Abstract
Objective:
To describe dosing patterns and to compare the drug costs per month spent in progression-free survival (PFS) among patients with advanced renal cell carcinoma (aRCC) treated with everolimus or axitinib following a first tyrosine kinase inhibitor (TKI).
Methods:
A medical record retrospective review was conducted among medical oncologists and hematologists/oncologists in the US. Patient eligibility criteria included: (1) age ≥18 years; (2) discontinuation of first TKI (sunitinib, sorafenib, or pazopanib) for medical reasons; (3) initiation of axitinib or everolimus as a second targeted therapy during February 2012–January 2013. Real-world dosing patterns were summarized. Dose-specific drug costs (as of October 2014) were based on wholesale acquisition costs from RED BOOK Online. PFS was compared between everolimus and axitinib using a multivariable Cox proportion hazards model. Everolimus and axitinib drug costs per month of PFS were compared using multivariable gamma regression models.
Results:
A total of 325 patients received everolimus and 127 patients received axitinib as second targeted therapy. Higher proportions of patients treated with axitinib vs everolimus started on a higher than label-recommended starting dose (14% vs 2%) or experienced dose escalation (11% vs 1%) on second targeted therapy. The PFS did not differ significantly between patients receiving everolimus or axitinib (adjusted hazard ratio (HR) = 1.16; 95% confidence interval [CI] = 0.73–1.82). After baseline characteristics adjustment, axitinib was associated with 17% ($1830) higher drug costs per month of PFS compared to everolimus ($12,467 vs $10,637; p < 0.001).
Limitations:
Retrospective observational study design and only drug acquisition costs considered in drug costs estimates.
Conclusions:
Patients with aRCC receiving axitinib as second targeted therapy were more likely to initiate at a higher than label-recommended dose and were more likely to dose escalate than patients receiving everolimus. With similar observed durations of PFS, drug costs were significantly higher—by 17% per month of PFS—with axitinib than with everolimus.
Introduction
The treatment of advanced renal cell carcinoma (aRCC) has changed dramatically in the last decade, with targeted therapies becoming the standard of careCitation1. A total of four tyrosine kinase inhibitors (TKIs: axitinib, sorafenib, sunitinib, and pazopanib), two mammalian target of rapamycin inhibitors (mTORis: everolimus and temsirolimus), and one vascular endothelial growth factor (VEGF) inhibitor (bevacizumab) have been approved for the treatment of aRCCCitation2.
Eventual resistance to treatment with initial targeted agents is commonCitation3. National Comprehensive Cancer Network (NCCN) treatment guidelines currently recommend sequential administration of targeted therapiesCitation2, with multiple agents available at each line of treatment. Given the diversity of choicesCitation2,Citation4, there is a need to evaluate the clinical and economic impacts of different treatment sequences. Currently, everolimus and axitinib are the only two targeted therapies with a category 1 recommendation for use after the failure of first TKIsCitation5–8. Both everolimus and axitinib have demonstrated efficacy as second targeted therapies for aRCC in the phase 3 RECORD-1 and AXIS trials, respectivelyCitation9–11. While no head-to-head clinical trial has directly compared everolimus and axitinib, indirect comparisons of RECORD-1 and AXIS trialsCitation12 and real-world observational studiesCitation13,Citation14 have found comparable effectiveness between these two drugs in terms of time on treatment (TOT), progression-free survival (PFS), and overall survival (OS).
In addition to efficacy, other factors including safety, dosing patterns, and drug costs should be considered in the evaluation of treatment sequencesCitation15,Citation16. The present study focuses on dosing and drug costs. To date, there has been limited information on real-world dosing patterns and associated costs for these treatmentsCitation17. The RECORD-1 and AXIS trials have reported dose reduction rates of 7%Citation9 for patients treated with everolimus and 34%Citation10 for axitinib. A 38%Citation8 dose escalation rate has also been reported for patients treated with axitinib—although, due to different designs of these two trials, such dosing change rates cannot be directly compared and may not be reflective of real-world practice. A recent review of the economic evidence for second targeted therapies for aRCC found that everolimus could be considered cost-effective compared to sorafenib, with an incremental cost-effectiveness ratio (ICER) of $64,155 per life-year (LY) gained or $89,160 per quality-adjusted life-year (QALY) gained, which fell below the ICER for many commonly-used oncology medicationsCitation17. In another cost-effectiveness model, compared with sorafenib, axitinib was associated with an ICER of over $1.8 million per LY gained or $31.9 million per QALY gained, thus would not be considered a cost-effective optionCitation18. In a separate study, Perrin et al.Citation19 modeled the lifetime costs of sunitinib-refractory aRCC patients treated with axitinib or everolimus in the second line and concluded that everolimus treatment was associated with lower total healthcare costs compared to axitinib, mostly due to the higher drug acquisition costs of axitinib. As an economic model, this analysis inherently included a number of assumptions about the clinical outcomes and dosing patterns of these two drugs.
