Abstract
Astrocytes are the main neural cell type responsible for the maintenance of brain homeostasis. They form highly organized anatomical domains that are interconnected into extensive networks. These features, along with the expression of a wide array of receptors, transporters, and ion channels, ideally position them to sense and dynamically modulate neuronal activity. Astrocytes cooperate with neurons on several levels, including neurotransmitter trafficking and recycling, ion homeostasis, energy metabolism, and defense against oxidative stress. The critical dependence of neurons upon their constant support confers astrocytes with intrinsic neuroprotective properties which are discussed here. Conversely, pathogenic stimuli may disturb astrocytic function, thus compromising neuronal functionality and viability. Using neuroinflammation, Alzheimer's disease, and hepatic encephalopathy as examples, we discuss how astrocytic defense mechanisms may be overwhelmed in pathological conditions, contributing to disease progression.
Los astrocitos constituyen el principal tipo celular neural responsable del mantenimiento de la homeostasis cerebral. Ellos forman áreas anatómicas altamente organizadas que están interconectadas en extensas redes. Estas caracierísticas, junto con la expresión de una gran variedad de receptores, transportadores y canales iónicos, los favorece de manera ideal para detectar y modular dínámicamente la actívídad neuronal. Los asirocitos cooperan con las neuronas a varios níveles, incluyendo el tránsito y reciclaje de neurotransmisores, la homeostasis iónica, la neuroenergética y la defensa contra el estrés oxidativo. Las neuronas dependen en forma crítica de su soporte constante, lo que le confiere a los astrocitos propiedades neuroprotectoras intrinsecas, las cuales también se discuten aqui. A la inversa, los estímulos patogénicos pueden alterar la función astrocítica, comprometiendo así la funcionalidad y la viabilidad neuronal. Se utilizan como ejemplos la neuroinflamación, la Enfermedad de Alzheimer y la encefalopatía hepática para discutir cómo los mecanismos de defensa de los astrocitos pueden estar sobrepasados en las condiciones patológicas, lo que contribuye a la progresión hacia la enfermedad.
Les astrocytes sont le principal type de cellules neuronales responsables de l'entretien de l'homéostasie cérébrale. Ils s'interconnectent en réseaux étendus, formant des régions anatomiques très organisées. Cette organisation qui s'accompagne de toute une série de récepteurs, transporteurs et canaux ioniques, les met en position idéale pour pressentir et moduler de façon dynamique l'activité neuronale. Les astrocytes coopèrent avec les neurones à différents niveaux, dont le recyclage et la circulation des neurotransmetteurs, l'homéostasie ionique, la neuroénergétique et la défense contre le stress oxydant. Les neurones sont très dépendants du soutien constant des astrocytes, ce qui donne à ces derniers des propriétés neuroprotectrices que nous analysons dans cet article. À l'opposé, lorsque des stimuli pathogènes troublent la fonction astrocytaire, la fonctionnalité et la viabilité des neurones sont compromises. En prenant pour exemples la neuro-inflammation, la maladie d'Alzheimer et l'encéphalopathie hépatique, nous montrerons comment les mécanismes de défense astrocytaires peuvent être débordés en situation pathologique, participant ainsi à la progression de la maladie.
In the last two decades, intense research efforts aiming to provide a better understanding of astroglial cell function have revealed a number of previouslyunsuspected roles for these neural cells, which were long considered as relatively passive structural elements of the brain. It has now become quite clear that a plethora of cooperative metabolic processes and interdependencies exist between astrocytes and neurons. As a result of the growing appreciation of the role of astrocytes in both the normal and diseased brain, the traditional neuroncentric conception of the central nervous system (CNS) has been increasingly challenged.
Astrocytes are territorial cells: they extend several processes with little overlap between adjacent cells, forming highly organized anatomical domainsCitation1-Citation3 which are interconnected into functional syncytia via abundant gap junctions.Citation4 These astrocytic processes closely ensheath synapses and express a wide range of receptors for neurotransmitters, cytokines, and growth factors, as well as various transporters and ion channels.Citation5-Citation11 In addition, astrocytes project specialized astrocytic endfeet which are in close contact with intraparenchymal blood vessels, almost entirely covering their surface.Citation12,Citation13 Together, these cytoarchitectural and phenotypical features ideally position astrocytes to fulfill a pivotal role in brain homeostasis, allowing them not only to sense their surroundings but also to respond to - and consequently modulate - changes in their microenvironment. Indeed, astrocytes can respond to neurotransmitters with transient increases in their intracellular Ca2+ levels, which can travel through the astrocytic syncytium in a wavelike fashion.Citation14,Citation15 These Ca2+ signals can trigger the release of neuroactive molecules from astrocytes (or gliotransmitters), such as glutamate, D-serine, or adenosine triphosphate (ATP) which in turn modulate synaptic activity and neuronal excitability (see ref 16 for review). This process, for which the term “gliotransmission” has been coined, marks the emergence of an exciting new notion that information processing may not be a unique feature of neurons.
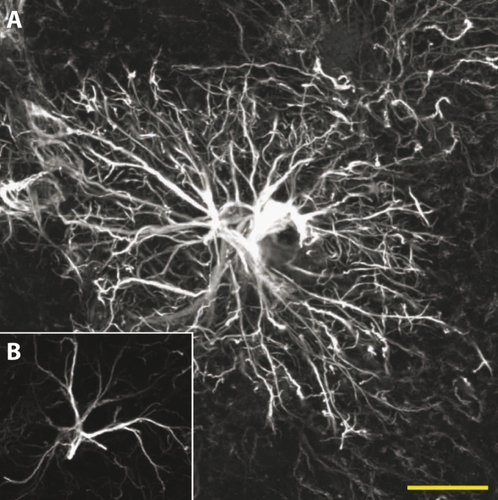
Remarkably, the phylogenetic evolution of the brain correlates with a steady increase of the astrocyte-toneuron ratio - going from about 1/6 in nematodes to 1/3 in rodents, and reaching up to 1.65 astrocytes per neuron in the human cortex.Citation3,Citation17 Importantly, more than simplyoutnumbering their rodent counterparts, human astrocytes are also strikingly more complex, both morphologically and functionally. In comparison, human neocortical astrocytes are 2.5 times larger, extend 10 times more processes, and display unique microanatomical features () Citation2. In addition, they generate more robust intracellular Ca2+ responses to neurotransmitter receptor agonists and display a 4-fold increase in Ca2+ wave velocity.Citation2 In light of these evolution-driven modifications, it is tempting to hypothesize that the astrocytic contribution to the overall neural network complexitymay in part provide the fine tuning necessary to take information processing to a higher level of competence, such as that seen in humans. At the very least, the evolutionary pressure exerted on astrocytes highlights the importance of this glial cell type in sustaining normal brain function as the brain itself becomes more complex. A continuously growing body of evidence demonstrates that astrocytes are essential sentinels and dynamic modulators of neuronal function. Considering the strong metabolic cooperation that exists between these two cell types, it is not surprising that alterations in astrocytic function have been shown to have potentially- cata strophic consequences for neurons. In the present review we discuss the intrinsically protective role of astrocytes in the normal brain, and examine how these defense mechanisms may be overwhelmed in pathological conditions, contributing to disease progression.
Astrocytes in the normal brain: maintenance of extracellular homeostasis
Despite the fact that the brain has a very high metabolic rate, neurons are by nature particularly sensitive to minute changes in their microenvironment. In this context, neuronal function and viability would rapidly be compromised without effective mechanisms for the supply of metabolic substrates and - equally as important - for the removal of waste products. In this respect, astrocytes play an essential role through a number of cellular processes; some of the most important are outlined in the following section.
Glutamate uptake and recycling
Astrocytic processes surrounding synaptic elements express transporters for a variety of neurotransmitters and neuromodulators including glutamate, y-aminobutyric acid (GABA), glycine, and histamine.Citation5-Citation8 These transporters participate in the rapid removal of neurotransmitters released into the synaptic cleft, which is essential for the termination of synaptic transmission and maintenance of neuronal excitability. In the specific case of glutamate, its uptake by astrocytes is also crucial in protecting neurons against glutamate-induced excitotoxicity. Indeed, although glutamate is the primary excitatory neurotransmitter in the brain, overstimulation of glutamate receptors is highly toxic to neurons (reviewed in detail by Sattler and Tymianski).Citation18 While basal extracellular glutamate levels are maintained in the low micromolar range, they increase dramatically during glutamatergic neurotransmission, reaching up to 1 mM for a few milliseconds in the synaptic cleft.Citation19 This concentration of glutamate would cause extensive neuronal injury in the absence of highly efficient mechanisms for its removal at the synapse. This is primarily achieved by the astrocyte-specific sodium-dependent high-affinity glutamate transporters GLT-1 and G LAST (corresponding to human EAAT2 and EAAT1 , respectively) and to a lesser extent by the neuronal glutamate transporters EAAC1 (human EAAT3) and EAAT4.Citation7 A number of in vitro and in vivo studies demonstrate the primary importance of astrocytic glutamate uptake in preventing glutamate-induced exciloloxicily.Citation20-Citation23 A good example is provided by the phenotypical changes displayed byknockout mice for the various glutamate transporters. Indeed, knockout mice for GLT-1, considered the main astrocytic glutamate transporter, suffer lethal spontaneous seizures and selective hippocampal neuronal degeneration,Citation24 whereas knockout mice for the neuronal EAAC1 display no apparent neurodegeneration.Citation25 Interestingly, beta-lactam antibiotics have been shown to upregulate the expression of GLT-1 and to prevent neuronal loss both in vitro and in vivo in models involving excitotoxicity.Citation26 This suggests that modulation of the glutamate uptake capacity of astrocytes may be achievable in vivo with classical pharmacological tools, thus representing a promising therapeutic target for pathologies involving excitotoxicity.
