ABSTRACT
Exposures of occupants in school buses to on-road vehicle emissions, including emissions from the bus itself, can be substantially greater than those in outdoor settings. A dual tracer method was developed and applied to two school buses in Seattle in 2005 to quantify in-cabin fine particulate matter (PM2.5) concentrations attributable to the buses' diesel engine tailpipe (DPMtp) and crankcase vent (PMck) emissions. The new method avoids the problem of differentiating bus emissions from chemically identical emissions of other vehicles by using a fuel-based organometallic iridium tracer for engine exhaust and by adding deuterated hexatriacontane to engine oil. Source testing results showed consistent PM:tracer ratios for the primary tracer for each type of emissions. Comparisons of the PM:tracer ratios indicated that there was a small amount of unburned lubricating oil emitted from the tailpipe; however, virtually no diesel fuel combustion products were found in the crankcase emissions. For the limited testing conducted here, although PMck emission rates (averages of 0.028 and 0.099 g/km for the two buses) were lower than those from the tailpipe (0.18 and 0.14 g/km), in-cabin PMck concentrations averaging 6.8 μg/m3 were higher than DPMtp (0.91 μg/m3 average). In-cabin DPMtp and PMck concentrations were significantly higher with bus windows closed (1.4 and 12 μg/m3, respectively) as compared with open (0.44 and 1.3 μg/m3, respectively). For comparison, average closed- and open-window in-cabin total PM2.5 concentrations were 26 and 12 μg/m3, respectively. Despite the relatively short in-cabin sampling times, very high sensitivities were achieved, with detection limits of 0.002 μg/m3 for DPMtp and 0.05 μg/m3 for PMck.
PM2.5 measurements in two Seattle school buses showed average concentrations of 26 and 12 μg/m3 with windows closed and open, respectively. Virtually all PM2.5 was car bonaceous. Tracer measurements showed that bus self-pollution contributed approximately 50% of total PM2.5 concentrations with windows closed and 15% with windows open, with over three-quarters of these contributions attributed to crankcase emissions. Maintaining ventilation in buses clearly reduces total PM2.5 exposures and that from the buses' own emissions. The dual tracer method now offers researchers a new technique for explicit identification of single source contributions in settings with multiple sources of carbonaceous emissions.
INTRODUCTION
In-vehicle exposures to vehicle-related emissions of fine particulate matter (PM2.5) and other species inside vehicles have been shown to be high relative to urban outdoor environments.Citation1,Citation2 Particular emphasis has been given to concentrations of diesel exhaust particulate matter (PM) inside school buses that are attributable to the buses' own exhaust. Such source attribution efforts are difficult in purely observational studies because of the presence of emissions from other vehicles, which are chemically identical to those of the bus, and the presence of chemically similar PM emitted by nonvehicular sources. A method was therefore sought that could allow definitive identification of the emissions of a single bus. Previous studiesCitation3–8 have produced estimates of exhaust PM inside school buses that are attributable to the buses' own exhaust ranging from 0.2 to as much as 20 μg/m3. With the exception of Ireson et al.,Citation5 these studies inferred bus exhaust contributions from measurements of species emitted by diesel engines and other sources and did not measure the emission rates of the buses tested. They were therefore unable to explicitly identify the buses' contribution relative to other sources of the marker species. One study did use a tracer in exhaust, but it did not attempt to maintain a constant ratio of tracer to emissions.Citation6,Citation7 As a result, although suggestive, these studies do not definitively quantify bus emission contributions.
To allow specific identification of a single bus' emissions, a previous studyCitation5,Citation9 extended the use of a fuel-based iridium (Ir) tracer method for diesel tailpipe exhaust (DPMtp)Citation10,Citation11 by conducting chassis dynamometer measurements of the tailpipe emission rates of PM2.5 and particle-bound Ir. The mass ratio of these two species in the exhaust (DPMtp:Ir) was used in conjunction with in-cabin Ir measurements to calculate the in-cabin concentration of DPMtp. The study presented here, an initial phase of a larger monitoring campaign,Citation12 tested two buses using the Ir tracer and added a new second tracer to the bus lubricating oil to specifically quantify crankcase PM emission contributions. An on-board dilution tunnel system was used to measure PM and tracer emission rates from the tailpipe and crankcase under actual operating conditions. The primary objective of this paper is to document this new method for use and extension by others. Results for two buses are presented to demonstrate the capabilities of the method.
Historically, relatively little attention has been given to crankcase contributions, which were generally considered to be significantly lower than tailpipe emissions. Hill et al.Citation8 conducted a series of experiments using nonspecific optical methods to measure PM in individual buses with and without retrofit PMck controls. They showed that crankcase emissions may contribute significantly to in-cabin concentrations of particles at the upper end of the PM2.5 size range. To accurately quantify this contribution, a deuterated al-kane (d-alkane) was used as an explicit marker for lubricating oil. Emissions testing and analysis of crankcase (PMck) particulate emissionsCitation13 and DPMtp were conducted to determine the PM mass-to-tracer ratios for both Ir and the d-alkane. In-cabin concentrations attributable to the crankcase and tailpipe emissions were quantified using direct measurements of the two tracers during routine bus operations.
Design of the study was guided by two primary objectives: (1) to unambiguously quantify DPMtp and PMck concentrations in school buses under typical operating conditions, and (2) to ensure adequate sensitivity to measure ambient contributions of tailpipe and crankcase emissions at levels well below the minimum concentrations of interest.
