Abstract
Objective: In the phase III SECURE trial, isavuconazole was non-inferior to voriconazole for all-cause mortality for the primary treatment of invasive mold disease (IMD) caused by Aspergillus spp. and other filamentous fungi. This analysis assessed whether hospital resource utilization was different between patients treated with isavuconazole vs voriconazole in SECURE. Methods: The analysis population comprised adults with proven/probable/possible IMD enrolled in SECURE. The primary endpoint was hospital length of stay (LOS) in the overall trial population. Patients were also stratified by estimated glomerular filtration rate-modification of diet in renal disease category (< 60 mL/min/1.73 m2 [moderate-to-severe impairment] and ≥60 mL/min/1.73 m2 [mild or no impairment]), body mass index (BMI; <25, ≥25–<30, and ≥30 kg/m2), and age (≤45, >45–≤65, and >65 years). Results: Data from 516 patients (258 per arm) were evaluated. Overall, median LOS was not statistically significantly different between the isavuconazole (15.0 days) and voriconazole (16.0 days; p = 0.607) arms. Median LOS was statistically significantly shorter in patients with moderate-to-severe renal impairment treated with isavuconazole (9.0 days) vs voriconazole (19.0 days; hazard ratio [HR]: 3.44; 95% confidence interval [CI] = 1.51–7.83). Median LOS was shorter, but not significantly, in patients with a BMI ≥30 kg/m2 (isavuconazole 13.5 days vs voriconazole 22 days; HR = 1.57; 95% CI = 0.70–3.52) or aged >65 years (isavuconazole 15.0 days vs voriconazole 20.0 days; HR = 1.37; 95% CI = 0.87–2.16). Limitations: As the patient subgroups analyzed were small, sub-group findings should be interpreted with caution in light of the lack of statistical significance for each sub-group-by-treatment interaction. Conclusions: Isavuconazole may reduce hospital LOS in certain subgroups of patients with IMD, especially those with moderate-to-severe renal impairment.
Introduction
Invasive mold disease (IMD) caused by Aspergillus spp. and other filamentous fungi represents a serious medical challenge in immunocompromised patients, including those with malignanciesCitation1 and patients who have received either solid organCitation2 or hematopoietic stem cell transplantsCitation3. Despite current treatment options and advances in diagnostic testing, these infections remain associated with significant morbidity and mortalityCitation4. As a consequence, the financial burden of IMD is highCitation5–9, especially as many patients require protracted hospital staysCitation5,Citation6,Citation8,Citation9. Patients with a primary diagnosis of invasive aspergillosis are calculated to spend an average of 12.0 days in the hospital and incur an average cost of US$33,253 per inpatient episodeCitation10. For patients diagnosed with this infection while in the intensive care unit, the burden is estimated to be even higher, with an average length of stay of 26.9 days, and total hospital costs of US$76,235Citation5. Additional evidence suggests hospital costs in some patient groups may be up to US$59,356 higher for patients with invasive aspergillosis vs otherwise similar patients without this infectionCitation9.
Voriconazole, a second-generation triazole anti-fungal agent, is currently recommended for the first-line treatment of invasive aspergillosisCitation11. However, this agent exhibits non-linear pharmacokineticsCitation12, is associated with multiple drug–drug interactionsCitation12, and has a potential for renal toxicity owing to the use of a cyclodextrin-based vehicle in its intravenous formulationCitation13. There is also controversy over the need for therapeutic drug monitoring with this agent in high-risk patientsCitation12,Citation14. Isavuconazonium sulfate is a novel, broad-spectrum, cyclodextrin-free triazole antifungal prodrug, which was approved in 2015 by the US Food and Drug Administration for the primary treatment of invasive aspergillosis and mucormycosisCitation15. It is rapidly converted to the active moiety isavuconazole, which displays linear pharmacokinetics and few clinically relevant drug–drug interactions in phase I trialsCitation16.
In the phase III SECURE trial, isavuconazole was shown to be non-inferior to voriconazole for all-cause mortality through Day 42 (19% vs 20%, respectively) for the primary treatment of IMDCitation17. Isavuconazole was also well tolerated compared with voriconazole, with statistically significantly fewer drug-related adverse events, particularly skin, eye, and hepatobiliary disorders. Given the high financial burden of IMD, we conducted an analysis of hospital outcomes to assess whether resource use was affected by the administration of isavuconazole vs voriconazole in the SECURE trial.