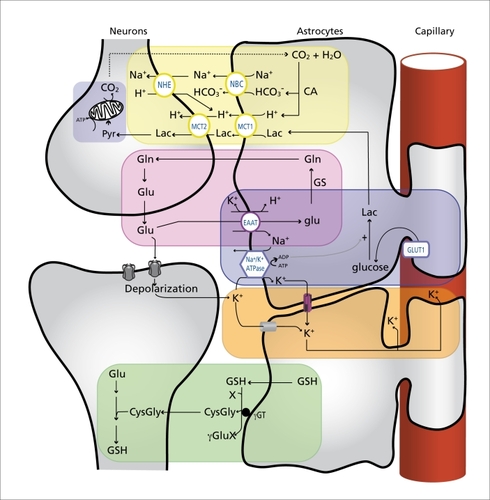
Astrocytes also play a central role in the transfer of glutamate back to neurons following its uptake at the synapse. Failure to do so would result in the rapid depletion of the glutamate pool in presynaptic neurons and subsequent disruption of excitatory neurotransmission. This transfer is achieved by the well-described glutamate-glutamine cycle ( , pink box).Citation27,Citation28 In short, glutamate is converted to glutamine by the astrocytespecific enzyme glutamine synthetase (GS).Citation29 Glutamine is then transferred to neurons in a process most likely involving the amino acid transport systems N, L, and ASC in astrocytes and system A in neurons.Citation27 Glutamine is then converted back to glutamate via deamination by phosphate-activated glutaminase which is enriched in the neuronal compartment. The ammonia produced in the process is thought to be shuttled back to astrocytes following its incorporation into leucine and/or alanine.Citation27 It is important to note that glutamate can be metabolized in a number of different pathways in astrocytes and neurons, including oxidation in the tricarboxylic acid (TCA) cycle.Citation28 Astrocytes are responsible for the replenishment of brain glutamate, as they are the only neural cell type expressing pyruvate carboxylase, a key enzyme in the main anaplerotic pathway in the brain, effectively allowing them to synthesize glutamate from glucose.Citation30,Citation31 This represents another level of cooperation between astrocytes and neurons.
K+ buffering
Apart from the release of neurotransmitters which have to be rapidly removed from the synaptic cleft, neuronal activity and the resulting propagation of action potentials causes substantial local increases of extracellular potassium ions (K+) in the restricted extracellular space. Without tight regulatory mechanisms, this could dramatically alter the neuronal membrane potential, leading to neuronal hyperexcitability and seriously compromising CNS function.Citation32 Such a scenario is prevented by the buffering of extracellular K+ by glial cellsCitation33,Citation34 (Figure 2, orange box). Indeed, astrocytes have a strongly negative resting potential and express a number of potassium channels, resulting in a high membrane permeability to K+.Citation35 These features, in conjunction with the action of the Na+/K+ ATPase, enable astrocytes to accumulate the excess extracellular K+ Citation36, which can then travel in the astrocytic syncitium through gap junctions down its concentration gradient.Citation34,Citation35 This allows for the spatial dispersion of K+ from areas of high concentration to areas of lower concentration where it can be extruded either into the extracellular space or the circulation, thus maintaining the overall extracellular K+ concentration within the physiological range. In addition to spatial buffering, other mechanisms such as the transient storage of K+ ions appear to contribute to the potassium-buffering capacity of astrocytes.Citation32
Supply of energy substrates
Although the brain represents only 2% of the body weight, it is responsible for the consumption of an estimated 25% of all glucose in the body.Citation37 This disproportionate energy need compared with other organs can be largely explained by the energetic cost of maintaining the steep ion gradients necessary for the transmission of action potentials.Citation38 For this reason, neurons in particular have very high energy requirements, and are therefore highly dependent upon a tight regulation of energy substrate supply in order to sustain their normal function and cellular integrity.
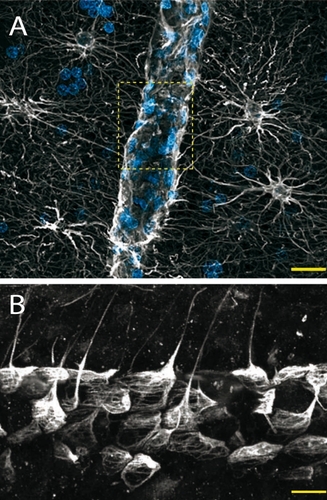
As mentioned previously, the morphological features of astrocytes ideally position them to sense neuronal activity at the synapse and respond with the appropriate metabolic supply via their astrocytic endfeet which almost entirely enwrap the intracerebral blood vessels (). In line with this, an increasing body of evidence suggests that astrocytes play a key role in the spatiotemporal coupling between neuronal activity and cerebral blood flow (known as functional hyperemia) in a process that involves transient neurotransmitterinduced increases of [Ca2+]i in astrocytes, the subsequent propagation of Ca2+ waves through the astrocytic syncitium and the release of vasoactive substances (such as arachidonic acid metabolites or ATP) by astrocytic endfeet.Citation13 Importantly, the role of astrocytes in functional hyperemia does not preclude a concerted contribution of neurons via the release of vasoactive substances such as neurotransmitters, nitric oxide, H+, and K+ to name a few.Citation39
Although neurons can import glucose directly from the extracellular space, astrocytes have been proposed to play an instrumental role in coupling neuronal activity and brain glucose uptake through a mechanism referred to as the astrocyte-neuron lactate shuttle (ANLS) (Figure 2, blue boxes).Citation40,Citation41 In brief, according to the ANLS, glutamate uptake into astrocytes following synaptic release causes a stimulation of anaerobic glycolysis and glucose uptake from the circulation via GLUT1, a glucose transporter expressed specifically by glial and capillary endothelial cells in the brain.Citation42 Lactate produced by astrocytes as an end result of glycolysis is released into the extracellular space and taken up by neurons via monocarboxylate transporters (MCTs) expressed on astrocytes and neurons.Citation42 Once into neurons, lactate can be used as an energy substrate via its conversion to pyruvate by the action of lactate dehydrogenase and subsequent oxidation in the mitochondrial TCA cycle. The existence of a lactate shuttle between astrocytes and neurons is supported by a number of experimental studies (reviewed in ref 41). For instance, in an elegant study by Rouach and colleagues,Citation43 it was recently demonstrated that 2-NBDG (a fluorescent glucose analogue) injected into a single astrocyte in hippocampal slices traffics through the astrocytic network as a function of neuronal activity. The diffusion of 2-NBGD across the astrocytic syncitium was indeed reduced when spontaneous neuronal activity was inhibited with tetrodotoxin, whereas increasing neuronal activity by means of epileptiform bursts or stimulation of the Schaffer collaterals resulted in the trafficking of 2-NBDG to a larger number of astrocytes.Citation43 They next went on to show that during glucose deprivation which resulted in a 50% depression of synaptic transmission in hippocampal slices, glucose delivery into a single astrocyte and its subsequent (and necessary) diffusion through the astrocytic syncitium could rescue neuronal activity. This effect was mimicked by lactate but was abolished in the presence of the MCT inhibitor acyano-4-hydroxycinnamic acid (4-CIN), demonstrating that glucose present in the astrocytic network is metabolized to lactate, transported out of astrocytes, and used by neurons to sustain their activity.Citation43 Interestingly, lactate has also been shown to preserve neuronal function in experimental models of excitotoxicity,Citation44 posthypoxic recovery,Citation45,Citation46 cerebral ischemia,Citation47 and energy deprivation,Citation48 highlighting the importance of astrocyte-derived lactate for neuronal function and viability.
