Abstract
Background:
While literature has focused on the impact of bleeding beginning outside the hospital setting among patients with atrial fibrillation (AF), there is little information regarding bleeding that first occurs within a hospital setting. This study was performed to determine the association between hospital-associated bleeding in patients admitted for AF on outcomes of length of stay (LOS) and total hospitalization cost.
Methods and results:
The Premier research database was queried to identify adult inpatients discharged between 2008–2011 having a primary diagnosis code for AF where a bleeding diagnosis code was not present on admission. Regression was used to adjust for baseline differences in patients to estimate outcomes comparing patients with and without a hospital-associated bleed. There were 143,287 patients that met the study criteria. There were 2991 (2.1%) patients identified with a hospital associated bleed. After adjustment for covariates, the mean estimated LOS was significantly greater in the bleed group, at 6.0 days (95% CI = 5.8–6.1) vs the no bleed group at 3.3 days (95% CI = 3.3–3.3) (p < 0.0001). Similarly, the adjusted mean estimated total hospitalization cost was also significantly greater in the bleed group, $12,069 (95% CI = $11,779–$12,366) vs $6561 (95% CI = $6538–$6583) in the no bleed group (p < 0.0001).
Conclusions:
After adjustments for baseline differences the data show that the 2.1% (n = 2991) of patients with hospital associated bleeding accounted for an estimated additional 8106 hospitalization days and $16.4 million dollars in cost over the study period compared to non-bleeders.
Introduction
There are an estimated 5.8 million individuals in the US that suffer from non-valvular atrial fibrillation (NVAF), making it one of the most common cardiac issues encounteredCitation1. NVAF is a major cause of morbidity, and increases the risk for mortalityCitation2,Citation3, strokeCitation3,Citation4, and congestive heart failure (CHF)Citation3. Studies have also established that risk factors for the development of AF include age, diabetes, myocardial infarction (MI), CHF, valvular heart disease, and hypertensionCitation5–7. Lifetime risk for development of AF is 1:4 for men and women aged 40 and older. This risk remains high, at 1:6, even when CHF and MI are not presentCitation8.
Treatment of thromboembolic complications related to NVAF can vary, with the benefits and risks dependent upon the type of therapy utilized. Overall bleeding rates in treated patients range from 9.0–12.0%Citation9–11, while major bleeds can range from 3.6–7.8%Citation7,Citation12, and up to 10.8 per 100 person-yearsCitation13 dependent upon the particular pharmacotherapy used and the age of the patient. In older individuals (≥75), the rate of major bleeding in a treated patient year can be as high as 4.97%Citation14. While use of oral anti-coagulation results in a 64% reduction in stroke and a 26% reduction in all-cause mortality compared to placeboCitation15 in NVAF patients, the benefit can come at the cost of a higher risk for major bleedingCitation16, especially in elderly patientsCitation7,Citation17. In patients who have experienced a first cardiac event, addition of anti-thrombotic therapy necessary to protect from a second event can result in a 2-fold increase in the risk of bleedingCitation18.
Prior work in this area has focused on patient risk for bleeding events that originate outside of acute care settings and its causesCitation7,Citation9–11 and less on the association of bleeding during inpatient hospitalizations with regard to economic outcomes. The objective of this study was to quantify the association of hospital associated bleeding on length of stay (LOS) and total hospitalization cost in patients admitted for NVAF.
Methods
Study design
A retrospective observational study of hospitalized non-valvular atrial fibrillation (NVAF) patients using the Premier research database was conducted. Descriptive analyses were used to characterize those patients with and without hospital associated bleeding events. Multivariable analyses were used to examine the incremental impact of bleeding on outcomes after adjusting for differences in baseline characteristics across admissions identified from January 1, 2008 through May 31, 2011.
Data source
Hospitalizations were identified within the Premier research database, a large US hospital-based, service-level, all-payer comparative database. Hospitalizations from more than 600 hospitals, primarily non-profit, non-governmental, community and teaching hospitals and health systems, from the year 2000 to mid-2011 were contained within the database with data from standard hospital discharge files, including a patient’s demographic and disease state and information on billed services, including inpatient medications, laboratory, diagnostics and therapeutic services in de-identified patient daily service records. In addition, information on hospital characteristics, including geographic location, bed size and teaching status, is also available.
The Premier research database has been statistically de-identified to comply with the Health Insurance Portability and Accountability Act privacy regulations. Institutional review board (IRB) approval for this study was not required per Title 45 CFR (Code of Federal Regulations) Part 46 of the US, the exemption that this research involved the study of existing data and that the information was recorded in such a manner that the individuals could not be identified directly or through identifiers linked to individualsCitation19. Therefore, IRB approval was not sought.