With evidence of similar durations of PFS for everolimus and axitinib after failure of a first TKI, and potential differences in dosing patterns and associated drug costs, it is of interest to assess which therapy provides better value in terms of drug costs per unit of time spent in PFS. The current study addresses this question using real-world data from a multi-practice, nationwide review of medical records.
Methods
Data source
Medical oncologists and hematologists/oncologists were recruited from a nationwide panel of physicians in the US in order to participate in a retrospective review of medical records from June 2014 to July 2014. To participate, physicians were required to have treated ≥3 patients with aRCC in the past year. Each eligible physician was invited to randomly select up to five patients meeting pre-specified patient inclusion criteria. A standardized electronic case report form (eCRF) was developed by the study investigators, pilot-tested by two oncologists, and then used by the eligible physicians to extract relevant chart information, during which real-time error checking was implemented. Abstracted patient data were anonymous and non-identifiable; a waiver from full ethics review was provided by the New England Institutional Review Board.
Study population
To be included in the study, patients were required to (1) be adults (≥18 years old) diagnosed with aRCC; (2) be treated for aRCC with a TKI (sunitinib, sorafenib, or pazopanib) as first targeted therapy; (3) have discontinued the first TKI for medical reasons (e.g., disease progression, non-response without progression, drug intolerance); (4) have subsequently initiated everolimus or axitinib as second targeted therapy between February 1, 2012 and January 31, 2013; and (5) have their medical records available for review for the entire period encompassing initiation from first targeted therapy until the most recent follow-up or death.
Study measures
The index date was defined as the date of initiation of everolimus or axitinib as second targeted therapy. The study period was defined as the period of time from the index date to the most recent follow-up or death.
Patients’ baseline characteristics included demographics (age, gender) at initial aRCC diagnosis, health status (comorbidities and Eastern Cooperative Oncology Group [ECOG] performance status at index date), tumor characteristics (metastasis at initial RCC diagnosis, clear cell RCC histology, sites of metastases, and duration of aRCC at index date), and treatment characteristics (prior nephrectomy for RCC, type, duration, response, and progression on first targeted therapy).
The initial dose of second targeted therapy was categorized based on whether it was above, on, or below the recommended starting dose of everolimus (10 mg once per day) or that of axitinib (5 mg twice per day). Dose escalation and de-escalation were defined as any subsequent dose increase or decrease during the entire study period, respectively, in the initially prescribed dose of everolimus or axitinib; the dates and levels of all recorded dose changes were collected from patient medical records.
PFS was defined as the time from the index date to progression or death; PFS for patients without a recorded date of progression or death was censored at the last recorded follow-up date. Progression was determined by physicians based on radiographic evidence indicating progression of tumor lesions or occurrence of new lesions, physical exams indicating worsening performance status, worsening hypercalcemia, growth of subcutaneous mass or palpable mass, or cancer-related symptoms (e.g., increased pain, fever, and weight loss).
Statistical methods
Baseline characteristics were summarized for patients treated with everolimus and axitinib and were compared using Wilcoxon rank-sum tests for continuous variables and Chi-square tests for categorical variables.