Another key feature of astrocytes is their capacity to store glucose in the form of glycogen. Indeed, in the CNS glycogen is almost exclusively present in astrocytes and virtually constitutes the only energy reserve.Citation37,Citation49 Interestingly, it has recently been demonstrated that neurons also possess the enzymatic machinery to synthesize glycogen, but that it normally is tightly suppressed.Citation50 Failure to do so results in neuronal apoptosis, suggesting that intracellular glycogen is actually toxic to neurons.Citation50 In astrocytes, glycogen can be rapidly mobilized in response to neuronal activity.Citation51,Citation52 The glycosyl units resulting from glycogen breakdown are fed into the glycolytic pathway of astrocytes, and released into the extracellular space in the form of lactate which can be used to face the transiently elevated energy requirements associated with neuronal activation.Citation49,Citation52-Citation54 Storage of energy in the form of glycogen is also essential for the preservation of neuronal viability in situations where glucose becomes scarce. For example, it has been demonstrated that brain glycogen levels are increased following mild hypoxic preconditioning in vivo, resulting in significant protection from brain damage as a result of subsequent cerebral hypoxic-ischemic injury.Citation55 Beyond lactate, it is of interest to note that astrocytes may also transfer other energy substrates to neurons. Indeed, evidence suggests that in certain conditions, astrocytes may be able to metabolize fatty acids or leucine to produce ketone bodies which are know to be readily used by neurons as an energy substrate.Citation56-Citation58 It has been suggested that this pathway may also serve a neuroprotective purpose by scavenging nonesterified phospholipids which can lead to the production of proapoptotic sphingolipids.Citation58,Citation59
pH buffering
Another instrumental function of astrocytes in supporting proper neuronal function is their contribution to pH regulation of the brain microenvironment (Figure 2, yellow box).Citation60-Citation62 Several neuronal processes are strongly affected by relatively small shifts in pll, including energymetabolism, membrane conductance, neuronal excitability, synaptic transmission, and gap junction communication.Citation60,Citation62 The main feature of glial cells, endowing them with a high pH buffering capacity, is their enriched expression of carbonic anhydrase (CA) which converts CO2 into H+ and HCO3 - - effectively allowing them to act as a CO2 sink. Indeed, CA is preferentially expressed in astrocytes and oligodendrocytes,Citation63,Citation64 although lowactivity levels are also observed in neurons and in the extracellular space.Citation62 A coupling mechanism which integrates synaptic transmission, pH regulation, and energy supply between neurons and glia has been proposed by J. W Deiter.Citation61,Citation65 According to this model, during periods of high neuronal activity, the CO2 produced by elevated (mostly neuronal) oxidative metabolism diffuses into glial cells and is converted to H+ and HCO3 by the action of glial CA. Two HCO3 - can then be transported into the extracellular space along with one Na+ via the Na+- HCO3 - cotransporter (NBC), thereby increasing the extracellular buffering power. The protons left in the glial compartment could be used to drive the transport of lactate outside of astrocytes through MCT-1 and -4 and its subsequent transport by MCT-2 into neurons, since MCTs exploit proton gradients for the transport of lactate.Citation41,Citation61 As previously discussed, according to the ANLS hypothesis, this lactate can then be used as an energy substrate by neurons.Citation40,Citation41 Alternatively, protons released into the extracellular space may also be reconverted to CO2 and water by the action of extracellular CA at the expense of one HCO3 -.Citation61 This model suggests that pH buffering taking place in glial cells during neuronal activation may also act cooperatively to: i) contribute, via the Na+- HCO3 - cotransporter, to the extrusion against its concentration gradient of the excess intracellular Na+ resulting from glutamate uptake in astrocytes, thereby alleviating the metabolic burden on the glial Na+/K+ ATPase; and ii) drive the efflux of lactate which is produced in response to glutamate uptake in astrocytes, thus providing an energy substrate for the neuronal TCA cycle,Citation61,Citation65
Defense against oxidative stress
Oxidative stress occurs as a result of an imbalance between the production of reactive oxygen species (ROS) and antioxidant processes. It is known to be involved in a number of neuropathological conditions, including neurodegenerative diseases, traumatic brain injury, and stroke,Citation66 suggesting that the CNS is particularly vulnerable to oxidative injury. This can be explained by the brain's high rate of oxidative energy metabolism (which inevitably generates ROS), combined with a relatively low intrinsic antioxidant capacity.Citation67 Compared with neurons, astrocytes display a much more effective artillery against ROS. Accordingly, cooperative astrocyte-neuron defense mechanisms against oxidative stress seem to be essential for neuronal viability.Citation68 This is supported by a number of studies demonstrating that when cultured in the presence of astrocytes, neurons show increased resistance to toxic doses of nitric oxide,Citation69,Citation70 hydrogen peroxide,Citation71-Citation73 superoxide anion combined with nitric oxide,Citation69,Citation74 or iron.Citation69,Citation74
This neuroprotective capacity of astrocytes may derive from the fact that they possess significantly higher levels of a variety of antioxidant molecules (including glutathione, ascorbate, and vitamin E) and display greater activities for ROS-detoxifying enzymes (including glutathione S-transferase, glutathione peroxidase, and catalase).Citation68,Citation72,Citation75 Citation78 In addition, it appears that astrocytes may also play an active role in preventing the generation of free radicals by redox active metals, as they participate in metal sequestration in the brain.Citation79 This is achieved in part through their high expression levels of metallothioneins and ceruloplasmin, which are involved in metal binding and iron trafficking, respectively.Citation80-Citation82
Glutathione (GSII) is the most important antioxidant molecule found in the brain.Citation83 This thiol compound can act as an electron donor, and thus fulfills its antioxidant role either by directly reacting with ROS or by acting as a substrate for glutathione S-transferase or glutathione peroxidase, Both neurons and astrocytes can synthesize the GSH tripeptide (L-glulamyl-Lcysteinylglycine) by the sequential action of glutamate cysteine ligase and glutathione synthetase. However, neurons are highly dependent on astrocytes for their own GSPI synthesis, as illustrated by the fact that GSPI levels are higher in neurons when they are cultured in the presence of astrocytes.Citation84 Astrocytes release GSII in the extracellular space, where it is cleaved by the astrocytic ectoenzyme γ-glutamyl transpeptidase (γGT) to produce CysGly, which can then be taken up by neurons directly or after undergoing further cleavage by extracellular neuronal aminopeptidase N to form glycine and cysteine.Citation83 This shuttling of GSPI between astrocytes and neurons is essential in providing precursors for neuronal GSII synthesis (Figure 2, green box). This is especially true for cysteine, the rate-limiting substrate for GSPI synthesis, since neurons, unlike astrocytes, cannot use the cysteine-oxidation product cystine as a precursor.Citation83 The importance of this cooperative process for neuronal defense against oxidative stress is evidenced by the reduced ability of GSPIdepleted astrocytes to protect neurons against oxidative injury.Citation85,Citation86 Conversely, increasing the capacity to synthesize GSPI specifically in astrocytes by increasing their capacity to uptake cystine significantly enhances the neuroprotective effect of astrocytes against oxidative stress.Citation87
The recycling of ascorbate is another example of cooperation between astrocytes and neurons for antioxidant defense. Ascorbate can directly scavenge ROS, and is also an important cofactor for the recycling of oxidized vitamin E and GSH.Citation68 Astrocytes are responsible for the uptake of the oxidation product of ascorbate, dehydroascorbic acid, from the extracellular space and its recycling back to ascorbic acid. The latter can then either be used intracellularly in astrocytes, or released into the extracellular space to be utilized by neurons for their own antioxidant defense.Citation68
Astrocytes in the diseased brain: a fine balance
Considering the extensive functional cooperativity that exists between neurons and astrocytes, one can expect that alterations of astrocytic pathways in response to pathological stimuli will result in (or at least contribute to) neuronal dysfunction. Interestingly, several neurological diseases share common pathogenic processes, such as oxidative stress, excitotoxicity, metabolic failure, or inflammation - many of which are known to be counteracted by the function of astrocytes in the normal brain (see previous sections). This may reflect a common underlying phenomenon by which disease progression is associated with chronic and/or escalating harmful stimuli that eventually exhaust the neuroprotective mechanisms of astrocytes. Even worse, deleterious pathways may then be turned on in astrocytes, directly contributing to the pathogenic process. A role of astrocytes has been described in a number of brain pathologies, and a complete review is beyond the scope of this article (see refs 88-90). Instead, we focus on three pathological processes that well illustrate the dual role of astrocytes in neuroprotection and neurotoxicity, namely neurointlammation, Alzheimer's disease, and hepatic encephalopathy.