Patients and methods
Study overview
The SECURE trial (ClinicalTrials.gov Identifier: NCT00412893) was a global, phase III, multi-center, randomized, double-blind, parallel-group, non-inferiority trial conducted globally from 2007–2013 to evaluate the efficacy and safety of isavuconazole compared with voriconazole for the primary treatment of IMD caused by Aspergillus spp. and other filamentous fungi. The trial was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonisation Guidelines for Good Clinical Practice, and the independent ethics committee or institutional review board at each participating site. Written, informed consent was obtained from all individual participants (or legally-authorized representatives) included in the study. The study design, treatment regimen, and patient population have been described previously (see )Citation17.
Outcome measures
The primary endpoint of this analysis was the hospital length of stay (LOS) in all patients with proven/probable/possible IMD caused by Aspergillus spp. and other filamentous fungi (as defined by the European Organization for the Research and Treatment of Cancer/Mycoses Study Group [EORTC/MSG] criteria)Citation18, who received at least one dose of isavuconazole or voriconazole (intent-to-treat [ITT] population). Hospital LOS was defined as the time (days) from the beginning of randomized therapy to the date of discharge or death during hospitalization, whichever occurred first. Other endpoints analyzed included: 30-day all-cause hospital readmission rate, defined as at least one other hospital readmission within 30 days post-discharge date of initial hospitalization during which study drug was initiated; ratio of total days as an inpatient on IV study drug therapy to total days as an inpatient on IV plus oral study drug therapy; and, the total number of additional days on non-study antifungal drugs (excluding prophylaxis) for patients who switched from the study drugs to a potentially mold-active systemic antifungal therapy after end of study treatment.
Pre-specified subgroup analyses were conducted to assess LOS in the following subgroups of clinical relevance: renal impairment (estimated glomerular filtration rate-modification of diet in renal disease [eGFR-MDRD] category <60 and ≥60 mL/min/1.73 m2), body mass index (BMI; strata: <25, ≥25 to <30, and ≥30 kg/m2), age (strata: ≤45, >45 to ≤65, and >65 years), gender (male or female), race (White, Black or African American, Asian, and Other), ethnicity (Hispanic or Latino, or Non-Hispanic or Latino), baseline neutropenic status (yes or no), and successful therapy followed by prophylaxis with a mold-active antifungal agent (yes or no).
Classification of renal function was based on the guidelines issued by the National Kidney Foundation Kidney Disease Outcomes Quality InitiativeCitation19. Patients with moderate renal impairment included those with eGFR-MDRD values 30–59 mL/min/1.73 m2, whereas those with values 15–29 mL/min/1.73 m2 were classified as having severe renal impairment. Following a protocol amendment in November 2010, patients with moderate-to-severe renal dysfunction were specifically excluded from the SECURE trial for safety purposes. The majority of patients with moderate-to-severe renal impairment included in the current analysis were enrolled prior to this date.
Safety
The frequency and severity of treatment-emergent adverse events (TEAEs) were monitored and assessed throughout the SECURE trial. A TEAE was defined as any untoward medical occurrence in a patient administered a study drug that started after the first administration of the study drug until 28 days after the last dose of the drug. Serious TEAEs included those that required inpatient hospitalization or led to prolongation of hospitalization (hospitalization for treatment, observation, or examination caused by a TEAE was considered to be a serious TEAE).
Statistical analysis
The SECURE analysis plan for resource utilization was finalized prior to treatment unblinding. No consideration was given to the endpoints of this analysis when the sample size was determined for this trial. The sample-size calculation conducted for the SECURE trial was based on a non-inferiority design with a primary trial endpoint of all-cause mortality through Day 42Citation17. Approximately 255 patients per group were to be enrolled to ensure at least 80% power to demonstrate that the upper bound of the 95% confidence interval (CI) for a treatment difference in favor of the comparator was no larger than 10%.
