;38:140-9
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613-19
- American Diabetes Association. Standards of medical care in diabetes-2014. Diabetes Care 2014;37(1 Suppl):S14-80
- Owens DR. Clinical evidence for the earlier initiation of insulin therapy in type 2 diabetes. Diabetes Technol Ther 2013;15:776-85
- Nauck M, Marre M. Adding liraglutide to oral antidiabetic drug monotherapy: efficacy and weight benefits. Postgrad Med 2009;121:5-15
- Gallwitz B, Vaag A, Falahati A, et al. Adding liraglutide to oral antidiabetic drug therapy: onset of treatment effects over time. Int J Clin Pract 2010;64:267-76
- Garber A, Mathews D, Zinman B, et al. The effect of disease stage, indicated by number of previous oral antidiabetic agents, on the response to liraglutide in type 2 diabetes. Diabetes 2011;60(1 Suppl):A265
- Heymann A, Maor Y, Goldstein I, et al. Efficacy of liraglutide in a real-life cohort. Diabetes Ther 2014;5:193-206
- Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med 2000;342:1887-92
- Mezquita Raya P, Reyes Garcia R. Is treatment with liraglutide efficient? Endocrinol Nutr 2014;61:202-8
Appendix