Ncuroinflammation
The brain can mount an immune response as a result of various insults such as infection, injury, cellular debris, or abnormal protein aggregates. In most cases, it constitutes a beneficial process aiming to protect the brain from potentially deleterious threats. In some situations, however, the insult may persist and/or the inflammatory process may get out of control. Chronic neuroinflammation sets in as a result, and may negatively affect neuronal function and viability, thus contributing to disease progression. Neuroinflammation has indeed been implicated in several neuropathologies including Alzheimer's disease, Parkinson's disease, amyotrophic lateral sclerosis, multiple sclerosis, and stroke.Citation91
While microglial cells are generally considered the main resident immune cells of the brain, it is important to note that astrocytes are immunocompetent cells as well, and that they act as important regulators of brain inflammation. Like microglia, astrocytes can become activated - a process known as astrogliosis, which is characterized by altered gene expression, hypertrophy, and proliferation.Citation92 Activated astrocytes can release a wide array of immune mediators such as cytokines, chemokines, and growth factors, that may exert either neuroprotective or neurotoxic effects.Citation93 Additionally, activated astrocytes can release potentially deleterious ROS and form a glial scar which may impede axon regeneration and neurite outgrowth.Citation94 This has led to considerable debate as to whether activation of astrocytes is beneficial or detrimental to neighbouring neurons. The most likely answer is that it is neither exclusively one nor the other, and that the overall consequences of an immune activation of astrocytes is the result of a complex interplay between pro- and anti-inflammatory - as well as neurotoxic and neurotrophic - processes.
Cytokines, for instance, are major effectors in this fine balance as they exert a dual role, potentially sustaining or suppressing neuroinflammation (hence their traditional labeling as pro - or anti-inflammatory). In this regard, dissecting out the exact neuroprotective and neurotoxic contributions of astrocytes in neuroinflammatory processes has proven to be extremely challenging because they are capable of releasing such an extensive repertoire of cytokines in response to various stimuli (some examples include interleukin (IL)-iβ,TNFα, IL6, IL-10, IL-15, INFβ, and TGFβ).Citation93 Adding another level of complexity, astrocytes express several cytokine receptors and can therefore also be a target of cytokine signaling through autocrine or paracrine mechanisms.Citation11
While cytokines are categorized as proinflammatory or anti-inflammatory, understanding their exact individual effect is far more complex, as many of them interact with each other (either antagonistically or synergistically) and may additionally have pleiotropic effects.Citation11,Citation95 As a result, cytokines can potentially mediate both neuroprotective and neurotoxic processes at once. For example, ample evidence indicates that IL-iβ may exacerbate neuronal injury both in vivo and in vitro.Citation96-Citation99 In contrast, IL-iβ has also been implicated in neuroprotective processes such as remyelination,Citation100 blood-brain barrier repair,Citation101 ischemic tolerance,Citation102 and neurotrophic factor production.Citation103-Citation106
Importantly, astrocytes can themselves respond to IL-iβ by releasing a number of potentially neuroprotective trophic factors such as nerve growth factor (NGF), ciliary neurotrophic factor (CNTF), glial cell-line derived neurotrophic factor GDNF, and fibroblast growth factor (FGF)-2.Citation11,Citation107-Citation109
Taken together, studies such as those mentioned above provide important information about the multiple effects of individual cytokines. However, they also have major limitations, in that they can only take into account a few pro- and anti-inflammatory pathways at a time. As such, they may only reflect a small fraction of an infinitely more intricate process in which astrocytes take part. For this reason, the use of genetically manipulated animal models specifically preventing the proliferation of reactive astrocytes or the activation of their core inflammatory pathways, has provided important new insight into their overall role in response to brain injury. For instance, it has been demonstrated that the selective attenuation of astrocytic proinflammatory processes, through genetic inactivation of the transcription factor NF-kB specifically in this cell type, affords substantial neuroprotection following spinal cord injury.Citation110 By contrast, using a transgenic mouse model in which dividing reactive astrocytes were selectively ablated, Sofroniew and colleagues have demonstrated that following various types of brain injury, reactive astrocytes play an essential role in temporally and spatially restricting neurointlammation, as well as in promoting blood-brain barrier repair, limiting brain edema, and preserving neuronal viability.Citation94,Citation111-Citation113
Consistent with a role of astrocytes in containing neuroinflammation, it is interesting to note that astrocytes appear to participate in the suppression of microglial activation through negative feedback loops. Activated microglial cells release high levels of proinflammatorycytokines and toxic ROS which may negatively impact neuronal survival.Citation114 Several in vitro studies have demonstrated that astrocyte-conditioned medium or the presence of astrocytes attenuates microglial activation in response to various proinflammatory stimuli.Citation115-Citation117 The exact nature of the astrocyte-derived factors involved has not been fully elucidated, but transforming growth factor (TFG)β is thought to contribute to this process.Citation115 This may in part explain the neuroprotective effect of TGFp in experimental models of excitotoxicity or ischemia.Citation118-Citation120
To summarize, if inflammatory activation of astrocytes unquestionably has consequences for neuronal function and viability, it must be emphasized that the overall effect is dependent on the fine balance between a number of factors including the type, duration, and severity of the insult, the complex interplay between the various cytokines released by astrocytes and surrounding cells, and the receptors for cytokines and growth factors expressed by these neighboring cells.
Alzheimer's disease
Alzheimer's disease (AD), the most prevalent neurodegenerative disorder, is characterized by the progressive decline of cognitive functions including memory and mental processing, and by disturbances in behavior and personality.Citation121 Typical histopathological features of the AD brain are amyloid-β (Aβ) plaques which may contain dystrophic neurites, intracellular neurofibrillary tangles, vascular amyloidosis, neuronal and synaptic loss, and reactive gliosis. Though the exact pathophysiological mechanisms leading to synaptic loss and the resulting cognitive decline have not been fully elucidated, a central role of Aβ peptides in concert with neuroinflammation is generally accepted.Citation122 Alois Alzheimer himself in 1910 suggested that glial cells may participate in the pathogenesis of dementiaCitation123; however, their exact role is still a matter of debate, as available evidence can argue both for neuroprotective or neurotoxic effects.
Reactive astrocytes, like microglia, are observed in close association with Aβ plaques in the brains of AD patients,Citation124,Citation125 and both cell types have been shown to be capable of internalizing and degrading Aβ peptides.Citation126-Citation128
This is thought to be a neuroprotective mechanism by contributing to the clearance of Aβ from the extracellular space, thus avoiding the accumulation of toxic extracellular Aβ. Several observations support an active role of astrocytes in Aβ clearance. For example, astrocytes surrounding plaques in autopsy material from the brain of AD patients contain intracellular Aβ deposits.Citation128,Citation130 In addition, when exogenous astrocytes were transplanted into the brain of Aβ plaque-bearing transgenic mice, they migrated towards Aβ deposits and internalized Appositive material.Citation129 Similarly in ex vivo studies, binding, internalization, and degradation of Aβ could be observed when cultured astrocytes were seeded on top of plaque-bearing sections prepared either from the brains of AD patients or transgenic mice models of AD.Citation127,Citation129 The physiological importance of Aβ clearance by glial cells in vivo is evidenced by the increased Ap accumulation and premature death observed in a transgenic mouse model of AD when microglial activation was impaired.Citation131 Interestingly, glial cell activation and astrocytic accumulation of Aβ can be observed even preceding plaque formation,Citation128,Citation132 suggesting that astrocyte cells attempt to scavenge Aβ early in the progression of the disease, which likely reflects an effort to limit its extracellular deposition.
Although their contribution to the clearance of Aβ deposits is thought to be protective, there is also evidence to suggest that microglia and astrocytes contribute to the progression of AD. One obvious explanation is that the physiological functions of astrocytes may be directly affected by Aβ. For instance, in a elegant study using fluorescence imaging microscopy in live mice bearing AD-like pathology, intracellular Ca2+ signaling was reported to be abnormally increased in astrocytes, sometimes propagating as intracellular calcium waves.Citation133 These Ca2+ transients were only observed after the mice developed senile plaques and were uncoupled from neuronal activity, suggesting that Aβ interacts directly with the astrocytic network.Citation133
The involvement of glial cells in the pathogenesis of AD is supported by several in vitro studies demonstrating that their interaction with Aβ impairs neuronal viability or worsens the neurotoxic effect of Aβ.Citation134 Citation138 Upon their activation by Aβ, astrocytes and microglia can release a number of inflammatory mediators which may be toxic for surrounding neurons. Examples include proinflammatory cytokines such as IL-1β and IL-6, and reactive oxygen and nitrogen species (RN/ROS) such as NO and O2 -.Citation132,Citation139-Citation143
Proinflammatory cytokines have been shown to exacerbate the microglial response to Aβ and to enhance its neurotoxic effects.Citation144-Citation146 Moreover, it appears that proinflammatory cytokines can also increase the expression of the amyloid precursor protein and its processing through amyloidogenic pathways.Citation147-Citation149 Aβ accumulation may therefore establish a vicious circle whereby neuronal stress and glial activation initiates an inflammatory response, which in turn promotes the synthesis and accumulation of more Aβ, thus perpetuating glial cell activation. This may in part explain why age is the most important risk factor for developing AD since increased neuroinflammation is associated with normal aging.Citation150 This enhancement of the basal inflammatory state, together with the gradual accumulation of Aβ which is also seen in the normal aging brain, may provide the trigger necessary for this vicious circle to set in. Because of their central role in neuroinflammation (see previous section), glial cells may provide a valuable therapeutic target for the treatment of AD. This is supported by studies testing newly identified antiinflammatory molecules which selectively suppress proinflammatory cytokines production in glia, resulting in a significant attenuation of synaptic dysfunction and neurodegeneration and in behavioral improvements in experimental models of AD.Citation151,Citation152
Besides proinflammatory cytokines, RN/ROS produced by activated astrocytes and microglia may contribute to disease progression by inducing oxidative stress, a hallmark of AD.Citation142,Citation153 Astrocytes have been proposed to take part in this process. For example, Aβ causes intracellular Ca2+ transients and stimulates the production of ROS by NADPH oxidase in astrocytes but not in neurons.Citation154 -Citation156 In mixed cultures, these effects were accompanied bydecreases in GSII levels in both astrocytes and neurons, resulting in neuronal cell death.Citation154-Citation156
Conversely, in the presence of microglia, astrocytes may provide significant protection through the negative regulation of microglial reactivity following exposure to Aβ.Citation137,Citation157 However, this must be interpreted with caution since, as previously discussed, increased microglial phagocytosis associated with their activated state maybe neuroprotective. In line with this, microglial phagocytosis was shown to be markedly suppressed in the presence of astrocytes, which resulted in increased persistence of senile plaques when presented to microglia in vitro.Citation158
In summary, the apparently conflicting roles of astrocytes in the progression of AD may be explained by the coexistence of potentially protective and deleterious pathways in activated astrocytes. As the disease progresses, the overwhelming combined effect of Aβ accumulation, neuroinflammation, and oxidative stress may tip the scales away from the neuroprotective functions of astrocytes and towards the activation of deleterious pathways.