EXPERIMENTAL PROCEDURES
A sampling campaign was conducted during summer 2005 to evaluate several independent methods for estimating penetration of bus emissions into the cabin, as described by Liu et al.Citation12 The tracer study presented here was conducted as part of that campaign to provide the in-cabin DPMtp and PMck concentrations against which the other methods were evaluated. In-cabin sampling and source testing were conducted using two conventional (front-engine) diesel school buses—a 2003 Bluebird and a 2000 International. Odometer readings for the buses were 48,900 and 78,400 mi, respectively, and both were equipped with diesel oxidation catalysts. Buses were taken in “as-received” condition, and no repairs (e.g., door or window seal replacement) were made, even when some evidence of wear was observed. The buses operated over a 10-km Seattle school bus route with each complete in-cabin sampling run consisting of forward and reverse routes. Typical ambient PM2.5 concentrations in the study area are lower than many urban areas, ranging from approximately 5 to 15 μg/m3. Each route was of approximately 1 hr in duration. Drivers were instructed to follow their standard practices, including the number and duration of door-opening events to on- and off load students. This route is predominately on residential streets, with little arterial and no freeway driving, and average speeds were less than 15 km/hr. There were frequent periods of low-speed, high engine load operations. Emissions testing was conducted on route segments selected to characterize, as closely as possible, the duty cycle of the school buses during in-cabin sampling.
Tracers, Equipment, and Sampling Media
Several studies have used metals as intentional fuel-based tracers for atmospheric dispersion and transport and exposure experiments.Citation14–17 Wu et al.Citation10 demon-strated the use of Ir in fuel as a means of investigating the exposures of students to diesel exhaust from the public transit fleet used by urban high school students. A fuel-based Ir tracer was initially selected by Ireson et al.Citation5 for three primary reasons: (1) individual Ir atoms are bound in exhaust particles and emitted at the same rate as fuel combustion, which is closely related to the diesel PM (DPM) emission rate; (2) the natural abundance of Ir is extremely low, and it is not commonly used in commercial or industrial processes that lead to its emission; and (3) it can be quantified in amounts as low as 50 fg (1 fg = 10−15g) using instrumental neutron activation analysis (INAA).Citation18
In the selection of a tracer for lubricating oil, trace metals were considered but rejected because it was unclear if a metallic oil additive would behave similarly to the naturally occurring components of lubricating oil. PMck emissions were assumed to consist primarily of the higher molecular weight hydrocarbons in oil that were aerosolized by blow-by past the piston rings or valve guides or were vaporized and then condensed into fine particles. Deuterated organic compounds are commonly used in gas chromatography (GC)/mass spectroscopy (MS) because (1) they do not occur naturally, (2) they elute from GC columns at nearly the same time as their nondeuterated analogs, and (3) they are readily differentiated from their nondeuterated analogs by MS. Normal (i.e., unbranched, straight carbon chain) hexatriacontane (n-C36H74) is a naturally occurring higher molecular weight component of lubricating oils, and its fully deuterated form, n-C36D74, was selected as the tracer.
Analyses of source and in-cabin samples included gravimetric mass, organic carbon (OC) and elemental carbon (EC), INAA for Ir, and GC/MS for the d-alkane. These analyses require Teflon (for gravimetric mass and INAA) and quartz (for particulate carbon and GC/MS) filter media. Both media were used for all types of samples.
The nominal sensitivity achievable for DPMtp on an integrated filter sample depends on several factors, including the Ir concentration in background PM, the analytical sensitivity of INAA, the sample volume, and the DPMtp:Ir ratio. The latter is determined in part by the DPM emission rate of the engine and the spike level in the fuel. Previous studiesCitation10,Citation11 showed that urban ambient concentrations of Ir were typically on the order of 100 fg/m3. High flow rate samplers designed and constructed at the University of Maryland were used to increase the sensitivity achievable for relatively short-duration samples. This “UMd sampler” uses an inlet impactor and 47-mm filter holders. The impactor inlet is designed to achieve a particle size cut point of approximately 2.3 μm at a flow rate of 120 L/min.Citation19 Sample flow rates ranged from 98 to 120 L/min (average 114 L/min), resulting in actual sample-specific cut points between 2.3 and 2.5 μm. On the basis of typical school bus fuel consumption rates, emission rates, and speeds, a conservative nominal spike level of 2.6 mg of Ir per liter of fuel was selected to achieve a target sensitivity of 0.001 μg/m3 DPMtp.
The crankcase d-alkane tracer spike level selected was 100 g of the tracer for the lubricating oil volume of typical school bus engines (∼22 L). This level was selected based on preliminary calculations and assumptions regarding the expected mass fraction of tracer in crankcase PM emissions. For 1-hr integrated samples at a flow rate of 120 L/min and an assumed nominal abundance of tracer in crankcase emissions, the anticipated sensitivity for ambient PMck measurements was approximately 0.1 μg/m3.
