Abstract
Continuing to discover how the brain works is one of the great challenges ahead of us. Although understanding the brain anatomy and its functional organization provided a first and indispensable foundation, it became clear that a static view was insufficient. To understand the complexity of neuronal communication, it is necessary to examine the chemical nature of the neurotransmission and, using the example of the acetylcholine receptors, follow the different layers of networks that can be distinguished. The natural alkaloid nicotine contained in tobacco leaves acts as an agonist with a subclass of acetylcholine receptors, and provides an interesting tool to approach brain functions. Analysis of the nicotinic acetylcholine receptors, which are ligand gated channels, revealed that these receptors are expressed at different critical locations on the neurons including the synaptic boutons, neurites, cell bodies, and even on the axons. These receptors can modulate the activity at the microcircuit synaptic level, in the cell processing of information, and, by acting on the velocity of action potential, the synchrony of communication between brain areas. These actions at multiple levels of brain organization provide an example of the complexity of brain neurocircuitry and an illustration of the relevance of this knowledge for psychiatry.
Uno de los grandes desafíos que se nos presenta es el descubrimiento del funcionamiento íntimo de nuestro cerebro. Si bien el análisis de la anatomía del sistema nervioso y de su organizatión estructural permite establecer los fundamentos indispensables; también es claro que una visión estática resulta insuficiente. Para comprender la complejidad de las comunicaciones neuronales nos debemos abocar a la naturaleza de la neurotransmisión y analizar, por ejemplo, el funcionamiento de un sistema de neurotransmisión como es la acetilcolina. La nicotina, que es el alcaloide natural contenido en las hojas de tabaco, activa un subtipo de receptores de acetilcolina que son canales ionotrópicos. El análisis de estos canales activados por la nicotina demuestra que ellos se expresan en diferentes zonas críticas de las neuronas, incluyendo los botones sinápticos, los cuerpos celulares e incluso los axones. La activatión de esos receptores permite modular la transmisión sináptica, la actividad de los microcircuitos neuronales como también la velocidad de propagatión de los potenciales de acción en la sustancia blanca y además modula la sincronía entre diferentes áreas cerebrales. Estos niveles de acción proporcionan un buen ejemplo de la importancia de la plasticidad de los circuitos neuronales que participan en la elaboratión de las señales cerebrales.
Découvrir le fonctionnement intime de notre cerveau représente l'un des grands défis que nous devons franchir. Si l'analyse de l'anatomie du système nerveux et de son organisation structurelle ont permis d'établir des fondements indispensables, il est clair qu'une vue statique reste cependant insuffisante. Pour comprendre la complexité des communications neuronales, nous devons nous pencher sur la nature de la neurotransmission en analysant, par exemple, le fonctionnement d'un système de neurotransmetteur comme l'acétylcholine. La nicotine qui est un alcaloïde naturel contenu dans les feuilles de tabac active un sous-type de récepteurs à l'acétylcholine qui sont des canaux ionotropes. L'analyse de ces canaux activés par la nicotine démontre qu'ils sont exprimés dans différentes zones critiques des neurones incluant les boutons synaptiques, les neurites, les corps cellulaires ou encore les axones. L'activation de ces récepteurs permet de moduler la transmission synaptique, l'activité de microcircuits neuronaux ainsi que la vitesse de propagation des potentiels d'action dans la matière blanche et module la synchronie entre différentes aires du cerveau. Ces niveaux d'action fournissent un bon exemple de l'importance de la plasticité des circuits neuronaux qui participent à l'élaboration des signaux cérébraux.
Understanding how the brain functions requires both a detailed comprehension of its anatomical organization and of the properties of neurons and their communications. A review of the findings on establishing how the brain is organized and on its fundamental properties allows us to contemplate the major progress made and the magnitude of the road that still lies ahead. The first layout of brain organization was provided by studies relying on the abnormalities resulting from lesions of the neuronal tissue, caused either by accidents or by hemorrhages, such as that of the area identified by Paul Broca (1824-1880). The design of the first brain atlas provided a building block in our comprehension of brain structure, with the definition of the Brodmann areas and the design of the first brain atlas (1909). Progress in the knowledge of the fine structure of the brain was marked by the appearance of detailed anatomies, with the description of neurons and their projections carried out by S. Ramón Y Cajal (1852-1934). In spite of the progress made possible by the refinement of brain atlases, and then confirmed by functional magnetic resonance imaging (fMRI), our knowledge of brain function and dysfunction is only slowly progressing.
