Abstract
The mood-improving effect of sleep deprivation (SD) in depression is even today still not fully understood. Despite the fact that mood and cognitive functions are lowered by prolonged sleep loss and despite convincing data that insomnia is a strong risk factor for subsequent depression,Citation1 acute SD for one night or even partial SD in the second half of the night improves mood in about 60% of depressed patients the day after.Citation2,Citation3 In this respect, among alt types of antidepressant treatments, SD elicits the fastest results, faster even than electroconvulsive therapy. Many authors correlate the likelihood of responding to SD with clinical variables. A summary of predictors is listed in Table I.
The main limitation is the transient nature of the effect, since the majority - but not all - of the improved patients experience a relapse after the next night of sleep.Citation2
Despite the rapid effects and low risk of relevant side effects (Table I),Citation2-Citation9 the method has remained an “orphan drug” or “orphan method.” This may be explained not only by the effort and motivation needed by the patient and by the frequent relapses after the next night of sleep, but also by the lack of funding for nonpharmacological and nonneurochemical research. Nevertheless, some progress has been made within the last few years. A variety of studies have focused on the problem of how to avoid relapses occurring after the next night of sleep and additionally treated the patients with light therapy, lithium, or other drugs. Lower relapse rates after SD were found when SD was combined with one of these therapeutic options (Table II).Citation10-Citation20
Table I. Clinical predictors of an antidepressant response to sleep deprivation (SD) in depressed subjects and side effects. *Not based on systematic documentation.
Table II. Therapeutic strategies to avoid relapses after successful sleep deprivation in depression (selected papers).
A further strategy has been to advance the sleep period to an ”unphysiological“ time. Several uncontrolled studies in small numbers of patients have indicated that this phase advance procedure per se acts as an antidepressant. More recent studies have combined SD with a subsequent phase advance of the sleep period, over the course of either six or three nights and consistently found that a phase advance of the sleep period stabilizes the antidepressant effect of SD in about 60% of those patients who responded positively to SD.Citation17-Citation20 Only one study also included a control group which participated in a phase-delay protocol after SD instead of a phaseadvance protocol.Citation18 Significantly more patients relapsed in the phase-delay protocol compared with the phase advance protocol (). This indicates that the high response rate after SD and phase advance cannot be explained by a placebo phenomenon alone and supports the hypothesis that, in depressed subjects, sleeping at certain phases of the circadian rhythm, ie, especially late in the night and in the morning, has depressogenic effects. Unfortunately, one major issue has been almost completely neglected by researchers: docs SD produce any lasting effects after 4 to 6 weeks, which is the typical period for measuring the effects of antidepressants? There is only one controlled study using such a design.Citation21 Twenty-four patients received amitriptylinc without additional SD, whereas 27 patients received amitriptyline plus a series of 6 partial SDs. Observer ratings, but not patient ratings, demonstrated superiority of the combined treatment after 4 weeks. By the standards of evidence -based medicine, there is little evidence to date that SD therapy has lasting effects over the course of several weeks.
![Figure 1 Antidepressant effects of total sleep deprivation (TSD) in one night with a consecutive phase advance of the sleep period (blue circles) in comparison with a phase delay of the sleep period (gray circles). In the phase-advance group, the antidepressant effect of SD (between day 0 and day 1 ) was stabilized until day 8, whereas in the phase-delay group mood worsened again (mood was measured by a short version of the Hamilton Depression Rating Scale [HDRS], containing 6 items). This scale is suitable for frequent ratings, whereas the 21 -item HDRS would not have been adequate within the study design. Citation18](/cms/asset/e1a939ee-e62b-455a-bae0-8f15358415fc/tdcn_a_12130537_f0001_oc.jpg)
Neurobiology of SD in depression
There is no generally accepted hypothesis concerning the mechanism of action of SD, nor an explanation for the observation that subsequent sleep after SD leads to relapses. A variety of neurobiological effects point toward potential mechanisms of action of the procedure (Table III).Citation22-Citation32
Table III. Neurobiological effects of sleep deprivation. In humans some of the studies were performed in depressed patients, while other studies were in healthy subjects or in depressed patients and healthy subjects.