Source testing of vehicle emissions has traditionally been conducted using stationary engine or chassis dynamometers with large constant volume sampling (CVS) dilution tunnels. For this study, an alternative approach was used that allowed collection of PM emissions samples under the same bus operating conditions as those of the in-cabin sampling. Tailpipe and crankcase vent emissions testing was conducted using the Ride Along-Vehicle Emissions Measurement (RAVEM) system developed by Engine Fuel & Emissions Engineering, Inc. The RAVEM is a portable dilution tunnel system based on proportional partial-flow CVS from the vehicle exhaust pipe. Isokinetic sampling in the tailpipe and subsequent dilution produces a diluted exhaust stream comparable to that of full-flow CVS systems used in chassis and engine dynamometer testing, but it allows measurements to be made on-board a moving vehicle. Teflon and quartz filter samples were collected from the dilution tunnel using University Research Glass PM2.5 cyclone inlets and 47-mm filter holders operated at their design flow rate of 16.7 L/min. Comparative testing of the RAVEM with full-flow CVS systems shows good general agreement between the two methods.Citation20–22 For carbon dioxide (CO2) and oxides of nitrogen emissions, the agreement is generally very close. For PM emissions, the differences between RAVEM results and those of specific full-flow CVS laboratories are generally between 0 and 25%, which is less than the range of differences between different full-flow CVS systems in round-robin testing.Citation23 Although the RAVEM shows some tendency toward under-reporting of PM emission rates, the key calculations from RAVEM samples are the PM: tracer ratios, which are based on analyses of the same filter. As discussed later in this paper, this minimizes potential bias in tracer-based estimates of in-cabin DPMtp and PMck concentrations that might result from calculations based on concurrent measurements from different media or methods.
For testing, buses were operated on ultralow sulfur diesel fuel to which a solution of tris(norbornadiene)iridium-(III)acetylacetonate in toluene was added. This tracer is an organometallic chelate that is readily soluble in toluene and soluble in diesel fuel. Because single Ir atoms in a positive oxidation state are bound in the complex and distributed uniformly through the fuel, the Ir atoms will be captured and bound within the fine carbonaceous particles formed in the diesel combustion chamber. For each bus, a solution of approximately 2 g of tracer compound (0.66 g of Ir) in 200 mL of toluene was added to the nearly empty fuel tank (expected capacity of 190–280 L), which was then refueled to mix the tracer. The bus was then driven for several miles to mix the spiked fuel through the system before testing.
The n-C36D74 tracer (100 g) was dissolved in 19 L of new motor oil and added to the first test bus, with new oil added as needed to bring the oil to its normal operating level (20 –25 L). At the conclusion of testing of the first bus, the spiked oil was drained and transferred to the second bus, again with the addition of new oil to bring the level up to its normal operating range.
In-Cabin On-Road Sampling
For each bus, in-cabin sampling was conducted during two sets of forward- and reverse-route pairs with windows closed and another two sets with windows open. The windows-closed tests were conducted with all windows, vents, and bus ventilation system closed and turned off. Integrated filter samples were collected inside of the bus and inside of a lead vehicle (LV) that traveled the same route a few minutes ahead of the bus. Samples from the LV could then be used to determine the background level (if any) of the tracers. Near-roadway PM2.5 samples were collected and analyzed by INAA several months before the tracer study to verify that background Ir levels were sufficiently low so as not to compromise the desired sensitivity for DPMtp.
In-bus and LV integrated samples were collected using the UMd samplers. Power for the sampling pumps was provided by 12 VDC inverters and banks of four lead-acid marine batteries. Concurrent samples were collected on Teflon and prefired quartz filters. Two pairs of samplers were operated on the bus, with one pair collecting samples for the approximately 1-hr duration of each forward and reverse route and the other pair collecting samples for the approximately 2-hr duration of full forward and reverse routes. Thus, for each bus, four Teflon/quartz filter pairs were collected for each 2-hr forward/reverse route run, and eight filter pairs were collected during the 1-hr forward and reverse routes. A single pair of samplers was operated in the LV, with a pair of samples collected for each route (forward or reverse). For bus 1, the samplers for 1-hr forward and reverse runs were mounted in the second and third seats from the rear of the bus on the left side, and the sampler pair for 2-hr samples was mounted in the second seat from the rear on the right side. For bus 2, the 1-hr samplers were mounted in the second and fourth seats from the front on the right side, and the 2-hr sampler pair was mounted in the third seat from the front on the right side. This change was made for bus 2 to bring these samplers closer to the other sampling equipment that was part of the larger study.Citation12 Filters were stored at −20 °C between sampling and laboratory analyses. Teflon filters were analyzed gravimetrically and by INAA. Quartz filters were analyzed for OC and EC by thermal optical reflectance (TOR) for a punch taken from each filter and for a range of alkanes (including the d-alkane tracer), hopanes, steranes, polycyclic aromatic hydrocarbons, and nitroarenes by extraction and GC/MS.Citation13 Teflon and quartz field blanks were also collected and analyzed.
Source Testing
Tailpipe and crankcase vent emissions testing using the RAVEM was conducted after the conclusion of in-cabin sampling to avoid the possibility of inadvertent contamination of the bus interior with either tracer. Tail-pipe sampling was conducted using the standard RAVEM proportional partial flow approach, with a small fraction of the total exhaust being extracted into the dilution tunnel. The low flow rate from crankcase vents precludes this approach, so the PMck samples were collected using the RAVEM as a conventional full-flow CVS system. The RAVEM dilution tunnel module was mounted on the front bumper of each bus, and the full-flow CVS system from the crankcase vent was ducted directly into the tunnel. For each test run, Teflon and quartz samples were collected from the dilution tunnel. In addition, a third sample (alternately collected on Teflon and quartz filters) of the tunnel dilution air was collected upstream of the emission injection point in the tunnel to serve as a field blank. All filters were stored at −20 °C between sample collection and laboratory analyses (gravimetry and INAA for Teflon, OC/EC and GC/MS for quartz).
For each bus, samples were collected for three runs for tailpipe and crankcase emissions. Testing was conducted on three segments of the bus routes used in on-road sampling that were selected as being representative of average driving conditions over the full length of the two routes. Tailpipe emissions test runs ranged from 30 to 40 min each. Tailpipe tests ducted 0.6% of total exhaust flow into the tunnel with a dilution flow rate of 600 L/min, yielding a dilution equivalent to a full-flow CVS operating at 100 m3/min. Because this was the first test of its kind, crankcase emissions test durations were limited to 15–20 min for the first bus to prevent overloading of filters but were increased to 30 min each for the second bus. Tunnel dilution flows were set to 1500 L/min for the first two crankcase tests but were reduced to 800 L/min after it was determined that filter loading was not a problem.