All patients in the ITT population with adequate hospitalization data were included in the endpoint analyses. Hospital LOS was summarized using descriptive statistics. Additionally, LOS was also analyzed using Kaplan–Meier method. Hospital LOS was calculated from randomization to the date of discharge or death (whichever happened first). Patients who died during hospitalization when study drugs were administered were censored from the day of death. The comparison of isavuconazole vs voriconazole for LOS was tested using a stratified log-rank test at the 0.05 two-sided significance level. The 50th percentile of Kaplan–Meier estimates was used to estimate the median LOS, and a two-sided 95% CI was provided for this estimate.
For the sub-group analyses, the treatment effect with respect to hospital LOS and its 95% CI within each level of the sub-group was estimated by the hazard ratio (HR) from a stratified Cox regression model with the following factors: treatment group, geographical regions, allogeneic bone marrow/hematopoietic stem cell transplant (BMT/HSCT) status and active malignancy status. The stratification factors were pre-specified for the SECURE trial. For each sub-group, the interaction p-value was calculated from a likelihood ratio test which compared the full stratified Cox regression model, including treatment group, sub-group, and sub-group-by-treatment group interaction terms.
Thirty-day all-cause hospital readmission rates and exact 95% CIs for each treatment group were calculated. Treatment difference in the hospitalization readmission rate and its 95% CI were calculated by the stratified Cochran-Mantel-Haenszel method, where the 95% CI was based on a normal approximation. For the ratio of days on IV therapy to total number of days of IV plus oral therapy, the treatment inference was made from the van Elteren test, which is a stratified version of the non-parametric Wilcoxon rank-sum test. A two-sided p-value based on normal approximation at the level <0.05 was used to claim statistical significance between treatment groups. All statistical analyses were conducted using ™SAS version 9.3, Enterprise Guide 4.3 (SAS Institute Inc., Cary, NC, USA).
Results
Patients
A total of 532 patients were eligible to participate in the trial, 527 underwent randomization and 516 patients were included in the ITT population (258 patients in each treatment arm). Of these, up to 501 patients had sufficient hospital details for calculation of analysis endpoints (isavuconazole, n = 246; voriconazole, n = 255). Baseline demographics and risk factors for fungal infections of the patients were balanced between the two treatment groupsCitation17. Overall, 433 (83.9%) patients in the ITT population had hematological malignancy, 360 (69.8%) had active malignancy, and 105 (20.3%) patients had received a prior allogeneic bone marrow transplant.
Demographics and clinical characteristics were generally similar between treatment arms in patients stratified by renal impairment (see ), BMI (see Supplemental Table 1), and age (see Supplemental Table 2).
Table 1. Demographics and clinical characteristics by renal status.Table Footnote*
Outcomes
In the overall trial population, there was no difference in the median LOS between the treatment arms, as shown in (see Supplemental Table 3 for analysis of LOS in patients discharged alive). The unadjusted median (range) LOS for the isavuconazole group was 13.0 (1.0–371.0) days, whereas that for the voriconazole group was 15.0 (2.0–118.0) days. In the adjusted models, the median (95% CI) LOS for the isavuconazole and voriconazole groups was 15.0 (13.0–17.0) and 16.0 (14.0–17.0) days, respectively (p = 0.607). There was also no statistical difference in readmission rates between treatment arms, as shown in . The ratios of days inpatients were given IV formulation to total days of IV plus oral therapy were comparable between treatment arms (see ). Median (range) durations that patients were on potentially mold-active systemic antifungal therapy other than study drugs were 32.0 (3.0–35.0) days and 33.0 (3.0–35.0) days for isavuconazole and voriconazole, respectively.
Table 2. Outcomes in the ITT population.
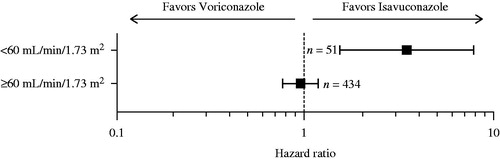
Median LOS for patients with moderate-to-severe renal impairment was statistically significantly shorter in the isavuconazole arm compared with the voriconazole arm (p = 0.0032) (see ). There were trends towards shorter median LOS in isavuconazole-treated patients with BMI ≥30 kg/m2 (isavuconazole 13.5 days vs voriconazole 22.0 days; HR = 1.57; 95% CI = 0.70–3.52) and those aged >65 years of age (isavuconazole 15.0 days vs voriconazole 20.0 days; HR = 1.37; 95% CI = 0.87–2.16), but without statistical significance. None of the other sub-group populations displayed differences in LOS between study arms (see Supplemental Table 4).