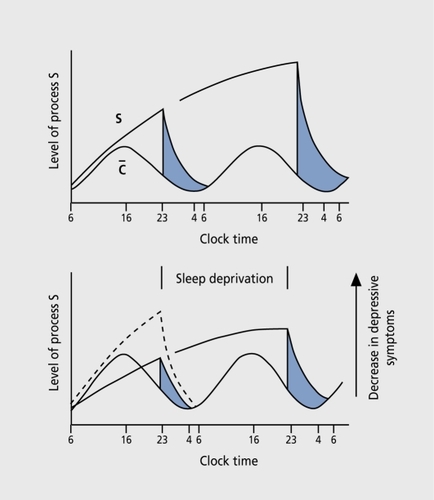
Based on the observations that hyperarousal and a high level of activation predict a favorable SD response,Citation4 the antidepressant effect was explained using the two-process model of sleep regulation ().Citation33 In this model, depressed patients have a deficiency of process S (ie, sleep need) with process C (circadian rhythm) remaining unaffected. Depression is characterized by a deficient build-up of process S (Figure 2) . SD transiently leads to an increase in process S to normal, whereas relapse occurs after “recovery sleep” due to a return to low levels of S.
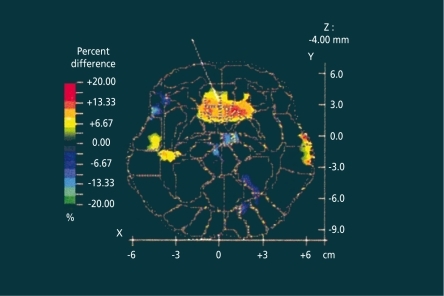
Several brain imaging studies have tried to correlate the SD response with metabolic states of certain brain areas. Two early studies using single photon emission computed tomography (SPECT)Citation22 and positron emission tomography (PPT),Citation23 respectively, found higher metabolic rates in limbic areas in responders compared with nonresponders. A more recent studyCitation24 confirmed these earlier findings: responders to SD had higher relative metabolic rates in the ventral anterior cingulate and in the medial prefrontal cortex (), as well as in the posterior subcallosal gyrus at baseline than depressed patients who did not respond to SD and normal volunteers. After SD, significant decreases in metabolic rates occurred in the medial prefrontal cortex and frontal pole in the patients who responded positively to SD. The brain imaging studies convincingly demonstrated that acute antidepressant SD is able to change metabolic states of brain areas that are involved in mood regulation.
Many studies have assessed endocrine parameters before and after SD. The results have been inconsistent, which may be partially explained by methodological shortcomings. Several authors favor the hypothesis that the hypothalamo-pituitary-thyroid (HPT) axis plays a key role in mediating the antidepressant effects of SD.Citation31,Citation32
Another issue is the impact of SD on the hypothalamo-pituitary-adrenal (HPA) axis. Increased activity of this axis is one of the most consistent abnormalities in depression and normalization of this hyperactivity is a correlate of clinical remission and has been suggested as the mechanism of action of antidepressant treatment.Citation34 In healthy humans, acute SD increases Cortisol secretion.Citation28,Citation29 In a study that we conducted ourselves, we found a significant stimulatory effect of acute SD on nighttime Cortisol in a group of unmedicated depressed subjects, which was not related to treatment response.Citation30 However, during the first half of the day after the night, SD responders in contrast to nonresponders had higher Cortisol concentrations compared with the day before SD. This finding does not necessarily contradict the above relationship between depression and HPA axis hyperactivity for two reasons. First, the acute effects of antidepressant treatments on the HPA axis may differ from the chronic effects. It has been shown that electroconvulsive treatment and antidepressants also initially stimulate the HPA axis. Second, two studies demonstrated acute antidepressant effects of Cortisol infusion compared with placebo.Citation35,Citation36
Another theory that possibly provides a link to the HPA effects of SD focuses on the psychostimulant effects. Earlier studies reported an increase in dopamine, norepinephrine, and serotonin after SD, ie, similar neurobiological effects as after the intake of psychostimulants like amphetamines (see reference 25 for an overview). Support for a psycho-stimulant theory also comes from brain imaging data, demonstrating effects of psychostimulants such as amphetamines on metabolic rates similar to those observed in SD.Citation37 Since there is a functional coupling of psychostimulant effects and the HPA axis,Citation38 a Cortisol increase following SD might therefore mediate psychostimulant-like actions of increased aminergic neurotransmitter release.