Patient selection
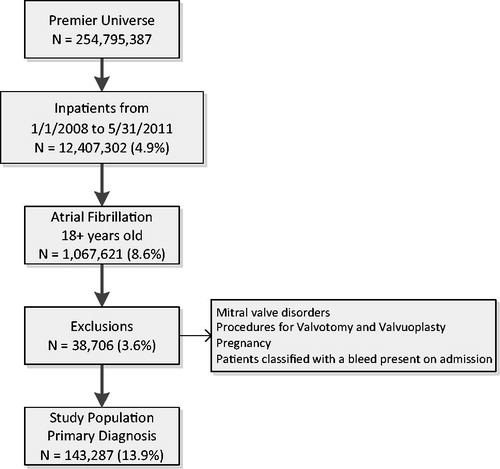
Hospitalized patients, at least 18 years of age, discharged between January 1, 2008 and May 31, 2011 with a primary discharge diagnosis code for AF using the International Classification of Diseases, Ninth Revision Clinical Modification (ICD-9 CM code 427.31) were included in the study. Patients with mitral valve disorders, procedures for valvotomy and valvuloplasty, pregnancy, and patients classified with a bleed present on admission identified by ICD-9 CM diagnosis codes were excluded. Additionally, patients having an extreme LOS outlier (defined as having a LOS greater than the 99th percentile) or without cost information were also excluded from the study population.
Measured outcomes
All outcomes were measured during the index visit, defined as a patient’s earliest inpatient discharge within the study window meeting the selection criteria. Primary outcomes were: hospital reported LOS (days) and total hospitalization cost (US$). Total hospitalization cost included all cost data as reported by the hospital associated with the admission. The costs did not have any inflation adjustments included across the 4 year time window as these are reported in aggregate comparing individuals with a hospital associated bleed to those without a bleed.
Covariate variable description
Patient and hospital characteristics
Patient demographic data selected from the Premier research database include age, gender, and race. Co-morbid conditions were identified using discharge ICD-9 CM diagnosis codes for acute coronary syndrome, renal insufficiency, hypertension, stroke, venous thromboembolism (DVT), and drug and alcohol use (). Hospital characteristics available in the Premier research database include bed size, teaching status, and urban vs rural status.
Table 1. Co-morbid condition definitions.
Anti-platelet and anticoagulant medications
Anti-platelet () and anticoagulant () medications were identified using the hospital charge master data associated with the index hospitalization. Medication utilization was categorized into the following mutually exclusive groups for modeling: anticoagulant only, anti-platelet only, both anticoagulant and anti-platelet, or none of the above.
Table 2. Anti-platelet medications.
Table 3. Anticoagulant medications.
Bleed definition
Inpatient bleeding was defined as the presence of one or more of the following: receipt of fresh frozen plasma or blood, as identified in the hospital charge master; a primary or secondary discharge ICD-9 CM diagnosis code related to bleeding; and/or an ICD-9 CM procedure code for a transfusion of blood or blood components. The diagnosis codes include those for intracranial and gastrointestinal bleeds.
Statistical analyses
The underlying hypothesis for this study was that hospital associated bleeding is associated with a significant increase in inpatient LOS and total hospitalization cost among NVAF patients. An alpha of 0.05 was considered statistically significant for the analyses in this study. For inclusion in multivariable models, clinical, as well as statistical significance was considered. In order to create a parsimonious model, statistical significance at an alpha of 0.10 was considered significant for model inclusion. Missing values for categorical variables were recoded to unknown and were excluded for continuous variables (e.g., LOS and total hospitalization cost).
The statistical methods included: (1) descriptive analyses of the study population to determine unadjusted differences between bleed and no bleed patients and (2) regression modeling to determine the statistical significance of variables on the end-points of interest.
Descriptive analysis
Descriptive analysis was used to gain an understanding of individual patients with NVAF. The analysis results, in conjunction with clinical relevance provided by subject matter experts, were used to determine multivariable analyses variables. Data measured on a continuous scale were expressed as mean, standard deviation, median, and range. Categorical data were expressed as numeric counts and percentages of patients in each category. Bivariate tables describe demographics, hospital characteristics, and outcomes of interest by comparing hospital associated bleed and no bleed patient populations. The overall bleeding rate was calculated. Chi-square or Fisher’s tests were used to test for statistical differences in categorical variables. Wilcoxon-Rank-Sum tests were utilized for determination of statistical differences in continuous variables.
Multivariable modeling
Multivariable modeling with least squares mean was performed to estimate adjusted outcomes for LOS and total hospitalization cost. Modeling estimated differences in the outcomes related to presence of bleeding during a hospitalization. Missing values for co-variates were not imputed and the associated records were not included in the models.