The proportions of patients starting on, above, or below the recommended starting dose of everolimus and axitinib, as well as the proportions of patients with any dose escalation or dose de-escalation, were summarized. For PFS, a multivariable-adjusted Cox proportional hazards model was used to assess the relationship between drug type (everolimus and axitinib as second targeted therapies) and PFS. The model adjusted for age, gender, the presence of hypercholesterolemia, ECOG performance status, the presence of aRCC at initial diagnosis, prior nephrectomy, type and duration of first targeted therapy, clinical benefit while on first targeted therapy (physician assessed), occurrence of progression while on first targeted therapy (physician assessed), duration of aRCC at second targeted therapy initiation, sites of metastases, tumor histological type (clear cell RCC or other), and years of practice of the treating physician; this methodology has been described in detail previouslyCitation20.
Total drug costs were estimated for everolimus and axitinib by applying dose-specific wholesale acquisition costs (WAC, obtained from RED BOOK Online in October 2014)Citation21 to the patient-level dosing histories noted during the progression-free interval. The WAC unit cost for one 10 mg tablet of everolimus was $342.44 (recommended dose: once per day) and for one 5 mg tablet of axitinib was $170.16 (recommended dose: twice per day). Drug costs per month of PFS were then calculated for each patient by dividing the pre-progression total drug costs by the duration of PFS, which was measured in months. Drug costs per month of PFS were compared between patients treated with everolimus and axitinib using both unadjusted and multivariable-adjusted generalized linear models (GLMs) with a gamma distribution and log link. The multivariable models adjusted for the same set of covariates as those included in the multivariable Cox proportional hazards model for PFS.
A two-sided p-value of 0.05 was used to determine statistical significance. All statistical analyses were performed using SAS v.9.3 (Cary, NC).
Results
Patient baseline characteristics
A total of 325 and 127 patients received second targeted therapy with everolimus and axitinib, respectively (). Baseline characteristics were similar between the two treatment groups: age (mean = 61 years for everolimus-treated patients, 60 years for axitinib-treated patients), gender (men = 70% and 65%), metastasis present at initial RCC diagnosis (51% and 59%), and follow-up duration after second targeted therapy initiation (15 months and 13 months). Patients receiving everolimus or axitinib had similar profiles of first targeted therapy in terms of type, duration, and occurrence of clinical benefit or clinical progression while on first targeted therapy. Finally, at the initiation of second targeted therapy, everolimus- and axitinib-treated patients were similar in terms of disease duration, comorbidities (except for hypercholesterolemia, which was significantly higher among patients treated with everolimus: 42% and 38%, p < 0.05), metastatic sites, presence of clear cell histology, and ECOG performance status.
Table 1. Patient characteristics.
Initiation dose and dose adjustment
The large majority of everolimus-treated patients (91%) started on the recommended dose of 10 mg once per day, while a small proportion started at a different dose: 2% started on a higher than recommended dose and 7% started on a lower than recommended dose (). A smaller proportion of axitinib-treated patients (84%) started on the recommended dose of 5 mg twice per day, while 14% of patients started on a higher than recommended dose and 2% started on a lower than recommended dose. Following the initiation of second targeted therapy, dose escalation occurred for 1% of patients receiving everolimus and 11% of patients receiving axitinib; dose de-escalation occurred for 7% of patients receiving everolimus and 2% of patients receiving axitinib ().
Table 2. Initial dose and dose adjustments of everolimus and axitinib as second targeted therapy.
Drug cost per month of PFS
PFS rates at 12 months were 60% and 56% for patients treated with everolimus and axitinib, respectively, based on Kaplan-Meier estimates. In a multivariable-adjusted Cox proportional hazards model, durations of PFS did not differ significantly between patients receiving everolimus or axitinib (multivariable-adjusted HR = 1.16; 95% CI = 0.85–1.59). A statistical test of the proportional hazards assumption revealed it to be consistent with the data.
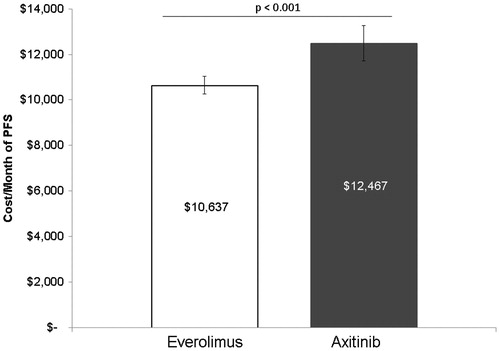
Average unadjusted drug costs per month of PFS were $12,951 for axitinib and $10,602 for everolimus (p < 0.001). After adjusting for baseline characteristics, axitinib was associated with 17% ($1830) higher drug costs per month of PFS compared to everolimus ($12,467 vs $10,637; p < 0.001, ). The goodness-of-fit metric—the Akaike information criterion (AIC) value—for the multivariable model was 10,524.13.