Figure 2. Comparison of median LOS (days) in the ITT population moderate-to-severe renal impairment category. Moderate-to-severe renal impairment: eGFR-MDRD category <60 mL/min/1.73 m2; p = 0.003. In patients with sufficient hospitalization and eGFR-MDRD data. eGFR-MDRD, estimated glomerular filtration rate-modification of diet in renal disease; ITT, intent-to-treat; LOS, length of stay. The error bars denote confidence intervals.
Safety
Overall, nine (3.5%) hospitalized patients in the isavuconazole arm and 18 (6.9%) hospitalized patients in the voriconazole arm (p = 0.112) experienced serious TEAEs that prolonged their hospital stay; while 46 (17.9%) isavuconazole-treated patients vs 59 (22.8%; p = 0.190) voriconazole-treated patients experienced serious TEAEs post-discharge which required their readmission to hospital. The most common types of serious TEAEs were those in the system organ class infections and infestations, i.e. 16 (6.2%) patients in the isavuconazole arm vs 30 (11.6%) patients in the voriconazole arm (p = 0.044).
In patients with no or mild renal impairment, more of those treated with voriconazole than isavuconazole experienced drug-related TEAEs (see ). Drug-related TEAEs and TEAEs were experienced by more patients treated with voriconazole than isavuconazole in the <25 kg/m2 BMI sub-group; more patients with BMI ≥25–30 kg/m2 treated with voriconazole than isavuconazole discontinued due to TEAEs (see Supplemental Table 5). More patients in the >45–≤65 years age group treated with voriconazole than isavuconazole experienced drug-related and serious TEAEs, as well as TEAEs that led to study discontinuation (see Supplemental Table 5). Drug-related TEAEs were also more common in patients aged ≤45 years treated with voriconazole vs isavuconazole.
Table 3. Safety by patient renal status.Table Footnote*
Discussion
In this prospective analysis of hospital LOS in isavuconazole and voriconazole-treated patients with IMD in the SECURE trial, we noted that treatment assignment did not affect post-randomization LOS or 30-day all-cause readmission rates. In the sub-group of patients with moderate-to-severe renal impairment receiving isavuconazole, hospital LOS was significantly shorter compared with patients receiving voriconazole. In addition, patients aged >65 years and those with BMI ≥30 kg/m2 had non-significant trends towards shorter LOS when treated with isavuconazole.
Renal impairment is a common comorbidity in patients with invasive fungal diseaseCitation20, and a risk factor for invasive aspergillosisCitation21. In a previous analysis, neutropenia, malnutrition, disseminated disease, and length of intensive care unit stay were identified as factors associated with increased hospital LOS in transplant patients with invasive aspergillosisCitation5. However, there were no apparent differences between treatment arms in patient factors in this sub-group that could account for the observed difference in LOS. Moreover, after 3 years of enrollment, patients with moderate-to-severe renal impairment were specifically excluded from SECURE following a study amendment owing to the voriconazole-labeled warning on the possibility of accumulation of the cyclodextrin-based vehicle used in the IV formulation of voriconazole; therefore, use of IV voriconazole is not recommended in patients with moderate-to-severe renal impairmentCitation13. In contrast, dose adjustment is not required for isavuconazole in this patient groupCitation15. As a consequence, patient numbers in this sub-group were low, limiting further speculation on reasons for the difference in LOS.
The prevalence of obesity is growing globally; however, no prospective studies have examined the impact of obesity on hospital resource use in patients with IMD. The global population is also agingCitation22. Older adults are at an increased risk of IMD due to immunosenescence and because they are more likely to receive aggressive chemotherapy regimens for cancer and immunosuppressive therapy for non-malignant conditions than younger patientsCitation23. Potential reasons underlying the trends observed in the current analysis towards a shorter LOS in elderly and obese patients treated with isavuconazole in the SECURE trial are unclear. The clinical characteristics of the patients were similar and the safety profile of isavuconazole appeared to be favorable compared with voriconazole in all age and BMI subgroups, rather than elderly or obese patients only. These findings merit further investigation in prospective trials.