Hepatic encephalopathy
Hepatic encephalopathy (HE), a neuropsychiatrie syndrome occurring as a result of chronic or acute liver failure, is one of the first identified neurological disorders involving astroglial dysfunction as its primary cause. In its acute form, the symptoms of HE can progress rapidly from altered mental status to stupor and coma, and may cause death within days. The most important cause of mortality in acute liver failure is brain herniation, which occurs as a result of cytotoxic swelling of astrocytes, leading to intracranial hypertension.Citation159 Although HE is a multifactorial disorder, ammonia is thought to play a central role in its pathogenesis.Citation159 Ammonia rapidly accumulates in the blood as a result of acute liver failure and can readily cross the blood-brain barrier. Because the brain does not possess an effective urea cycle, it relies almost exclusively on glutamine synthesis for the detoxification of ammonia.Citation159 As mentioned before, this is accomplished by the enzyme glutamine synthetase (GS) which is exclusively localized in astrocytes.Citation29 Ammonia detoxification is an essential homeostatic function of astrocytes, as excess hyperammonemia has profound effects on various brain functions.Citation159 However, the astrocytic accumulation of osmotically active glutamine as a result of ammonia detoxification is thought to contribute at least in part to the swelling of astrocytes in hyperammonemic conditions. This is supported by the demonstration that inhibition of GS with methionine sulfoxide prevents brain edema in experimental hyperammonemia.Citation160 Alternatively, glutamine may also induce astrocytic swelling via other mechanisms, including oxidative and nitrosative stress.Citation161 Interestingly, glutamine efflux from asctrocytes through the system N transporter appears to be negatively regulated by elevated extracellular glutamine in hyperammonemic conditions.Citation162 Such a mechanism may contribute to trap glutamine in astrocytes and promote swelling.
In contrast with its acute form, chronic hepatic encephalopathy, which is associated with more modest increases in brain ammonia, does not result in overt cerebral edema,Citation163 suggesting the existence of compensatory mechanisms taking place in astrocytes in order to prevent excessive swelling. This is thought to be accomplished by the release of osmolytes such as taurine and myo-inositol by astrocytes in response to glutamine accumulation. However, it appears that when osmolyte pools are depleted as a result of excessive hyperammonemia, for example during acute liver failure, this protective mechanism is exhausted and astrocytes swell as a result. This, together with an impaired capacity of astrocytes to fulfill their role in ammonia detoxification, seriously compromises brain function in acute liver failure.
Conclusion
Astrocytes are known to be the most important neural cell type for the maintenance of brain homeostasis. It is safe to assume that, as technology advances in the years to come, we will continue to uncover the multiple facets of astroglia. It has already become quite clear however that it is unrealistic to approach brain function and dysfunction from a uniquely neuronal standpoint. Because of their involvement in such a wide range of homeostatic functions, any brain insult is likely to have an impact on astrocytes. Their capacity to adapt to these changes weighs heavily in the fine balance between neuroprotection and neurotoxicity as illustrated by the three neuropathological conditions discussed above. In this context, understanding astrocytic function is key to providing a better grasp of brain function in general and how it may go awry. This may lead to the identification of better suited therapeutic targets, as they should take into account the multiple interactions and interdependencies between neural cell types.
Selected abbreviations and acronyms
| Aβ | = | amyloid-beta |
| AD | = | Alzheimer's disease |
| GSH | = | glutathione |
| MCT | = | monocarboxylate transporter |
| ROS | = | reactive oxygen species |
Acknowledgements: The authors wish to thank Drs Igor Allaman and Nicolas Aznavour for their help with the manuscript. Work in PJM's laboratory is supported by the Swiss National Science Foundation (grant no. 3100AO-108336/1 to PJM). MB was supported by the Fonds de la Recherche en Santé du Québec (FRSQ).
REFERENCES
- HalassaMM.FellinT.TakanoH.DongJH.HaydonPG.Synaptic islands defined by the territory of a single astrocyte.J Neurosci.2007276473647717567808
- OberheimNA.TakanoT.HanX.et al.Uniquely hominid features of adult human astrocytes.J Neurosci.2009293276328719279265
- NedergaardM.RansomB.GoldmanSA.New roles for astrocytes: redefining the functional architecture of the brain.Trends Neurosci.20032652353014522144
- RouachN.KoulakoffA.GiaumeC.Neurons set the tone of gap junctional communication in astrocytic networks.Neurochem int.20044526527215145542
- GadeaA.Lopez-ColomeAM.Glial transporters for glutamate, glycine, and GABA III. Glycine transporters.J Neurosci Res.20016421822211319765
- GadeaA.Lopez-ColomeAM.Glial transporters for glutamate, glycine, and GABA: II. GABA transporters,J Neurosci Res.20016346146811241581
- DanboltNC.Glutamate uptake.Prog Neurobiol.200165110511369436
- HusztiZ.PrastH.TranMH.FischerH.PhilippuA.Glial cells participate in histamine inactivation in vivo.Naunyn Schrniedebergs Arch Pharmacol.19983574953
- PorterJT.McCarthyKD.Astrocytic neurotransmitter receptors in situ and in vivo.Prog Neurobiol.1997514394559106901
- VerkhratskyA.SteinhauserC.Ion channels in glial cells.Brain Res Brain Res Rev.20003238041210760549
- JohnGR.LeeSC.BrosnanCF.Cytokines: powerful regulators of glial cell activation.Neuroscientist.20039102212580336
- KacemK.LacombeP.SeylazJ.BonventoG.Structural organization of the perivascular astrocyte endfeet and their relationship with the endothelial glucose transporter: a confocal microscopy study.Glia.1998231109562180
- ladecolaC.NedergaardM.Glial regulation of the cerebral microvasculature.Nat Neurosci.2007101369137617965657
- Cornell-BellAH.FinkbeinerSM.CooperMS.SmithSJ.Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling.Science.19902474704731967852
- WangX.LouN.XuQ.et al.Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo.Nat Neurosci.2006981682316699507
- HalassaMM.FellinT.HaydonPG.The tripartite synapse: roles for g Motransmission in health and disease.Trends Mol Med.200713546317207662
- SherwoodCC.StimpsonCD.RaghantiMA.et al.Evolution of increased glia-neuron ratios in the human frontal cortex.Proc Natl Acad Sci USA.2006103136061361116938869
- SattlerR.TymianskiM.Molecular mechanisms of glutamate receptormediated excitotoxic neuronal cell death.Mol Neurobiol.20012410712911831548
- ClementsJD.LesterRA.TongG.JahrCE.WestbrookGL.The time course of glutamate in the synaptic cleft.Science.1992258149815011359647
- RosenbergPA.AizenmanE.Hundred-fold increase in neuronal vulnerability to glutamate toxicity in astrocyte-poor cultures of rat cerebral cortex.Neurosci Lett.19891031621682570387
- SelkirkJV.NottebaumLM.VanaAM.et al.Role of the GLT-1 subtype of glutamate transporter in glutamate homeostasis: the GLT-1-preferring inhibitor WAY-855 produces marginal neurotoxicity in the rat hippocampus.Eur J Neurosci.2005213217322816026460
- RothsteinJD.JinL.Dykes-HobergM.KunclRW.Chronic inhibition of glutamate uptake produces a model of slow neurotoxicity.Proc Natl Acad Sci US A.199390659195
- RothsteinJD.Dykes-HobergM.PardoCA.et al.Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate.Neuron.1996166756868785064
- TanakaK.WataseK.ManabeT.et al.Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1.Science.1997276169917029180080