In these data, LOS and total hospitalization cost typically have a gamma distribution. Therefore, the SAS/STAT software GENMOD procedure was used with the log link function and gamma distribution option. The log link function alters the distribution curve and approximates a gamma distribution. Using this technique, commonly employed for data distributions that are heavily right skewed, ensured that we did not violate underlying requirements of regression modeling. These distributions were evaluated prior to the modeling to ensure there was a Gamma distribution. Backward selection with a p-value cut point of 0.10 was utilized to determine final models.
Sensitivity analyses
To address potential confounding, a series of sensitivity analyses were performed to address the following areas: selection bias, timing of anticoagulant/anti-platelet use during hospitalization and cardiac procedure prevalence in the population which is a known high risk population for bleeding.
A two-to-one (control-to-case) propensity score matching was utilized to adjust for potential selection imbalances in the patients within the groups (hospital associated bleed vs no bleed). The propensity score technique was used to statistically model treatment selection to minimize selection bias attributable to observed co-variates. Co-variates included in the score were based on a priori selection and on their ability to maximize the receiver operator characteristic curve of the selection model. These included patient and hospital characteristics, co-morbidities and the use of anti-platelet and/or anticoagulants. A second matching was performed and the anticoagulant/anti-platelet group was split into mutually exclusive categories of anticoagulant alone, anti-platelet alone, and a combination of anticoagulant and anti-platelet.
To determine the impact of the initialization of anticoagulant/anti-platelet use during the hospitalization on results, a ratio of the length of stay to index day of service for the anticoagulant/anti-platelet use was calculated for each patient. For example, if a patient has a LOS of 5 days and had their first dispensing of a drug on day 3, they would have a ratio of 5/3 (1.67). With this approach the smaller the number the later that the drug was started and the larger the number the earlier during their visit the drug was started.
The data analyses for this study were generated using SAS software (version 9.2). Copyright, SAS Institute Inc. SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc. (Cary, NC).
Results
There were 143,287 () patients meeting the patient selection criteria. The comparator cohorts were defined as patients identified with a hospital associated bleed (2991 patients, 2.1%) vs the no bleed population (140,296 patients, 97.9%). There were 1754 (58.6%) of the hospital associated bleed group that had an identified transfusion, 1239 (41.4%) with record of receiving fresh frozen plasma, and 612 (20.5%) with an identified ICD-9 code for bleeding.
Demographics
The mean age of the study population was 69.4 years, with 51% of the population being female. shows statistical differences in the demographic, hospital and payer characteristics between the bleed and no bleed patients. Patients identified with a hospital associated bleed were older (74.6 vs 69.3 years, p < 0.0001), more likely to be female (55.5% vs 50.4%, p < 0.0001) and more likely to have a government based payer (83.2% vs 68.3%, p < 0.0001) compared to those without a bleed.
Table 4. Demographic and hospital characteristic comparisons between patients with and without a hospital associated bleeding event in primary atrial fibrillation inpatient visits.
shows that patients having a hospital associated bleed were more likely (p < 0.0001) than no-bleed patients to have the following comorbidities: renal insufficiency, stroke, hypertension, ACS, and DVT. Unadjusted length of stay (2.5-times longer) and total hospitalization cost (2.8-times the cost) (both p < 0.0001) were higher among hospital-associated bleeding vs non bleeding patients ().
Table 5. Co-morbidities and cardiac procedure comparison between patients with and without a hospital associated bleeding event in primary atrial fibrillation inpatient visits.
Table 6. Unadjusted outcomes comparison between patients with and without a hospital associated bleeding event in primary atrial fibrillation inpatient visits.
Modeling
Different models were run to review the impact of covariates on the outcomes. Models included combinations of the following variables: gender, age, race, payer, rural/urban status, teaching status, bed size, use of any anticoagulant/anti-platelet, renal insufficiency, hypertension, stroke, anemia, DVT, ACS, drugs/alcohol use or abuse, admission status, and selected procedures.
describes the outcomes after multivariable modeling. After adjustment, the estimated mean values for LOS and total hospitalization cost remained significantly higher for the patients identified as having a hospital associated bleed vs those who did not. The difference in the adjusted mean length of stay between bleed and no bleed patients was 2.7 days (p < 0.0001), while the adjusted total hospitalization cost difference was $5508 (p < 0.0001).
Table 7. Adjusted outcomes comparisons between patients with and without a hospital associated bleeding event in primary atrial fibrillation inpatient visits.