Calculation of In-Cabin DPMtp and PMck Concentrations
The use of intentional tracers as quantitative markers for tailpipe and crankcase PM emissions allows direct calculation of DPMtp and PMck concentrations from in-cabin measurements. The mass ratios of these two parameters to the two tracers provide, in effect, a source profile for the two sources. Because there are no other sources of the tracers, the calculation of the two parameters' concentrations can be accomplished by solving a linear two-equation system:
The known values are the measured in-cabin concentrations of the tracers [Ir] and [d-alkane] and the mass:tracer ratios DPMtp :Ir, DPMtp :d-alkane, PMck :Ir, and PMck :dalkane. This approach explicitly accounts for the amounts of each tracer contributed by PM from both emission sources.
RESULTS
Source Testing
shows the summary results of emission testing of the two buses for tailpipe and crankcase emissions. The observed DPMtp ranged from 0.13 to 0.20 g/km, which is typical of expected rates for buses of this age and mileage. Bus 1 PMck averaged 0.028 g/km, or approximately 16% of the tailpipe emission rate. Crankcase emission rates for bus 2 were approximately 3 times higher, averaging 0.099 g/km, approximately 70% of the bus' tailpipe emission rate. The PM:tracer ratios were quite consistent for the primary tracer for each emissions source. For DPMtp:Ir ratios, the coefficients of variation (CV; the relative standard deviation across three samples for each bus) were 8%, and for the PMck:d-alkane ratio the CVs were 5% and 7%. For the two buses, the average DPMtp:d-alkane ratios were 10 and 24 times higher than the corresponding PMck:d-alkane ratio, and the PMck:Ir ratios were 500 and 1200 times higher than the corresponding DPMtp:Ir ratios. This suggests that, although there is some small amount of unburned lubricating oil being emitted from the tailpipe (in the range of 4–9% of mass emissions), there are virtually no diesel fuel combustion products in the crankcase emissions (<0.2%).
Table 1a. Tailpipe PM emission rates and PM:tracer mass ratios
Table 1b. Crankcase PM emission rates and PM:tracer mass ratios
For most sample pairs, there was good agreement between gravimetric and carbonaceous mass data. There was some evidence of vacuum-side sampling manifold leaks for 4 of the 24 total samples: 1 Teflon tailpipe sample, 1 quartz tailpipe sample, and 2 quartz crankcase samples. However, the effects of these problems were minimal because paired samples and observed consistency between filter pairs provided acceptable surrogates for the suspect data. The gravimetric mass for one bus 1 run showed tailpipe emission rates approximately one-half of the concurrent carbonaceous mass (estimated as EC + 1.2 × OC to reflect mass associated with hydrogen and other element fractions of OC). Other tailpipe tests showed gravimetric mass to be consistently approximately 7% higher than the carbonaceous mass. To avoid underestimating PM emissions for this run, the mass emission rate was estimated from the carbonaceous mass. The two suspect quartz crankcase samples had carbonaceous mass from 35 to 70% lower than gravimetric mass. All other crankcase samples showed gravimetric mass to be within 10% of the carbonaceous mass. Manifold leaks did not affect the PMck emission rates, which were determined from gravimetric analyses of Teflon filters. Although these possible leaks may have resulted in a sampler cut point larger than 2.5 μm, they are not expected to compromise the DPMtp:Ir or PMck:d-alkane because these were based on gravimetric and INAA analysis of the same Teflon filter and on OC/EC and GC/MS analysis of the same quartz filters. It is also possible that OC could include positive artifact mass,Citation24 resulting in some overestimation of actual carbonaceous mass.
The mass ratios of PM emissions to the tracers should ideally be calculated based on laboratory analyses of the same filter to avoid introducing errors because of sample volume or particle size cut point differences for two concurrent samples. PM:Ir ratios were calculated from the gravimetric and INAA analyses of individual Teflon filters. Because quartz filters were not analyzed gravimetrically, the PM mass loading of the quartz filters was calculated from the OC/EC data as the carbonaceous mass multiplied by the average ratio of gravimetric to carbonaceous mass. As noted above, the gravimetric:carbonaceous mass ratios averaged 1.07 for tailpipe sample pairs and 1.04 for crankcase samples. DPMtp:d-alkane ratios were calculated by multiplying carbonaceous mass:tracer ratios by 1.07. PMck:d-alkane ratios were calculated by multiplying carbonaceous mass:tracer ratios by 1.04.
The PM:tracer mass ratios were derived from several measurements. For the PM:tracer mass ratios, the CVs for the primary tracer ratios (DPMtp:Ir and PMck:dalkane) are indicative of the level of uncertainty carried in these parameters. DPMtp:Ir CVs were 8% for both buses, and the PMck:d-alkane CVs were 4 and 7% for the two buses. The gravimetric, INAA, and GC/MS analyses for source samples reported analytical uncertainties ranging from less than 1% (gravimetry) to 15–20% (dalkane). Reported EC and OC measurement uncertainties for source test filters were between 10 and 15%, and Ir measurement uncertainty was approximately 5%.