Discussion
Following the failure of a first TKI, everolimus and axitinib are the only two targeted therapies with NCCN category 1 recommendations for aRCC. While no head-to-head clinical trial has directly compared everolimus and axitinib, multiple sources of evidence, including indirect comparison of clinical trial dataCitation12,Citation22,Citation23 and real-world evidenceCitation13,Citation14, have shown that, overall, these two drugs have similar effectiveness, as measured by PFS and OS. In the absence of demonstrated superiority in effectiveness, other factors, including drug costs, may be considered when recommending a therapyCitation15. Prior to this study, the real-world dosing patterns and drug costs had not been compared for everolimus and axitinib.
In the current study, while most patients treated with both drugs started treatment at the label-recommended dose (everolimus = 10 mg q.d.; axitinib = 5 mg b.i.d.), patients treated with axitinib as second targeted therapy were more likely to start on a higher than recommended dose and were more likely to dose escalate than patients treated with everolimus. The current findings are consistent with the dosing patterns observed in the RECORD-1 trial for everolimus. The dose de-escalation rates are similar in the present study (6%) and the RECORD-1 trial (7%); the trial was associated with a mean dose intensity of 88% among patients who failed first TKI of sunitinibCitation19. However, the real-world findings from the present study differ substantially from the dosing patterns observed in the AXIS clinical trial for axitinib, which reported mean dose intensity of 99% in the trial publicationCitation11 and 102% in a 2011 FDA briefing documentCitation24. In particular, dose de-escalation and escalation rates for axitinib were much lower than those reported in the AXIS trial (2% vs 34% and 11% vs 38%, respectively). Differences between the present real-world study and clinical trial results may be due to differences in prior treatments (e.g., in both AXIS and RECORD-1 substantial proportions of patients had received cytokines but not prior targeted therapies) or to the effect of real-world decision-making free of a clinical study protocolCitation25.
The need for a larger amount of medication in order to control the disease in axitinib-treated patients could be reflected in higher drug costs. In our study, with similar durations of PFS, axitinib was associated with significantly higher (at least 17%) drug costs per month of PFS relative to everolimus. During a typical treatment course with 6 months of PFS, this would translate into a drug cost difference of $10,980. In a recent economic modeling study on the lifetime cost of treating patients with second targeted therapies after failure of sunitinib, axitinib was estimated to be associated with higher lifetime costs relative to everolimusCitation19. Although the model also included various medical costs, the cost advantage in favor of everolimus was largely driven by drug costs—axitinib was associated with a higher dose intensity (102%) relative to everolimus (88%), resulting in higher costs. Although not directly assessing dose intensity, our current findings did show that, compared to patients treated with everolimus, those treated with axitinib were more likely to initiate therapy at a higher-than-recommended dose and were more likely to dose escalate. Together with the modeling study by Perrin et al.Citation19, the current findings indicate that everolimus might be a less costly option in routine clinical practice relative to axitinib, which provide additional real-world evidence to help decision-making regarding the optimal treatment sequencing for aRCCCitation26–30.
This study is subject to several limitations, some of which are inherent to retrospective reviews of medical chart data. First, retrospective analyses are subject to selection bias because patients were not randomized to second targeted therapies. Multivariable comparative analyses in this study accounted for multiple known risk factors for aRCC progression that were available from medical charts. However, confounding due to unobserved or unknown risk factors is possible. Second, chart reviews may include data entry errors. To the extent that these factors affect both cohorts in a similar manner, they are not expected to substantially affect the comparative conclusions. Finally, the drug costs estimated in the present study may not reflect costs for all payers. Drug costs here were based on drug acquisition costs, which did not include manufacturer rebates or any cost-sharing arrangements. In addition, other drug cost components (e.g., patient out-of-pocket costs) or non-drug medical costs (e.g., costs for managing AEs or for physician and nurse visits) were not available. Further research into healthcare resource utilization and the costs associated with the use of axitinib and everolimus as second targeted therapy in aRCC is warranted.