The matching analyses performed had results which demonstrated similar direction and magnitude to the unmatched adjusted estimates produced. Within the sensitivity analysis of the ratio of length of stay to initiation of anticoagulation therapy, the majority of the patients that met the selection criteria initiated therapy near their discharge from the hospital, with a similar pattern in both the bleed and no bleed groups, indicating that anticoagulation therapy within the hospitalization was similar between the two groups. Additionally, sensitivity analyses were performed to determine if adjustments for cardiac procedures would impact the outcomes. As with the propensity matched review, these results were of similar magnitude and direction.
Discussion
In a nationally representative database of patients that had a primary diagnosis of NVAF identified, the prevalence of hospital associated bleeding was 2.1%. In these patients, we calculated the LOS and total hospitalization cost associated with bleeding after adjusting for demographic and co-morbid conditions. The incremental difference in adjusted mean LOS and total hospitalization costs between hospital-associated bleed and no bleed patients was derived from the estimate of LOS and total hospitalization cost attributed to individuals identified as having a bleed.
Bleeding is a common risk within individuals with cardiac diagnoses and procedures and is well researched and documented in areas such as percutaneous coronary intervention (PCI) with a bleeding rate of ∼2.4%Citation20. Bleeding is also an acknowledged risk in individuals with NVAF. Studies have shown that, in individuals with AF there is an estimated overall bleeding rate between 9.0–12.0%Citation9–11, dependent upon the type of anticoagulant dispensed. The current study focused only on bleeds that emerged during a hospitalization for AF and, thus, represents a portion of all the possible bleeding that patients with NVAF may be at risk for developing. Yet hospital associated bleeding represents an important outcome for both patients and providers as AF patients are frequently hospitalized, with inpatient costs accounting for the largest component of cost within an AF populationCitation21.
There is a preponderance of studies in the literature reviewing the association between bleeding and mortality among patients with cardiac diseases and proceduresCitation22–25. Conversely, there is little evidence supporting the association between bleeding and the outcomes of length of stay and total hospitalization cost, and none reviewing associations between these outcomes and hospital associated bleeding. Results from this study’s analyses indicate that, while hospital associated bleeding within a population admitted for AF accounts for a small proportion among many nosocomial risks, it is associated with significant LOS and total hospitalization cost consequences.
In the Healthcare Cost and Utilization Project (HCUP) projections for cardiac conditions, the mean LOS for Atrial Fibrillation vacillated ∼3.7 days between 2001–2010Citation25. This number was projected to decrease to ∼3.5 days in 2011 and 2012Citation25. The study findings are similar, with an unadjusted mean LOS of 3.5 days for the total primary NVAF population. The HCUP projections report the mean hospital cost increasing from $5500 to $8500 between 2001–2010 and a projection of ∼$9500 for 2012. The study findings are smaller, with an unadjusted total hospitalization cost of $7455 for this NVAF population. Using the adjusted estimates for the study population (n = 2991), there is an additional burden to the healthcare system represented by these data of an estimated 2000 days per year or an estimated 8120 hospital days for the identified time period and $4.13 million per year, which is an estimated $16.5 million dollars in total hospitalization cost when comparing hospital associated bleed to no bleed patients with a primary diagnosis for NVAF.
The study is subject to some limitations. The identification of bleeding was based on a definition that utilized a combination of ICD9-CM codes as well as billing charges identified in the hospital record. These data are primarily used for administrative purposes for payment. Because laboratory values were unavailable in the data, we chose to use bleeding diagnoses and blood product use in our definition. As such, there may have been some misclassification of patients who had surgery. A sensitivity analysis was run adjusting for surgery types and the direction and magnitude of the effects of the hospital associated bleed on the outcomes remained the same.
These analyses offer insight into the magnitude of differences in LOS and total hospitalization costs between patients with and without hospital associated bleeding among NVAF inpatients. Management of bleeding is an important goal both outside the hospital setting and within. These complications are associated with increased LOS and cost and other outcomes such as mortalityCitation22. In view of the higher LOS and hospitalization costs associated with managing patients with hospital associated bleeding, more comprehensive approaches should be explored to reduce these burdens to the patient and hospital. Options: include weighing the risk and benefit of bleeding and stroke prevention on individual patients; utilizing therapeutic options that are known to have a decreased risk of bleeding; and intense patient education to avoid risk factors contributing to bleeding. Each of these opportunities have their challenges in real-world practice setting.
Transparency
Declaration of funding
This study was sponsored by Bristol-Myers Squibb and Pfizer.
Declaration of financial/other relationships
BDB, BHJ, and SBR are employees of Premier Research Services which received funding from Bristol-Myers Squibb for conducting this study, analyzing the data, and developing this manuscript. AA received research funding from Pfizer for this work.