In summitry, the SD response in depressive patients remains a highly interesting issue Jor depression research, since, contrary to all antidepressant drugs, it may significantly ameliorate mood within one day. Understanding this effect and optimizing the duration of the effect, ie, preventing relapse after the response, might improve our ability to treat depression.
REFERENCES
- RiemannD.VoderholzerU.Primary insomnia: a risk factor to develop depression?J Affect Disord.200376247251
- WuJC.BunneyWE.The biological basis of an antidepressant response to sleep deprivation and relapse: review and hypothesis.Am J Psychiatry.199014714212403471
- Van den HoofdakkerRH.Total sleep deprivation: clinical and theoretical aspects. In: Honig A, van Praag HM, eds.Depression: Neurobiological, Psychopathological and Therapeutic Advances. Chichester, UK: John Wiley & Sons Ltd;1997563589
- Bouhuysvan den BurgW.van den HoofdakkerRH.The relationship between tiredness prior to sleep deprivation and the antidepressant response to sleep deprivation in depression.Biol Psychiatry.1995374574617786959
- GordijnMC.BeersmaDG.BouhuysReininkE.Van den HoofdakkerRH.A longitudinal study of diurnal mood variation in depression; characteristics and significance.J Affect Disord.1994312612737989641
- ReininkE.BouhuysN.Wirz-JusticeA.van den HoofdakkerR.Prediction of the antidepressant response to total sleep deprivation by diurnal variation of mood.Psychiatry Res.1990321131242367598
- BarbiniB.ColomboC.BenedettiF.CamporiE.BellodiL.SmeraldiE.The unipolar-bipolar dichotomy and the response to sleep deprivation.Psychiatry Res.19987943509676825
- ColomboC.BenedettiF.BarbiniB.CamporiE.SmeraldiE.Rate of switch from depression into mania after therapeutic sleep deprivation in bipolar depression.Psychiatry Res.19998626727010482346
- BenedettiF.ZanardiR.ColomboC.SmeraldiE.Worsening of delusional depression after sleep deprivation: case reports.J Psychiatr Res.199933697210094242
- ElsengaS.van den HoofdakkerRH.Clinical effects of sleep deprivation and clomipramine in endogenous depression.J Psychiatr Res.1982-8317361747187779
- Baxter LRJr.ListonEH.SchwartzJM.et al.Prolongation of the antidepressant response to partial sleep deprivation by lithium.Psychiatry Res.19861917233097691
- GrubeM.HartwichP.Maintenance of antidepressant effect of sleep deprivation with the help of lithium.Eur Arch Psychiat Neurol Sci.19902406061
- SzubaMP.Baxter LRJr.AltshulerLL.et al.Lithium sustains the acute antidepressant effects of sleep deprivation: preliminary findings from a controlled study.Psychiatry Res.1994512832958208874
- SmeraldiE.BenedettiF.BarbiniB.CamporiE.ColomboC.Sustained antidepressant effect of sleep deprivation combined with pindolol in bipolar depression. A placebo-controlled trial.Neuropsychopharmacology.19992038038510088139
- NeumeisterA.GoesslerR.LuchtM.KapitanyT.BamasC.KasperS.Bright light therapy stabilizes the antidepressant effect of partial sleep deprivation.Biol Psychiatry.19963916218719121
- ColomboC.LuccaA.BenedettiF.BarbiniB.CamporiE.SmeraldiE.Total sleep deprivation combined with lithium and light therapy in the treatment of bipolar depression: replication of main effects and interaction.Psychiatry Res.200095435310904122
- BergerM.VollmannJ.HohagenF.et al.Sleep deprivation combined with consecutive sleep phase advance as a fast-acting therapy in depression: an open pilot trial in medicated and unmedicated patients.Am J Psychiatry.19971548708729167521
- RiemannD.KönigA.HohagenF.et al.How to preserve the antidepressant effect of sleep deprivation: a comparison of sleep phase advance and sleep phase delay.Eur Arch Psychiatry Clin Neurosci.199924923123710591988