Conclusions
In a retrospective review of medical charts of patients with aRCC in the US, patients who received axitinib as second targeted therapy were more likely to initiate therapy at a higher-than-recommended dose and were more likely to escalate their starting dose compared to patients who received everolimus. With similar observed durations of PFS, drug costs per month of PFS were 17% higher for patients who received axitinib relative to those who received everolimus.
Transparency
Declaration of funding
Funding for this research was provided by Novartis Pharmaceuticals Corporation, East Hanover, NJ.
Declaration of financial/other relationships
SKP has been a consultant for Novartis, Pfizer, Aveo, Dendreon, and Myriad and has spoken at Novartis, Pfizer, and Medivation. EJ has been a consultant for Novartis, GSK, and Pfizer, as well as received grant funding from Exelixis, GSK, Novartis, and Pfizer. JES, WMR, and NL are employees of Analysis Group Inc., which has received consultancy fees from Novartis Pharmaceuticals Corporation for this project. ZL and JRP are employees of Novartis Pharmaceuticals Corporation and may own stock/stock options. NJV has been a consultant for Novartis, Amgen, Celgene, Medivation, Eisai, Exelixis, Roche, has spoken at Novartis, Astellas, Johnson and Johnson, Pfizer, Dendreon, Bayer/Algeta, GSK, and Veridex/Janssen, and has received research support from Novartis, Bayer, Exelixis, Progenics, Bavarian Nordic, and Viamet. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
Medical writing assistance was provided by Ana Bozas, PhD, an employee of Analysis Group, Inc.
References
- Randall JM, Millard F, Kurzrock R. Molecular aberrations, targeted therapy, and renal cell carcinoma: current state-of-the-art. Cancer Metastasis Rev 2014;33:1109-24
- National Comprehensive Cancer Network. Kidney Cancer v. 3.2015. Fort Washington, PA: National Comprehensive Cancer Network; 2015 http://www.nccn.org/professionals/physician_gls/PDF/kidney.pdf. Accessed August 1, 2015
- Pracht M, Berthold D. Successes and limitations of targeted therapies in renal cell carcinoma. Prog Tumor Res 2014;41:98-112
- Sun M, Larcher A, Karakiewicz PI. Optimal first-line and second-line treatments for metastatic renal cell carcinoma: current evidence. Int J Nephrol Renovasc Dis 2014;7:401-7
- Escudier B, Kataja V, Group EGW. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21(5 Suppl):v137-9
- Ljungberg B, Hanbury DC, Kuczyk MA, et al. Renal cell carcinoma guideline. Eur Urol 2007;51:1502-10
- Motzer RJ, Agarwal N, Beard C, et al. NCCN clinical practice guidelines in oncology: kidney cancer. J Natl Compr Canc Netw 2009;7:618-30
- Nathan P, Wagstaff J, Porfiri E, et al. UK guidelines for the systemic treatment of renal cell carcinoma. Br J Hosp Med (Lond) 2009;70:284-6
- Motzer RJ, Escudier B, Oudard S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer 2010;116:4256-65
- Motzer RJ, Escudier B, Tomczak P, et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol 2013;14:552-62
- Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 2011;378:1931-9
- Sherman SA, Wang X, Amzal B, et al. A weighted-adjusted indirect comparison of everolimus (EVE) versus axitinib (AXI) in second-line metastatic renal cell carcinoma (mRCC) patients who previously failed sunitinib therapy. J Clin Oncol 2014;32:abstr 491
- Signorovitch JE, Pal SK, Reichmann WM, et al. Comparative effectiveness of everolimus (EVE) and axitinib (AXI) for 2nd targeted therapy (TT) of metastatic renal cell carcinoma (mRCC) in the US: a retrospective chart review. J Clin Oncol 2015;33:abstr e15612
- Li JR, Yang CK, Wang SS, et al. First-line treatment result influence second–line regimen selection in targeted therapy for metastatic renal cell carcinoma. Anticancer Res 2014;34:5643-7