Fuel samples collected from each bus at the conclusion of testing were analyzed by INAA. This analysis showed the Ir spike level in both buses to be approximately 3.2 mg/L. Source tests for both buses showed the Ir emission rate to be only approximately 0.32 mg/L. Most of the Ir in the fuel (∼90%) appears to be captured in the engines or exhaust systems (which included diesel oxidation catalyst emission controls). This effect was noted, although to a lesser degree, in the single tracer study; however, that bus was not equipped with a diesel oxidation catalyst.Citation5 There are several possible mechanisms by which Ir could be captured, including being trapped in lubricating oil after making contact with cylinder walls; impaction on turbocharger blades; deposition on exhaust valves, manifolds, or exhaust pipes; and capture by the diesel oxidation catalyst. Once captured, Ir would not be collected on exhaust samples unless it were re-emitted in the PM2.5 size range.
The potential effect of this Ir loss cannot be definitively determined but would be expected to be small. It is possible that the DPMtp:Ir ratio varies with engine operating mode on a short-term basis. However, the time scale of engine operating transients is small (on the order of seconds), whereas the time scales of dispersion processes outside of the bus are substantially longer. Because the PM:tracer ratios were determined under typical operating conditions, such variability would be expected to have little effect on longer-term averages. Also, because the DPMtp:Ir ratio was empirically determined from source testing at the tailpipe, the loss of Ir does not affect the accuracy of estimates of DPMtp derived from on-road test data, and the use of such average ratios is consistent with current practices in receptor modeling.
In-Cabin Sampling
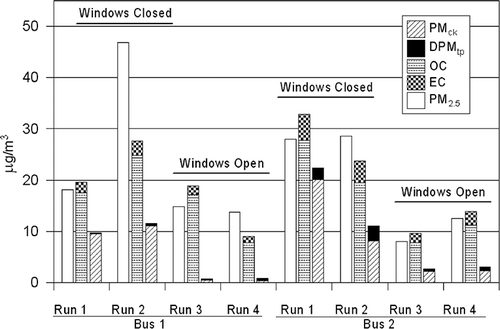
presents the mass- and tracer-based results for all samples, and shows the time-weighted run average in-bus concentrations. Because of time constraints, the Teflon and quartz filter pair for the reverse portion of one run (bus 1 run 4) could not be prepared, so two sample pairs were collected for forward and reverse routes.
Table 2. Bus 1 in-cabin PM2.5, carbonaceous PM, DPMtp, and PMck concentrations (μg/m3)
The observed PM2.5 concentrations in the buses and the LV averaged between approximately 14 and 22 μg/m3. This is substantially lower than in-vehicle concentrations reported in other areas (some in excess of 100 μg/m3).Citation1–3,Citation5 This is certainly due in part to the intentional selection of a light-traffic, residential route and the lower urban background concentrations in Seattle as compared with other areas. PM2.5 concentrations were roughly comparable between buses and between the buses and the LV. However, PM2.5 concentrations were lower in the bus than the LV when windows were open and higher when windows were closed. Concentrations of EC, OC, and other parameters exhibit different relationships, as discussed by Liu et al.Citation12 Tracer measurements on samples collected in the LV were insignificant with the exception of a 0.05-ng/m3 d-alkane concentration observed during the first bus 2 run. This was assumed to be the result of the bus at some point being close enough and upwind of the LV to generate a measurable level of tracer, so this value was not treated as actual background and was not subtracted during the course of the tracer analyses.
and present results for bus 1 and 2, respectively, and the averages of all tests are presented in . The right-most columns in these tables present the calculated carbonaceous mass, DPMtp, and PMck. The carbonaceous PM2.5 concentrations are close to, and in some cases higher than, the corresponding gravimetric mass. This is particularly apparent for the 2-hr samples for run 2 of bus 1 and for run 2 of bus 2. The generally close agreement between carbonaceous mass and PM2.5, and the generally large differences between carbonaceous mass and the mass explained by DPMtp and PMck, suggest that the dominant sources of in-cabin PM2.5 concentrations are emissions from other vehicles or other sources of carbonaceous material.
Table 3. Bus 2 in-cabin PM2.5, carbonaceous PM, DPMtp, and PMck concentrations (μg/m3)
Table 4. Average in-cabin PM2.5, carbonaceous PM, DPMtp, and PMck concentrations (μg/m3)
During testing, some difficulty was reported in loading and sealing filter cassettes, and it is possible that undetected leaks in the cassettes resulted. It is also possible that the carbonaceous mass estimates are influenced by positive OC artifact.Citation24 The bus 1 run 3 forward-route sample exhibited an abnormally high value for one of the four OC fractions, suggesting it may have been affected by contamination. Other than these three cases, PM2.5 and carbonaceous mass are in general agreement for all filter pairs. The 2-hr sample concentrations are often lower but in some cases higher than those of the corresponding 1-hr samples. There were not enough sampling locations in each bus to establish consistent patterns, but it was suspected that the observed differences were related to differences among the pathways by which different pollutants enter the bus. For example, because the PMck release point is at the front of the bus, its concentration gradients within the bus will differ from those of DPMtp (emitted at the rear bumper of the bus) and PM from other sources. This suggests that the different locations of the sampling equipment within the buses may have contributed to the observed differences between the 2-hr and the two 1-hr sampler concentrations.