Acknowledgment
The authors would like to thank Carol Cohen who provided editorial feedback for this manuscript.
Previous presentation
This work was presented in part at the American Heart Association Annual Scientific Session, Los Angeles, CA, November 2012.
References
- Colilla S, Crow A, Simon T, et al. Projected estimates of prevalence and annual growth rate of atrial fibrillation in the united states from a dynamic age-period progression model. Circ Cardiovasc Qual Outcomes 2012;5:A2056
- Benjamin EJ, Wolf PA, D’Agostino RB, et al. Impact of atrial fibrillation on the risk of death: The Framingham Heart Study. Circulation 1998;98:946-52
- Stewart S, Hart CL, Hole DJ, et al. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med 2002;113:359-64
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22:983-8
- Benjamin EJ, Levy D, Vaziri SM, et al. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA 1994;271:840-4
- Psaty BM, Manolio TA, Kuller LH, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation 1997;96:2455-61
- Lip GY, Frison L, Halperin JL, et al. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J Am Coll Cardiol 2011;57:173-80
- Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation 2004;110:1042-6
- Hansen ML, Sorensen R, Clausen M, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med 2010;170:1433-41
- Nieuwlaat R, Connolly BJ, Hubers LM, et al. Quality of individual INR control and the risk of stroke and bleeding events in atrial fibrillation patients: a nested case control analysis of the ACTIVE W study. Thromb Res 2012;129:715-19
- Poli D, Antonucci E, Grifoni E, et al. Bleeding risk during oral anticoagulation in atrial fibrillation patients older than 80 years. J Am Coll Cardiol 2009;54:999-1002
- Wallentin L, Yusuf S, Ezekowitz MD, et al. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalized ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet 2010;376:975-83
- Amin A, Stokes, M, Wu N, et al. Application of randomized clinical trial data to actual practice: apixaban therapy for reduction of stroke risk in on-valvular atrial fibrillation patients. Curr Med Res Opin 2013;29:1253-61
- Deitelzweig S, Amin A, Jing Y, et al. Medical costs in the US of clinical events associated with oral anticoagulant (OAC) use compared to warfarin among non-valvular atrial fibrillation patients >75 and <75 years of age, based on the ARISTOTLE, RE-LY, and ROCKET-AF trials. J Med Econ 2013;16:1163-8
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146:857-67
- van Walraven C, Hart RG, Singer D, et al. Oral anticoagulants vs aspirin in nonvalvular atrial fibrillation: an individual patient meta-analysis. JAMA 2002;288:2441-8
- Mant J, Hobbs FD, Fletcher K, et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet 2007;370:493-503
- Hutten BA, Lensing AW, Kraaijenhagen RA, et al. Safety of treatment with oral anticoagulants in the elderly. A systematic review. Drugs Aging 1999;14:303-12
- Hutten, B. A., Lensing, A. W. A., Kraaijenhagen, R. A., Prins, M. H. Regulations and Ethical Guidelines: Title 45 Public Welfare Department of Health and Human Services, Part 46 Protection of Human Subjects. 2009. http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.html. Accessed September 11, 2013 Department of health and human services Washington DC, USA
- Mehta SK, Frutkin AD, Lindsey JB, et al. Bleeding in patients undergoing percutaneous coronary intervention: the development of a clinical risk algorithm from the National Cardiovascular Data Registry. Circ Cardiovasc Interv 2009;2:222-9
- Kim MH, Johnston SS, Chu BC, et al. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes 2011;4:4313-20
- Suh JW, Mehran R, Claessen BE, et al. Impact of in-hospital major bleeding on late clinical outcomes after primary percutaneous coronary intervention in acute myocardial infarction: the HORIZONS-AMI Trial. J Am Coll Cardiol 2011;58:1750-6
- Rao SV. Hemorrhage in patients with acute coronary syndrome: from annoying observation to major challenge. Rev Esp Cardiol 2010;63:1-4
- Ruiz-Nodar JM, Marin F, Roldan V, et al. Should we recommend oral anticoagulation therapy in patients with atrial fibrillation undergoing coronary artery stenting with a high HAS-BLED bleeding risk score? Circ Cardiovasc Interv 2012;5:459-66
- Steiner C, Andrews R, Barrett M, et al. HCUP Projections: cardiovascular/cerebrovascular conditions and procedures 2011 to 2012. HCUP Projections Report# 2012-02. U.S. Agency for Healthcare Research and Quality, 2012. http://www.hcup-us.ahrq.gov/reports/projections/2012-02.pdf. Accessed September 11, 2013 Washington DC, USA