- PeghiniP.JanzenJ.StoffelW.Glutamate transporter EAAC-1deficient mice develop dicarboxylic aminoaciduria and behavioral abnormalities but no neurodegeneration.EMBOJ.19971638223832
- RothsteinJD.PatelS.ReganMR.et al.Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression.Nature.2005433737715635412
- BakLK.SchousboeA.WaagepetersenHS.The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer.J Neurochem.20069864165316787421
- McKennaMC.The glutamate-glutamine cycle is not stoichiometric: fates of glutamate in brain.J Neurosci Res.2007853347335817847118
- NorenbergMD.Martinez-HernandezA.Fine structural localization of glutamine synthetase in astrocytes of rat brain.Brain Res.197916130331031966
- YuAC.DrejerJ.HertzL.SchousboeA.Pyruvate carboxylase activity in primary cultures of astrocytes and neurons.J Neurochem.198341148414876619879
- ShankRP.BennettGS.FreytagSO.CampbellGL.Pyruvate carboxylase: an astrocyte-specific enzyme implicated in the replenishment of am ino acid neurotransmitter pools.Brain Res.19853293643673884090
- WalzW.Role of astrocytes in the clearance of excess extracellular potassium.Neurochem int.20003629130010732996
- OrkandRK.NichollsJG.KufflerSW.Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia.J Neurophysiol.1966297888065966435
- HolthoffK.WitteOW.Directed spatial potassium redistribution in rat neocortex.Glia.20002928829210642755
- KofujiP.NewmanEA.Potassium buffering in the central nervous system.Neuroscience.20041291045105615561419
- D'AmbrosloR.GordonDS.WinnHR.Differential role of KIR channel and Na(+)/K(+)-pump in the regulation of extracellular K(+) in rat hippocampus.J Neurophysiol.2002878710211784732
- MagistrettiPJ.Brain energy metabolism. In: Squire LR, Bloom FE, McConnell SK, Roberts JL, Spitzer NC, Zigmond MJ, eds.Fundamental Neuroscience. 3rd ed. San Diego, CAL: Academic Press2008271292
- SokoloffL.Energetics of functional activation in neural tissues.Neurochem Res.1999243213299972882
- DrakeCT.ladecolaC.The role of neuronal signaling in controlling cerebral blood flow.Brain Lang.200710214115217010421
- MagistrettiPJ.PellerinL.RothmanDL.ShulmanRG.Energy on demand.Science.19992834964979988650
- PellerinL.Bouzier-SoreAK.AubertA.et al.Activity-dependent regulation of energy metabolism by astrocytes: an update.Glia.2007551251126217659524
- SimpsonIA.CarruthersA.VannucciSJ.Supply and demand in cerebral energy metabolism: the role of nutrient transporters.J Cereb Blood Flow Metab.2007271766179117579656
- RouachN.KoulakoffA.AbudaraV.WilleckeK.GiaumeC.Astroglial metabolic networks sustain hippocampal synaptic transmission.Science.20083221551155519056987
- MausM.MarinP.IsraelM.GlowinskiJ.PremontJ.Pyruvate and lactate protect striatal neurons against N-methyl-D-aspartate-induced neurotoxicity.Eur J Neurosci.1999113215322410510185
- SchurrA.PayneRS.MillerJJ.RigorBM.Brain lactate is an obligatory aerobic energy substrate for functional recovery after hypoxia: further in vitro validation,J Neurochem.1997694234269202338
- SchurrA.PayneRS.MillerJJ.RigorBM.Glia are the main source of lactate utilized by neurons for recovery of function posthypoxia.Brain Res.19977742212249452213
- SchurrA.PayneRS.MillerJJ.TsengMT.RigorBM.Blockade of lactate transport exacerbates delayed neuronal damage in a rat model of cerebral ischemia.Brain Res.200189526827211259789
- CaterHL.BenhamCD.SundstromLE.Neuroprotective role of monocarboxylate transport during glucose deprivation in slice cultures of rat hippocampus,J Physiol.200153145946611230518
- BrownAM.RansomBR.Astrocyte glycogen and brain energy metabolism.Glia.2007551263127117659525
- VilchezD.RosS.CifuentesD.et al.Mechanism suppressing glycogen synthesis in neurons and its demise in progressive myoclonus epilepsy.Nat Neurosci.2007101407141317952067
- SwansonRA.MortonMM.SagarSM.SharpFR.Sensory stimulation induces local cerebral glycogenosis: demonstration by autoradiography.Neuroscience.1992514514611465204
- BrownAM.TekkokSB.RansomBR.Glycogen regulation and functional role in mouse white matter.J Physiol.200354950151212679378
- DringenR.GebhardtR.HamprechtB.Glycogen in astrocytes: possible function as lactate supply for neighboring cells.Brain Res.19936232082148221102
- BrownAM.SickmannHM.FosgerauK.et al.Astrocyte glycogen metabolism is required for neural activity during aglycemia or intense stimulation in mouse white matter.J Neurosci Res.200579748015578727
- BrucklacherRM.VannucciRC.VannucciSJ.Hypoxic preconditioning increases brain glycogen and delays energy depletion from hypoxiaischemia in the immature rat.Dev Neurosci.20022441141712640180
- AuestadN.KorsakRA.MorrowJW.EdmondJ.Fatty acid oxidation and ketogenesis by astrocytes in primary culture.J Neurochem.199156137613862002348
- BixelMG.HamprechtB.Generation of ketone bodies from leucine by cultured astroglial cells.J Neurochem.199565245024617595539
- GuzmanM.BlazquezC.Ketone body synthesis in the brain: possible neuroprotective effects.Prostaglandins Leukot Essent Fatty Acids.20047028729214769487
- EscartinC.PierreK.ColinA.et al.Activation of astrocytes by CNTF induces metabolic plasticity and increases resistance to metabolic insults,J Neurosci.2007277094710417611262
- DeitmerJW.RoseCR.pH regulation and proton signalling by glial cells.Prog Neurobiol.199648731038737439
- DeitmerJW.A role for CO(2) and bicarbonate transporters in metabolic exchanges in the brain.J Neurochem.20028072172611948234
- ObaraM.SzeligaM.AlbrechtJ.Regulation of pH in the mammalian central nervous system under normal and pathological conditions: facts and hypotheses.Neurochem int.20085290591918061308
- AgnatiLF.TinnerB.StainesWA.VaananenK.FuxeK.On the cellular localization and distribution of carbonic anhydrase II immunoreactivity in the rat brain.Brain Res.199567610247796160
- CammerW.TanseyFA.The astrocyte as a locus of carbonic anhydrase in the brains of normal and dysmyelinating mutant mice,J Comp Neurol.198827565753139721
- DeitmerJW.Glial strategy for metabolic shuttling and neuronal function.Bioessays.20002274775210918305
- SlemmerJE.ShackaJJ.SweeneyMl.WeberJT.Antioxidants and free radical scavengers for the treatment of stroke, traumatic brain injury and aging.CurrMedChem.200815404414
- DringenR.Metabolism and functions of glutathione in brain.Prog Neurobiol.20006264967110880854
- WilsonJX.Antioxidant defense of the brain: a role for astrocytes.Can J Physiol Pharmacol.199775114911639431439
- TanakaJ.TokuK.ZhangB.IshiharaK.SakanakaM.MaedaN.Astrocytes prevent neuronal death induced by reactive oxygen and nitrogen species.Glia.199928859610533053
- GeggME.BeltranB.Salas-PinoS.et al.Differential effect of nitric oxide on glutathione metabolism and mitochondrial function in astrocytes and neurones: implications for neuroprotection/neurodegeneration?J Neurochem.20038622823712807442
- LangeveldCH.JongenelenCA.SchepensE.StoofJC.BastA.DrukarchB.Cultured rat striatal and cortical astrocytes protect mesencephalic dopaminergic neurons against hydrogen peroxide toxicity independent of their effect on neuronal development.Neurosci Lett.199519213167675299
- DesagherS.GlowinskiJ.PremontJ.Astrocytes protect neurons from hydrogen peroxide toxicity.J Neurosci.199616255325628786431
- FujitaT.Tozaki-SaitohH.InoueK.P2Y1 receptor signaling enhances neuroprotection by astrocytes against oxidative stress via IL-6 release in hippocampal cultures.Glia.20095724425718756525
- LuciusR.SieversJ.Postnatal retinal ganglion cells in vitro: protection against reactive oxygen species (ROS)-induced axonal degeneration by cocultured astrocytes.Brain Res.199674356629017230
- MakarTK.NedergaardM.PreussA.GelbardAS.PerumalAS.CooperAJ.Vitamin E, ascorbate, glutathione, glutathione disulfide, and enzymes of glutathione metabolism in cultures of chick astrocytes and neurons: evidence that astrocytes play an important role in antioxidative processes in the brain.J Neurochem.19946245537903354