Individual DPMtp sample concentrations in this study ranged from 0.24 to 3.7 μg/m3 with windows closed (averages of the 2-hr samples and two 1-hr samples across runs were of 0.33 and 2.6 μg/m3, respectively) and from 0.17 to 0.81 μg/m3 with windows open (averages of the 2-hr samples and two 1-hr samples across runs were 0.33 and 0.54 μg/m3, respectively). The averages of all DPMtp concentrations for the two buses were 0.33 and 1.5 μg/m3. PMck concentrations are substantially higher than those of DPMtp for both buses. Hill et al.Citation8 suggested this result. However, the magnitude is somewhat surprising considering the relative mass emission rates of the two sources, particularly for bus 1 (see ). With windows closed, PMck concentrations ranged from 6.8 to 23 μg/m3 (averages of 10 and 14 μg/m3 for bus 1 and 2, respectively), and from 0.2 to 2.9 μg/m3 with windows open (averages of 0.4 and 2.3 μg/m3 for bus 1 and 2, respectively). The overall average PMck concentrations for the two buses were 5.3 and 8.2 μg/m3—approximately 16 and 5 times greater than the corresponding DPMtp concentrations, respectively. Factors likely to contribute to this result include (1) the crankcase vent location inside of the engine compartment, below the engine; (2) the tailpipe location at the rear of the bus; and (3) the substantial positive air pressure gradient from inside of the engine compartment to inside of the bus while driving, resulting in enhanced flow of air from the engine compartment through the firewall in spaces left for engine and bus controls. The passenger door at the front right of the cabin is also a possible point of entry for crankcase emissions that is well away from the tailpipe. Intrusion via the firewall and the passenger door would also be likely to carry emissions from vehicles ahead of the bus and re-entrained road dust.
In-cabin PM2.5 concentrations from all sources averaged 10 μg/m3 with windows open and 26 μg/m3 with windows closed. As expected, these over-roadway concentrations are higher than typical ambient concentrations in Seattle but are low when compared with other large urban areas. For example, in-bus PM2.5 concentrations during the original Los Angeles Ir studyCitation5 averaged 72 μg/m3.
As for source testing, PM2.5, OC/EC, DPMtp, and PMck concentrations in the buses are subject to uncertainties in PM:tracer mass ratios, total sample volumes, and the individual analyses. Again, a 5% uncertainty can be assumed for sample volumes, and gravimetric and INAA analysis reported uncertainties were also approximately 5%. OC uncertainties were between 5 and 10%, and EC uncertainties were between 10 and 15%.Citation13 Uncertainties reported for d-alkane were 15–20% for most measurements,Citation13 increasing to 25% for some of the lower concentrations (open-window runs).
A question has been raised regarding the validity of the Ir tracer method because of possible suppression of DPM formation by Ir in the fuel because no emissions testing of the bus was conducted with unspiked fuel.Citation25 However, Clark et al.Citation26 conducted engine dynamometer testing using fuel with and without the Ir tracer and found that the tracer had no measurable effect on DPM emission rates (the final report is published at http://secure.awma.org/onlinelibrary/samples/10.3155-1047-3289.61.5.494_supplmaterial.pdf). Further, although busspecific DPMtp:Ir ratios were used to calculate in-cabin DPMtp concentrations for the specific buses tested, the Ir measurements themselves are definitive indicators of the amount of exhaust intrusion into the buses. They can be used to calculate the expected in-cabin DPMtp concentrations for any assumed DPM emission rate.
DISCUSSION
The results of this study are consistent with those of previous studies in showing that the highest in-cabin PM2.5 concentrations occur when buses are operated with windows closed. However, the DPMtp results of this and the previous Ir study differ from other results with regard to estimates of exhaust intrusion into school bus cabins. The 0.33- and 1.5-μg/m3 DPMtp concentrations found in the Seattle study are higher than the 0.22-μg/m3 average observed in the first Ir tracer study of a single Los Angeles bus.Citation5 Nevertheless, they are still substantially lower than estimates derived using concentrations of nonspecific markers or a tracer for which the relationship to DPMtp was variable and unquantified.Citation4,Citation7 This discrepancy is attributed to the inadvertent misattribution of some fraction of nonspecific indicator species (e.g., black carbon [BC]) to the bus, when it actually came from another source, or to undetected instrument calibration bias.Citation12 The substantially larger contribution of PMck, despite its lower emission rates, confirms the identification by Hill et al.Citation8 of crankcase emissions as potentially significant. The testing of the two Seattle buses does not fully resolve questions of representativeness of buses tested, but the low-wind, low-speed, high engine load test conditions encountered in Seattle are arguably more likely to result in high levels of DPMtp in bus cabins. Further study of additional buses in different settings is needed to determine whether the tracer-based findings to date are representative of the full range of in-cabin DPMtp and PMck concentrations.
Larson et al.Citation27,Citation28 used this study's data to explore the use of receptor modeling techniques for tailpipe and crankcase emissions, using OC, EC, and GC/MS-derived marker species including hopanes and steranes as potential tracers of opportunity for lubricating oil. In the evaluation of inferential methods against the tracer results presented here, Liu et al.Citation12 found that under the conditions of these tests, differential concentrations between in-bus and LV measurements of nonspecific markers such as EC, OC, and PM1 (PM ≤ 1 μm in aerodynamic diameter) may provide some useful information regarding self-pollution, although with a positive bias of up to 20%. In the absence of definitive evidence to the contrary, such approaches cannot be considered reliable or accurate without concurrent verification by tracers. The dual tracer method allowed explicit identification of a single engine's emissions at very low detection limits. The authors are unaware of any other method capable of accurately quantifying tailpipe and crankcase self-pollution in school buses or for addressing similar source attribution problems.