- BenedettiF.BarbiniB.CamporiE.FulgosiMC.PontiggiaA.ColomboC.Sleep phase advance and lithium to sustain the antidepressant effect of total sleep deprivation in bipolar depression: new findings supporting the internal coincidence model?J Psychiatr Res.20013532332911684139
- VoderholzerU.ValeriusG.SchaererL.et al.Is the antidepressive effect of sleep deprivation stabilized by a three day phase advance of the sleep period? A pilot study.Eur Arch Psychiatry Clin Neurosci.2003253687212799743
- KuhsH.FärberD.BorgstädtS.MrosekS.ToileR.Amitriptyline in combination with repeated late sleep deprivation versus amitriptyline alone in major depression. A randomised study.J Affect Disord.19963731418682976
- EbertD.FeistelH.BarockaA.Effects of sleep deprivation on the limbic system and the frontal lobes in affective disorders: a study with Tc-99mHMPAO SPECT.Psychiatry Res.1991402472511811242
- WuJC.GillinJC.BuchsbaumMS.HersheyT.JohnsonJC.Bunney WEJr.Effect of sleep deprivation on brain metabolism of depressed patients.Am J Psychiatry.19921495385431554042
- WuJ.BuchsbaumMS.GillinJC.et al.Prediction of antidepressant effects of sleep deprivation by metabolic rates in the ventral anterior cingulate and medial prefrontal cortex.Am J Psychiatry.19991561149115810450253
- EbertD.BergerM.Neurobiological similarities in antidepressant sleep deprivation and psychostimulant use: a psychostimulant theory of antidepressant sleep deprivation.Psychopharmacology.1998401109862396
- DingesDF.DouglasSD.HamarmanS.ZauggL.KapoorS.Sleep deprivation and human immune function.Adv Neuroimmunol.19955971107496616
- VoderholzerU.HohagenF.HerrA.et al.Effects of sleep deprivation on cytokines in healthy and depressed subjects. In: Sperner-Unterweger B, Fleischhacker WW, Kaschka WP, eds.Psychoneuroimmunology Hypotheses and Current Research. Advances in Biological Psychiatry. Basel, Switzerland: Karger;.20012098109
- LeproultR.CopinschiG.BuxtonO.Van-CauterE.Sleep loss results in an elevation of Cortisol levels the next evening.Sleep.1997208658709415946
- SpiegelK.LeproultR.Van-CauterE.Impact of sleep debt on metabolic and endocrine function.Lancet.19993541435143910543671
- VoderholzerU.WeskeG.KleinT.et al.Endocrine studies during sleep, sleep deprivation, and recovery sleep in depressed patients.Neuropsychopharmacology.200023(suppl 2)S82S83
- ParekhPI.KetterTA.AltshulerL.et al.Relationships between thyroid hormone and antidepressant responses to total sleep deprivation in mood disorder patients.Biol Psychiatry.1998433923949513756
- OrthDN.SheltonRC.NicholsonWE.et al.Serum thyrotropin concentrations and bioactivity during sleep deprivation in depression.Arch Gen Psychiatry.200158778311146761
- BorbelyAA.Wirz-JusticeA.Sleep, sleep deprivation and depression.Hum Neurobiol.198212052107185793
- HolsboerF.BardenN.Antidepressants and hypothalamic-pituitaryadrenocortical regulation.Endocr Rev.1996171872058706631
- GoodwinGM.MuirWJ.SecklJR.et al.The effects of Cortisol infusion upon hormone secretion from the anterior pituitary and subjective mood in depressive illness and in controls.J Affect Disord.19922673831332993
- DeBattistaC.PosenerJA.KalehzanBM.SchatzbergAF.Acute antidepressant effects of intravenous hydrocortisone and CRH in depressed patients: a double-blind, placebo-controlled study.Am J Psychiatry.20001571334133710910802
- VolkowND.WangGJ.FowlerJS.et al.Gender differences in cerebellar metabolism: text-retest reproducibility.Am J Psychiatry.199715450558988958
- MarinelliM.Rouge-PontF.DérocheV.et al.Glucocorticoids and behavioral effects of psychostimulants. I: Locomotor response to cocaine depends on basal levels of glucocorticoids.J Pharmacol Exp Ther.1997281139214009190875