- HuangJ.PhilbertMA.Distribution of glutathione and glutathionerelated enzyme systems in mitochondria and cytosol of cultured cerebellar astrocytes and granule cells.Brain Res.199568016227663973
- BolanosJP.HealesSJ.LandJM.ClarkJB.Effect of peroxynitrite on the mitochondrial respiratory chain: differential susceptibility of neurones and astrocytes in primary culture.J Neurochem.199564196516727722484
- DringenR.KussmaulL.GuttererJM.HirrlingerJ.HamprechtB.The glutathione system of peroxide detoxification is less efficient in neurons than in astroglial cells,J Neurochem.1999722523253010349863
- Tiffany-CastiglionE.QianY.Astroglia as metal depots: molecular mechanisms for metal accumulation, storage and release.Neurotoxicology.20012257759211770879
- KlompLW.FarhangraziZS.DuganLL.GitlinJD.Ceruloplasmin gene expression in the murine central nervous system.J Clin Invest.1996982072158690795
- OideT.YoshidaK.KanekoK.OhtaM.ArimaK.Iron overload and antioxidative role of perivascular astrocytes in aceruloplasminemia.Neuropathol Appl Neurobiol.20063217017616599945
- DringenR.BishopGM.KoeppeM.DangTN.RobinsonSR.The pivotal role of astrocytes in the metabolism of iron in the brain.Neurochem Res.2007321884189017551833
- DringenR.HirrlingerJ.Glutathione pathways in the brain.Biol Chern.2003384505516
- DringenR.PfeifferB.HamprechtB.Synthesis of the antioxidant glutathione in neurons: supply by astrocytes of CysGly as precursor for neuronal glutathione.J Neurosci.1999195625699880576
- McNaughtKS.JennerP.Altered glial function causes neuronal death and increases neuronal susceptibility to 1-methyl-4-phenylpyridinium- and 6-hydroxydopamine-induced toxicity in astrocytic/ventral mesencephalic cocultures.J Neurochem.1999732469247610582607
- ChenY.VartiainenNE.YingW.ChanPH.KoistinahoJ.SwansonRA.Astrocytes protect neurons from nitric oxide toxicity by a glutathionedependent mechanism.J Neurochem.2001771601161011413243
- ShihAY.ErbH.SunX.TodaS.KalivasPW.MurphyTH.Cystine/glutamate exchange modulates glutathione supply for neuroprotection from oxidative stress and cell proliferation.J. Neurosci.200626105141052317035536
- SeifertG.SchillingK.SteinhauserC.Astrocyte dysfunction in neurological disorders: a molecular perspective.Nat Rev Neurosci.2006719420616495941
- MarkiewiczI.LukomskaB.The role of astrocytes in the physiology and pathology of the central nervous system.Acta Neurobiol Exp Wars.20066634335817265695
- De KeyserJ.MostertJP.KochMW.Dysfunctional astrocytes as key players in the pathogenesis of central nervous system disorders.J Neurol Sci.200826731617935736
- AllanSM.RothwellNJ.Inflammation in central nervous system injury.Philos Trans R Soc Lond B Biol Sci.20033581669167714561325
- RidetJL.MalhotraSK.PrivâtA.GageFH.Reactive astrocytes: cellular and molecular cues to biological function.Trends Neurosci.1997205705779416670
- FarinaC.AloisiF.MeinlE.Astrocytes are active players in cerebral innate immunity.Trends Immunol.20072813814517276138
- SofroniewMV.Reactive astrocytes in neural repair and protection.Neuroscientist.20051140040716151042
- TrendelenburgG.DirnaglU.Neuroprotective role of astrocytes in cerebral ischemia: focus on ischemic preconditioning.Glia.20055030732015846804
- ReltonJK.RothwellNJ.lnterleukin-1 receptor antagonist inhibits ischaemic and excitotoxic neuronal damage in the rat.Brain Res Bull.1992292432461388088
- LawrenceCB.AllanSM.RothwellNJ.lnterleukin-1 beta and the interleukin-1 receptor antagonist act in the striatum to modify excitotoxic brain damage in the rat.Eur J Neurosci.199810118811959753187
- HailerNP.VogtC.KorfHW.DehghaniF.lnterleukin-1 beta exacerbates and interleukin-1 receptor antagonist attenuates neuronal injury and microglial activation after excitotoxic damage in organotypic hippocampal slice cultures.Eur J Neurosci.2005212347236015932594
- ThorntonP.PinteauxE.GibsonRM.AllanSM.RothwellNJ.Interleukin1-induced neurotoxicity is mediated by glia and requires caspase activation and free radical release.J Neurochem.20069825826616805812
- MasonJL.SuzukiK.ChaplinDD.MatsushimaGK.lnterleukin-1 beta promotes repair of the CNS.J Neurosci.2001217046705211549714
- HerxLM.YongVW.lnterleukin-1 beta is required for the early evolution of reactive astrogliosis following CNS lesion.J Neuropathol Exp Neurol.20016096197111589427
- OhtsukiT.RuetzlerCA.TasakiK.HallenbeckJM.lnterleukin-1 mediates induction of tolerance to global ischemia in gerbil hippocampal CA1 neurons.J Cereb Blood Flow Metab.199616113711428898685
- StrijbosPJ.RothwellNJ.lnterleukin-1 beta attenuates excitatory amino acid-induced neurodegeneration in vitro: involvement of nerve growth factor.J Neurosci.199515346834747538561
- DeKoskyST.StyrenSD.O'MalleyME.et al.lnterleukin-1 receptor antagonist suppresses neurotrophin response in injured rat brain.Ann Neurol.1996391231278572657
- HerxLM.RivestS.YongVW.Central nervous system-initiated inflammation and neurotrophism in trauma: IL-1 beta is required for the production of ciliary neurotrophic factor.J Immunol.20001652232223910925311
- JuricDM.Carman-KrzanM.lnterleukin-1 beta, but not IL-1 alpha, mediates nerve growth factor secretion from rat astrocytes via type I IL-1 receptor.Int J Dev Neurosci.20011967568311705672
- AppelE.KolmanO.KazimirskyG.BlumbergPM.BrodieC.Regulation of GDNF expression in cultured astrocytes by inflammatory stimuli.Neuroreport.19978330933129351662
- HoA.BlumM.Regulation of astroglial-derived dopaminergic neurotrophic factors by interleukin-1 beta in the striatum of young and middle-aged mice.Exp Neurol.19971483483599398477
- LibertoCM.AlbrechtPJ.HerxLM.YongVW.LevisonSW.Pro-regenerative properties of cytokine-activated astrocytes.J Neurochem.2004891092110015147501
- BrambillaR.Bracchi-RicardV.HuWH.et al.Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury.J Exp Med.200520214515615998793
- BushTG.PuvanachandraN.HornerCH.et al.Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scarforming, reactive astrocytes in adult transgenic mice.Neuron.19992329730810399936
- FaulknerJR.HerrmannJE.WooMJ.TanseyKE.DoanNB.SofroniewMV.Reactive astrocytes protect tissue and preserve function after spinal cord injury.J Neurosci.2004242143215514999065
- MyerDJ.GurkoffGG.LeeSM.HovdaDA.SofroniewMV.Essential protective roles of reactive astrocytes in traumatic brain injury.Brain.20061292761277216825202
- BlockML.ZeccaL.HongJS.Microglia-mediated neurotoxicity: uncovering the molecular mechanisms.Nat Rev Neurosci.20078576917180163
- VincentVA.TildersFJ.Van DamAM.Inhibition of endotoxin-induced nitric oxide synthase production in microglial cells by the presence of astroglial cells: a role for transforming growth factor beta.Glia.1997191901989063726
- HailerNP.WirjatijasaF.RoserN.HischebethGT.KorfHW.DehghaniF.Astrocytic factors protect neuronal integrity and reduce microglial activation in an in vitro model of N-methyl-D-aspartate-induced excitotoxic injury in organotypic hippocampal slice cultures.Eur J Neurosci.20011431532611553282
- MinKJ.YangMS.KimSU.JouI.JoeEH.Astrocytes induce hemeoxy genase-1 expression in microglia: a feasible mechanism for preventing excessive brain inflammation.J Neurosci.2006261880188716467537
- PrehnJH.BackhaussC.KrieglsteinJ.Transforming growth factor-beta 1 prevents glutamate neurotoxicity in rat neocortical cultures and protects mouse neocortex from ischemic injury in vivo.J Cereb Blood Flow Metab.1993135215258097519
- Henrich-NoackP.PrehnJH.KrieglsteinJ.TGF-beta 1 protects hippocampal neurons against degeneration caused by transient global ischemia. Dose-response relationship and potential neuroprotective mechanisms.Stroke.199627160916148784137