SUMMARY
Two conventional front-engine school buses were tested in a relatively clean urban setting using separate intentional tracers for diesel exhaust and crankcase vent PM. The results for in-bus concentrations of its tailpipe and crankcase vent emissions showed self-pollution to be higher with windows closed, and despite their lower emission rate, crankcase PM concentrations were substantially higher than those of tailpipe exhaust PM. The results also confirmed the specificity and sensitivity achievable by this method. The complete absence of ambient interferences in the Ir and d-alkane analyses and the sensitivities of analytical methods for these species made it possible to unambiguously quantify DPMtp and PMck concentrations as low as 0.002 and 0.05 μg/m3, respectively. For comparison, detection limits for EC or BC, the most commonly used indicator of DPMtp, are typically 0.1 μg/m3 or greater. Before this study, no methods were available to quantitatively distinguish PMck concentrations from those of similar carbonaceous PM sources.
The use of high flow rate in-cabin samplers allowed an approximately 6-fold increase in sensitivity relative to the standard 16.7-L/min samplers. The use of the RAVEM system allowed emissions testing under actual bus operating conditions. The samplers and the RAVEM system generally performed well, but they exhibited occasional evidence of leaks. Although some in-bus and source samples were potentially compromised by these leaks, a sufficient number of samples were collected, and there was sufficient redundancy of samples (including concurrent Teflon and quartz sampling) to generate acceptable surrogates for suspect values and to establish the validity of the overall findings of the field program.
It should be noted that future applications of the dual tracer method may not require the detection limits achieved here. Options include using lower flow rate in-cabin samplers, reducing sample durations, and reducing the tracer spike levels. In particular, the d-alkane tracer is extremely expensive. If lower sensitivity is acceptable, material cost savings from reducing the spike level in lubricating oil could be substantial, especially if multiple engines/vehicles are being tested. Also, if appropriate, quartz filter extractions could be composited to reduce GC/MS analytical costs.
The use of the d-alkane lubricating oil tracer provided clear confirmation and quantification of the apparent significance of PMck intrusion in bus cabins, as initially reported by Hill et al.Citation8 Although PMck is not diesel exhaust, in-vehicle concentrations of particles from all sources are of concern. PMck concentrations in the buses were disproportionately higher than DPMtp based on a comparison of the emission rates of the two pollutants. This suggests that the location of the crankcase vent may be an important factor in the amount of intrusion that occurs.
Supplementary Material
Download Zip (385.4 KB)ACKNOWLEDGMENTS
This study was partially sponsored by the National Institute of Environmental Health Sciences (1R01ES12657-01A1). Inclusion of the dual tracer experiments was made possible by funding provided by Navistar, Inc., and the U.S. Department of Energy Office of Vehicle Technologies through the National Renewable Energy Laboratory. The assistance of David Anderson of the Seattle School District Transportation Department, First Student, Inc., and the Puget Sound Clean Air Agency is gratefully acknowledged.
REFERENCES
- Rodes, C.; Sheldon, L.; Whitaker, D.; Clayton, A.; Fitzgerald, K.; Flanagan, J.; DiGenova, F.; Hering, S.; Frazier, C. Measuring Concentrations of Selected Air Pollutants inside California Vehicles; Research Triangle Institute: Research Triangle Park, NC, 1998 http://www.arb.ca.gov/research/abstracts/95-339.htm (http://www.arb.ca.gov/research/abstracts/95-339.htm) (Accessed: 2011 ).
- Fruin , S.A. , Winer , A. and Rodes , C. 2004 . Black Carbon Concentrations in California Vehicles and Estimation of In-Vehicle Diesel Exhaust Particulate Matter Exposures . Atmos. Environ. , 38 : 4123 – 4133 .
- Wargo , J. 2002 . Children's Exposure to Diesel Exhaust on School Buses , North Haven , CT : Environment and Human Health .
- Solomon , G.M. , Campbell , T. , Feuer , G. , Masters , J. , Samkian , A. and Paul , K. 2001 . No Breathing in the Aisles , New York : Natural Resources Defense Council and Coalition for Clean Air .
- Ireson, R.G.; Easter, M.; Lakin, M.; Ondov, J.; Clark, N.; Wright, D. Estimation of Diesel Particulate Concentrations in a School Bus Using a Fuel-Based Tracer: a Sensitive and Specific Method for Quantifying Vehicle Contributions; Trans. Res. Record 2004, 1880, 21–28 http://trb.metapress.com/home/main.mpx (http://trb.metapress.com/home/main.mpx) (Accessed: 2011 ).
- Fitz , D.R. , Winer , A. , Colome , S. , Behrentz , E. , Sabin , L. , Lee , S. , Wong , K. , Kozawa , K. , Pankratz , D. , Bumiller , K. , Gemmill , D. and Smith , M. 2003 . Characterizing the Range of Children's Pollutant Exposure during School Bus Commutes , Riverside , CA : University of California .
- Behrentz , E. , Fitz , D. , Pankratz , D. , Sabin , L. , Colome , S. , Fruin , S. and Winer , A. 2004 . Measuring Self-Pollution in School Buses Using a Tracer Gas Technique . Atmos. Environ. , 38 : 3735 – 3746 .
- Hill , L.B. and Gooch , J. 2009 . “ Exposures to Particulate Matter in Conventional and Retrofit School Buses in Five U.S. Cities ” . In Proceedings of A&WMA's 102nd Annual Conference & Exhibition , Pittsburgh , PA : A&wma . Paper A-363
- Estimated Concentrations of Diesel Particulates Inside a School Bus Based on a Tracer Added to its Fuel; California Ensight: Walnut Creek, CA, 2003 http://www.greendieseltechnology.com/tracer_091003.asp (http://www.greendieseltechnology.com/tracer_091003.asp) (Accessed: 2011 ).