In the overall SECURE trial population, median hospital LOS and 30-day hospital readmission rates were not statistically significantly different between the treatment arms. Patient risk factors and underlying illnesses were well balanced in the SECURE trialCitation17. Similar numbers of patients in the treatment arms had proven/probable disease (isavuconazole, n = 143; voriconazole, n = 129), and a similar distribution of pathogens was observed, with 49/143 (34%) vs 39/129 (30%) having infections caused by Aspergillus spp. only in the isavuconazole and voriconazole arms, respectivelyCitation17. In addition, we found that there were no statistically significant differences between the treatment arms in the numbers of patients who experienced serious TEAEs that required hospital readmission or who experienced serious TEAEs which prolonged hospitalization. Together, these findings suggest that isavuconazole and voriconazole are comparable with regards to hospital LOS and readmission.
This analysis has a number of limitations. Although these were prospectively-collected data, and a priori defined analyses and subgroups, SECURE was a clinical trial and its findings do not necessarily reflect real-world clinical practice. The SECURE trial population included a high proportion of patients with hematological malignancies and recipients of BMT/HSCT, which limits the generalizability of the results. The patient subgroups analyzed were small and the analyses were conducted on clinical trial endpoints, which were not a consideration for trial power. The sub-group findings should be interpreted with caution in light of the lack of statistical significance for each sub-group-by-treatment interaction. Additionally, eGFR may not always be an accurate predictor of kidney disease in patients with values near 60 mL/min/1.73 m2 Citation24. Therapeutic drug monitoring of voriconazole was not conducted, therefore, it is unknown whether supratherapeutic levels of voriconazole contributed to an increased frequency of TEAEs in obese patients or to the differences in outcomes observed in this study in elderly or obese patients between isavuconazole and voriconazole treated groups. Lastly, SECURE was an international trial and resource allocation and clinical practice vary widely between US and non-US centers, which may have affected outcomes. However, the numbers of patients enrolled in US and non-US centers were comparable between treatment arms.
Conclusions
Invasive fungal disease is an economic burden for hospitalsCitation5–9 and hospital LOS is the main determinant of hospital costsCitation9,Citation25. Patients with invasive aspergillosis have longer LOS in hospitals and higher costs of care compared with similar patients without this diseaseCitation6,Citation8,Citation9. Therefore, efficacy with reduced hospital LOS and costs are clearly desirable characteristics of new antifungal agents. The present analysis demonstrated that isavuconazole and voriconazole are generally comparable with regards to hospital LOS and 30-day hospital readmission rates; however, hospital LOS may be lower in certain sub-groups of patients, especially those with moderate-to-severe renal impairment. Further investigation of the subgroups identified in this analysis is warranted.
Transparency
Declaration of funding
This analysis was funded by Astellas Pharma Global Development, Inc.
Declaration of financial/other relationships
Isavuconazonium sulfate has been co-developed by Astellas Pharma Global Development, Inc. and Basilea Pharmaceutica International Ltd. DH has received consultation fees from Astellas Pharma Global Development, Inc., and is the principal of David Horn, LLC and CEO of Mid-Atlantic BioTherapeutics. DG has received funding from Astellas Pharma Global Development, Inc. AS has served as a consultant to, speaker for, and received research support from Astellas Pharma Global Development, Inc. He also has served as a consultant to, speaker for, and received research support from: AstraZeneca, Bayer, BMS, Cubist, Merck, Pfizer, Roche, Theravance, and Tetraphase. JS, NA, FS, and BF are employees of Astellas Pharma Global Development, Inc. NK was an employee of Astellas Pharma Global Development, Inc. at the time of the analysis. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplementary_Material
Download MS Word (29.7 KB)Acknowledgments
Editorial assistance was provided by Neil M. Thomas, PhD, CMPP, of Envision Scientific Solutions, funded by Astellas Pharma Global Development, Inc. The authors are grateful for the contributions of the investigators and staff who conducted the SECURE trial, and to the patients who volunteered for this study.