- RuoccoA.NicoleO.DocagneF.et al.A transforming growth factorbeta antagonist unmasks the neuroprotective role of this endogenous cytokine in excitotoxic and ischemic brain injury.J Cereb Blood Flow Metab.1999191345135310598939
- McKhannG.DrachmanD.FolsteinM.KatzmanR.PriceD.StadlanEM.Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease.Neurology.1984349399446610841
- SelkoeDJ.Alzheimer disease: mechanistic understanding predicts novel therapies.Ann intern Med.200414062763815096334
- RodriguezJJ.OlabarriaM.ChvatalA.VerkhratskyA.Astroglia in dementia and Alzheimer's disease.Cell Death Differ.20091637838519057621
- ShaoY.GearingM.MirraSS.Astrocyte-apolipoprotein E associations in senile plaques in Alzheimer disease and vascular lesions: a regional immunohistochemical study,J Neuropathol Exp Neurol.1997563763819100668
- SchwabC.McGeerPL.Inflammatory aspects of Alzheimer disease and other neurodegenerative disorders,J Alzheimers Dis.20081335936918487845
- FrautschySA.ColeGM.BairdA.Phagocytosis and deposition of vascular beta-amyloid in rat brains injected with Alzheimer beta-amyloid.Am J Pathol.1992140138913991376558
- Wyss-CorayT.LoikeJD.BrionneTC.et al.Adult mouse astrocytes degrade amyloid-beta in vitro and in situ.Nat Med.2003945345712612547
- NageleRG.D'AndreaMR.LeeH.VenkataramanV.WangHY.Astrocytes accumulate A beta 42 and give rise to astrocytic amyloid plaques in Alzheimer disease brains.Brain Res.200397119720912706236
- PihlajaR.KoistinahoJ.MalmT.SikkilaH.VainioS.KoistinahoM.Transplanted astrocytes internalize deposited beta-amyloid peptides in a transgenic mouse model of Alzheimer's disease.Glia.20085615416318004725
- KurtMA.DaviesDC.KiddM.beta-Amyloid immunoreactivity in astrocytes in Alzheimer's disease brain biopsies: an electron microscope study.Exp Neurol.199915822122810448435
- El KhouryJ.ToftM.HickmanSE.et al.Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease.Nat Med.20071343243817351623
- HenekaMT.SastreM.Dumitrescu-OzimekL.et al.Focal glial activation coincides with increased BACE1 activation and precedes amyloid plaque deposition in APP[V717I] transgenic mice,J Neuroinflammation.200522216212664
- KuchibhotlaKV.LattaruloCR.HymanBT.BacskaiBJ.Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice.Science.20093231211121519251629
- Malchiodi-AlbediF.DomeniciMR.ParadisiS.BernardoA.Ajmone-CatMA.MinghettiL.Astrocytes contribute to neuronal impairment in beta A toxicity increasing apoptosis in rat hippocampal neurons.Glia.200134687211284021
- DomeniciMR.ParadisiS.SacchettiB.et al.The presence of astrocytes enhances beta amyloid-induced neurotoxicity in hippocampal cell cultures.J Physiol Paris.20029631331612445911
- ParadisiS.SacchettiB.BalduzziM.GaudiS.Malchiodi-AlbediF.Astrocyte modulation of in vitro beta-amyloid neurotoxicity.Glia.20044625226015048848
- von BernhardiR.EugeninJ.Microglial reactivity to beta-amyloid is modulated by astrocytes and proinflammatory factors.Brain Res.2004102518619315464759
- SaezET.PeharM.VargasMR.BarbeitoL.MaccioniRB.Production of nerve growth factor by beta-amyloid-stimulated astrocytes induces p75NTR-dependent tau hyperphosphorylation in cultured hippocampal neurons,J Neurosci Res.2006841098110616862561
- ShengJG.MrakRE.GriffinWS.Neuritic plaque evolution in Alzheimer's disease is accompanied by transition of activated microglia from primed to enlarged to phagocytic forms.Acta Neuropathol.199794159224523
- AkamaKT.AlbaneseC.PestellRG.Van EldikLJ.Amyloid beta-peptide stimulates nitric oxide production in astrocytes through an NFkappaB-dependent mechanism.Proc Natl Acad Sci U S A.199895579558009576964
- BiancaVD.DusiS.BianchiniE.DalP.IRossiF.beta-amyloid activates the 0-2 forming NADPH oxidase in microglia, monocytes, and neutrophils. A possible inflammatory mechanism of neuronal damage in Alzheimer's disease.J Biol Chem.1999274154931549910336441
- LuthHJ.MunchG.ArendtT.Aberrant expression of NOS isoforms in Alzheimer's disease is structurally related to nitrotyrosine formation.Brain Res.200295313514312384247
- FarfaraD.LifshitzV.FrenkelD.Neuroprotective and neurotoxic properties of glial cells in the pathogenesis of Alzheimer's disease.J Cell Mol Med.20081276278018363841
- CraftJM.WattersonDM.HirschE.Van EldikLJ.Interleukin 1 receptor antagonist knockout mice show enhanced microglial activation and neuronal damage induced by intracerebroventricular infusion of human betaamyloid.J Neuroinflammation.200521515967035
- BateC.KempsterS.LastV.WilliamsA.Interferon-gamma increases neuronal death in response to amyloid-beta1-42.J Neuroinflammation.20063716569229
- RamirezG.ReyS.von BernhardiR.Proinflammatory stimuli are needed for induction of microglial cell-mediated AbetaPP_{244-C} and Abeta-neurotoxicity in hippocampal cultures.J Alzheimers Dis.200815455918780966
- GoldgaberD.HarrisHW.HlaT.et al.Interleukin 1 regulates synthesis of amyloid beta-protein precursor mRNA in human endothelial cells.Proc Natl Acad Sci U S A.198986760676102508093
- ForloniG.DemicheliF.GiorgiS.BendottiC.AngerettiN.Expression of amyloid precursor protein mRNAs in endothelial, neuronal and glial cells: modulation by interleukin-1.Brain Res Mol Brain Res.1992161281341334190
- BlaskoI.VeerhuisR.Stampfer-KountchevM.Saurwein-TeisslM.EikelenboomP.Grubeck-LoebensteinB.Costimulatory effects of interferongamma and interleukin-1 beta or tumor necrosis factor alpha on the synthesis of Abeta1-40 and Abeta1-42 by human astrocytes.Neurobiol Dis.2000768268911114266
- SparkmanNL.JohnsonRW.Neuroinflammation associated with aging sensitizes the brain to the effects of infection or stress.Neuroimmunomodulation.20081532333019047808
- CraftJM.WattersonDM.FrautschySA.Van EldikLJ.Aminopyridazines inhibit beta-amyloid-induced glial activation and neuronal damage in vivo.Neurobiol Aging.2004251283129215465624
- RalayRH.CraftJM.HuW.GuoL.et al.Glia as a therapeutic target: selective suppression of human amyloid-beta-induced upregulation of brain proinflammatory cytokine production attenuates neurodegeneration.J Neurosci.20062666267016407564
- PraticoD.Evidence of oxidative stress in Alzheimer's disease brain and antioxidant therapy: lights and shadows.Ann N Y Acad Sci.20081147707819076432
- AbramovAY.CanevariL.DuchenMR.Changes in intracellular calcium and glutathione in astrocytes as the primary mechanism of amyloid neurotoxicity,J Neurosci.2003235088509512832532
- AbramovAY.CanevariL.DuchenMR.Calcium signals induced by amyloid beta peptide and their consequences in neurons and astrocytes in culture.Biochim BiophysActa.200417428187
- AbramovAY.CanevariL.DuchenMR.Beta-amyloid peptides induce mitochondrial dysfunction and oxidative stress in astrocytes and death of neurons through activation of NADPH oxidase.J Neurosci.20042456557514724257
- SmitsHA.van BeelenAJ.de VosNM.et al.Activation of human macrophages by amyloid-beta is attenuated by astrocytes.J Immunol.20011666869687611359847
- DeWittDA.PerryG.CohenM.DollerC.SilverJ.Astrocytes regulate microglial phagocytosis of senile plaque cores of Alzheimer's disease.Exp Neurol.19981493293409500964
- FelipoV.ButterworthRF.Neurobiology of ammonia.Prog Neurobiol.2002672597912207972
- TakahashiH.KoehlerRC.BrusilowSW.TraystmanRJ.Inhibition of brain glutamine accumulation prevents cerebral edema in hyperammonemic rats.Am J Physiol.1991261H825H8291679605
- NorenbergMD.JayakumarAR.Rama RaoKV.PanickarKS.New concepts in the mechanism of ammonia-induced astrocyte swelling.Metab Brain Dis.20072221923417823859
- KanamoriK.RossBD.Suppression of glial glutamine release to the extracellular fluid studied in vivo by NMR and microdia lysis in hyperammonemic rat brain.J Neurochem.200594748515953351
- HaussingerD.SchliessF.Pathogenetic mechanisms of hepatic encephalopathy.Gut.2008571156116518628377