- Calvo E, Grunwald V, Bellmunt J. Controversies in renal cell carcinoma: treatment choice after progression on vascular endothelial growth factor-targeted therapy. Eur J Cancer 2014;50:1321-9
- Bracarda S, Sisani M, Marrocolo F, et al. GOAL: an inverse toxicity-related algorithm for daily clinical practice decision making in advanced kidney cancer. Crit Rev Oncol Hematol 2014;89:386-93
- Wong MK, Wang X, Chulikavit MJ, et al. Review of US comparative economic evidence for treatment of metastatic renal cell carcinoma after failure of first-line VEGF inhibitor therapy. Am Health Drug Benefits 2013;6:275-86
- Ozer-Stillman I, Keyser R, Ambavane A, et al. Sorafenib versus axitinib for second-line treatment of sunitinib-refractory patients with advanced renal cell carcinoma (RCC) in the United States (US): an economic evaluation. Paper presented at: ESMO Congress 2012; 28 Sep - 02 Oct, 2012; Vienna, Austria. http://oncologypro.esmo.org/Meeting-Resources/ESMO-2012/Sorafenib-versus-axitinib-for-second-line-treatment-of-sunitinib-refractory-patients-with-advanced-renal-cell-carcinoma-RCC-in-the-United-States-US. Accessed September 7, 2015
- Perrin A, Sherman S, Pal S, et al. Lifetime cost of everolimus vs axitinib in patients with advanced renal cell carcinoma who failed prior sunitinib therapy in the US. J Med Econ 2015;18:200-9
- Pal SK, Jonasch E, Signorovitch JE, et al. Real-world dosing and drug costs with everolimus and axitinib as second targeted therapies for metastatic renal cell carcinoma: a retrospective chart review. J Manag Care Specialty Pharm 2015;21:S15 abstract C13
- Truven Health Analytics. RED BOOK | Healthcare Drug Pricing Resource | Product Information. Greenwood Village, CO: Truven Health Analytics, 2015. http://www.redbook.com/redbook/online/. Accessed August 1, 2015
- Dranitsaris G, Schmitz S, Broom RJ. Small molecule targeted therapies for the second-line treatment for metastatic renal cell carcinoma: a systematic review and indirect comparison of safety and efficacy. J Cancer Res Clin Oncol 2013;139:1917-26
- Miguel LS, Luz R. Economic evaluation of axitinib for second line treatment in adult patients with advanced renal cell carcinoma – the Portuguese case. Paper presented at: ISPOR 17th Annual European Congress; November, 2014; Amsterdam, The Netherlands. http://www.ispor.org/ScientificPresentationsDatabase/Presentation/52700. Accessed August 1, 2015
- U.S. Food and Drug Administration. FDA Briefing Document – Oncologic Drugs Advisory Committee Meeting – NDA 202324 Axitinib (Inlyta). Silver Spring, MD: AG U.S. Food and Drug Administration, 2011. p 1-27. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/OncologicDrugsAdvisoryCommittee/UCM282290.pdf. Accessed August 1, 2015
- Rini BI, Melichar B, Fishman MN, et al. Axitinib dose titration: analyses of exposure, blood pressure and clinical response from a randomized phase II study in metastatic renal cell carcinoma. Ann Oncol 2015;26:1372-7
- Felici A, Bria E, Tortora G, et al. Sequential therapy in metastatic clear cell renal carcinoma: TKI-TKI vs TKI-mTOR. Expert Rev Anticancer Ther 2012;12:1545-57
- Heng DY, Signorovitch J, Swallow E, et al. Comparative effectiveness of second-line targeted therapies for metastatic renal cell carcinoma: a systematic review and meta-analysis of real-world observational studies. PLoS One 2014;9:e114264
- Kruck S, Bedke J, Kuczyk MA, et al. Second-line systemic therapy for the treatment of metastatic renal cell cancer. Expert Rev Anticancer Ther 2012;12:777-85
- Oudard S, Elaidi RT. Sequential therapy with targeted agents in patients with advanced renal cell carcinoma: optimizing patient benefit. Cancer Treat Rev 2012;38:981-7
- Porta C, Sabbatini R, Procopio G, et al. Primary resistance to tyrosine kinase inhibitors in patients with advanced renal cell carcinoma: state-of-the-science. Expert Rev Anticancer Ther 2012;12:1571-7