- Wu , C.C. , Suarez , A. , Lin , Z.-B. , Kidwell , C. , Borgoul , P. , Caffrey , P. , Ondov , J. and Sattler , B. 1998 . Application of an Ir Tracer to Determine Soot Exposure to Students Commuting to School on Baltimore Public Buses . Atmos. Environ. , 32 : 1911 – 1919 .
- Suarez , A.E. , Caffrey , P.F. , Borgoul , P.V. , Ondov , J.M. and Divita Jr , F. 1998 . Use of an Ir Tracer to Determine the Size Distribution of Aerosol Emitted from a Fleet of Diesel Sanitation Trucks . Environ. Sci. Technol. , 32 : 1522 – 1529 .
- Liu , L.-J. , S; Phuleria , H. , Webber , W. , Davey , M. , Lawson , D. , Ireson , R. , Zielinska , B. , Ondov , J. , Weaver , C. , Lapin , C. , Easter , M. , Hesterberg , T. and Larson , T. 2010 . Quantification of Self-Pollution from Two Diesel School Buses Using Three Independent Methods . Atmos. Environ. , 44 : 3422 – 3431 .
- Zielinska , B. , Campbell , D. , Lawson , D. , Ireson , R. , Weaver , C. , Hesterberg , T. , Davey , M. , Larson , T. and Liu , S. 2007 . Detailed Characterization and Profiles of Crankcase and Diesel Particulate Matter Exhaust Emissions Using Speciated Organics . Environ. Sci. Technol. , 42 : 5661 – 5666 .
- Lin , Z.C. , Ondov , J. , Kelly , W. and Stevens , R. 1992 . Tagging Diesel and Residential Oil Furnace Emissions in Roanoke, VA, with Enriched Isotopes of Samarium . Journal of the Air & Waste Management Association , 42 : 1057 – 1062 .
- Ondov , J.M. and Kelly , W. 1991 . Developing a New Tracer for Atmospheric Particles with Stable Rare Earth Isotopes . Anal. Chem. , 63 : 691A – 697A .
- Ondov , J.M. 1996 . Particulate Tracers for Source Attribution: Potential for Application to California's San Joaquin Valley . J. Aerosol Sci. , 27 : S687
- Horvath , H. , Kreiner , I. , Norek , C. and Preining , O. 1988 . Diesel Emissions in Vienna . Atmos. Environ. , 22 : 1255 – 1269 .
- Heller-Zeisler , S.F. , Borgoul , P. , Moore , R. , Smoliar , M. , Suarez , A. and Ondov , J. 2000 . Comparison of INAA and RNAA Methods with Thermal-Ionization Mass Spectrometry for Iridium Determinations in Atmospheric Tracer Studies . J. Radioanal. Nuc. Chem. , 244 : 93 – 96 .
- Marple , V.A. , Willeke , K. and Design , Impactor . 1976 . Atmos. Environ. , 10 : 891 – 896 .
- Weaver , C.S. and Petty , L. 2004 . Reproducibility and Accuracy of On-Board Emission Measurements Using the RAVEM System; Society of Automotive Engineers (SAE) Paper No. 2004-01-0965 , Warrendale , PA : SAE .
- Weaver , C.S. and Balam-Almanza , M.V. 2001 . Development of the “RAVEM” Ride-Along Vehicle Emission Measurement System for Gaseous and Particulate Emissions; Society of Automotive Engineers (SAE) Paper No. 2001-01-3644 , Warrendale , PA : SAE .
- Correlation between the Mexico City Government's RAVEM System and West Virginia University's Transportable Heavy Duty Emissions Laboratory; Report for Contract No. GDF-SMA-GEF-SC-027-06 to the Mexico City Secretariat of Environment; Engine, Fuel, and Emissions Engineering: Rancho Cordova, CA, 2006 http://www.efee.com/download/RAVEM-WVU%20Correlation%20Report.pdf (http://www.efee.com/download/RAVEM-WVU%20Correlation%20Report.pdf) (Accessed: 2011 ).
- Traver , M.L. , Tennant , C.J. , McDaniel , T.I. , McConnell , S.S. , Bailey , B.K. and Maldonado , H. 2002 . Interlaboratory Cross-Check of Heavy-Duty Vehicle Chassis Dynamometers. Society of Automotive Engineers (SAE) Paper No. 2002-01-2879 , Warrendale , PA : SAE .
- McDow , S.R. and Huntzicker , J. 1990 . Vapor Adsorption Artifact in the Sampling of Organic Aerosol: Face Velocity Effect . Atmos. Environ. , 24 : 2563 – 2571 .
- Behrentz , E. , Sabin , L. , Winer , A. , Fitz , D. , Pankratz , D. , Colome , S. and Fruin , S. 2006 . Reply to Comment on “Measuring Self-Pollution in School Buses Using a Tracer Gas Technique” . Atmos. Environ. , 40 : 5325 – 5327 .
- Clark , N. , Krishnamurthy , M. , Wayne , W.S. , Gautam , M. , McKain , D. , Thompson , G. and Lyons , D. 2011 . Final Report: Effect of Tracer on Diesel Engine PM Emissions; OSP Reference No. 07–080 , Morgantown , WV : Department of Mechanical and Aerospace Engineering; West Virginia University .
- Larson, T.; Webber, W.; Zielinska, B.; Ireson, R.; Liu, S. Source Apportionment of PM2.5 inside Two Diesel School Buses Using Weighted Partial Least Squares Regression and Chemical Mass Balance. In Proceedings of A&WMA's 102nd Annual Conference & Exhibition; A&WMA; Pittsburgh, PA, 2009; Abstract 704.
- in press . Squares Discriminant Analysis with Chemical Mass Balance . Atmos. Pollut. Res. ,