References
- Leventakos K, Lewis RE, Kontoyiannis DP. Fungal infections in leukemia patients: how do we prevent and treat them? Clin Infect Dis 2010;50:405–15
- Pappas PG, Alexander BD, Andes DR, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis 2010;50:1101–11
- Kontoyiannis DP, Marr KA, Park BJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis 2010;50:1091–100
- Azie N, Neofytos D, Pfaller M, et al. The PATH (Prospective Antifungal Therapy) Alliance® registry and invasive fungal infections: update 2012. Diagn Microbiol Infect Dis 2012;73:293–300
- Baddley JW, Stephens JM, Ji X, et al. Aspergillosis in Intensive Care Unit (ICU) patients: epidemiology and economic outcomes. BMC Infect Dis 2013;13:29
- Drgona L, Khachatryan A, Stephens J, et al. Clinical and economic burden of invasive fungal diseases in Europe: focus on pre-emptive and empirical treatment of Aspergillus and Candida species. Eur J Clin Microbial Infect Dis 2014;33:7–21
- Kim A, Nicolau DP, Kuti JL. Hospital costs and outcomes among intravenous antifungal therapies for patients with invasive aspergillosis in the United States. Mycoses 2011;54:e301–12
- Menzin J, Meyers JL, Friedman M, et al. Mortality, length of hospitalization, and costs associated with invasive fungal infections in high-risk patients. Am J Health Syst Pharm 2009;66:1711–17
- Slobbe L, Polinder S, Doorduijn JK, et al. Outcome and medical costs of patients with invasive aspergillosis and acute myelogenous leukemia-myelodysplastic syndrome treated with intensive chemotherapy: an observational study. Clin Infect Dis 2008;47:1507–12
- HCUP. Weighted national estimates from HCUP Nationwide Inpatient Samples (NIS). 2013. http://hcupnet.ahrq.gov/HCUPnet.jsp?Parms=H4sIAAAAAAAAAKtMLE4sSvTzDDY0TnWJMEzMSjQ0NE_yM05LdUnKTCpOKghJSkxKSkxOTMlOzEzLTATCNAhIzEpLS81MBABQdnF1QQAAAA537315005BE04B34B843875CE17815325DE08097. Accessed February 2016
- Walsh TJ, Anaissie EJ, Denning DW, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2008;46:327–60
- Laverdiere M, Bow EJ, Rotstein C, et al. Therapeutic drug monitoring for triazoles: a needs assessment review and recommendations from a Canadian perspective. Can J Infect Dis Med Microbiol 2014;25:327–43
- Pfizer Inc. VFEND® (voriconazole) prescribing information. 2014. http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/021266s037,021267s044,021630s027lbl.pdf. Accessed May 7, 2015
- Kuo IF, Ensom MH. Role of therapeutic drug monitoring of voriconazole in the treatment of invasive fungal infections. Can J Hosp Pharm 2009;62:469–82
- Astellas Pharma US Inc. CRESEMBA® (isavuconazonium sulfate) prescribing information. 2015. http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207500Orig1s000lbl.pdf. Accessed March 10, 2015
- McCormack PL. Isavuconazonium: first global approval. Drugs 2015;75:817–22
- Maertens JA, Raad II, Marr KA, et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet 2016;387:760–9
- De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clinical Infectious Diseases 2008;46:1813–21
- Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 2003;139:137–47
- Baddley JW, Andes DR, Marr KA, et al. Factors associated with mortality in transplant patients with invasive aspergillosis. Clin Infect Dis 2010;50:1559–67
- Herbrecht R, Bories P, Moulin JC, et al. Risk stratification for invasive aspergillosis in immunocompromised patients. Ann N Y Acad Sci 2012;1272:23–30
- Cohen JE. Human population: the next half century. Science 2003;302:1172–75
- Kauffman CA. Fungal infections in older adults. Clin Infect Dis 2001;33:550–5
- Stevens LA, Coresh J, Feldman HI, et al. Evaluation of the modification of diet in renal disease study equation in a large diverse population. J Am Soc Nephrol 2007;18:2749–57.
- Zilberberg MD, Shorr AF, Huang H, et al. Hospital days, hospitalization costs, and inpatient mortality among patients with mucormycosis: a retrospective analysis of US hospital discharge data. BMC Infect Dis 2014;14:310