Developments in the understanding the functional properties of neurons and their communications was marked by a series of fundamental steps. The first was the controversy between the Italian physicist Alessandro Volta (1745-1827) and his compatriot the physician Luigi Galvani (1737-1798), who posited for the first time the existence of “animal electricity.” Proper studies of the electrical properties of neurons had, however, to await the development of electronics, and it was only in 1952 that Alan Hodgkin and Aldous Huxley established the theory explaining action potential properties. It was indispensable to understand how neurons, which are the building blocks of our brain, communicate, and how the electrical signal is transmitted from one cell to another. A contribution to our understanding of neurotransmission was made by the French physiologist Claude Bernard (1813-1878) with his postulate about the existence of a chemical transmitter that relayed the information between the nerve and the muscle. He based his hypothesis on the observation that transmission of the nerve impulse, which normally provokes the contraction of the muscle, was blocked by the plant extract tubocurare, while the muscle still responded to direct electrical stimulation. Subsequently, Otto Loewi (1873-1961) identified that stimulation of the vagus nerve caused the release of a soluble factor that slowed down the heartbeat. First termed “vagus stoff” this substance was soon identified as acetylcholine, and it was found that this molecule activated the G-coupled muscarinic and the ionotropic nicotinic receptors, such as those expressed at the neuromuscular junction. The work of John Eccles (1903-1997) and Bernard Katz (1911-2003) with Ricardo Miledi provided the necessary steps to finally developing the general principles explaining synaptic transmission.
From these studies it became clear that brain function resulted from the electrical activity of neurons measured by the electroencephalogram (EEG). These observations confirmed the relevance of neuronal communications both within and across brain areas. As a consequence, different levels of complexities must be considered, with, on one hand the importance of microcircuits, which are restricted to the level of a group of neurons within a given area, and on the other hand the contribution of macrocircuits allowing communication between brain areas or brain hemispheres. As we shall see, nicotinic acetylcholine receptors significantly contribute to both micro- and macrocircuit levels. In this review we shall examine how acetylcholine can modulate brain function. While this neurotransmitter has a dual action at the G-coupled muscarinic and ionotropic nicotinic receptors, we shall restrict our analysis to ligand gated ion channels.
Nicotine and nicotinic acetylcholine receptors
Ever since the beginning of humanity, mankind has experienced the effect of food or natural substances on the mental state, and use or abuse of psychotropics were known even in the most ancient cultures. The mechanisms by which psychotropic substances exerted their effects were totally unknown; they indirectly showed the interaction of a compound with the brain function. Plants are endowed with many alkaloids, serving different purposes and in certain cases acting as natural insecticides.Citation1 The discovery of America marked the introduction into Europe of new plant species, among them the tobacco plants. Nicotine was named after the French physician Jean Nicot (1530-1600) who used it to treat queen Catherine de Medici's headache. Tobacco usage was progressively extended, becoming widely consumed by about half of the population. That smoking tobacco can become a central issue for many is illustrated by sentences such as the one by Mark Twain (1835-1910): “If I cannot smoke in heaven, then I shall not go.” While it had been recognized that at high concentrations nicotine could affect the neurotransmission at the neuromuscular junction and the conduction of the nerve impulse in ganglia, the action of nicotine on the central nervous system remained for a long time a theme of controversy. The development of molecular biology and the identification of an entire family of genes encoding for nicotinic receptor subunits settled this controversy with the demonstration of the broad expression of nicotinic acetylcholine receptors in the central nervous system. Today seventeen genes encoding for the muscle and neuronal nicotinic acetylcholine receptors (nAChRs) have been identified in mammals.Citation2 Muscle and neuronal nAChRs result from the assembly of five subunits around an axis of pseudosymmetry. At their simplest neuronal nAChRs are homomeric (constituted from five identical subunitsCitation3) while the more complex forms are heteromeric, composed of at least one α and one β subtype (reviewed in refs 2,4). Combination of various αs and βs allow for a large number of receptor subtypes to be formed. The physiological and pharmacological properties of the receptors depend upon both the α and β subunits expressed in the functional complexes.
Analysis of human, mouse, and other animal genomes revealed that nicotinic acetylcholine receptors have been conserved throughout evolution, and that ancestor genes appeared early, in the simplest forms of life. Indeed, more nicotinic receptor genes have been identified in mollusks than in vertebrates, indicating that these receptors may play an even larger role in invertebrates. The observation of this high degree of conservation called for further reflections about the role of these receptors in brain activity, and forced the design of new experiments to examine their function and dysfunction in the central nervous system. Labeling experiments carried out with different probes revealed that nicotinic receptors are widely expressed both in the cortex, white matter, and in groups of neurons in brain nuclei.Citation5 For example, intense labeling was observed in the fasciculus retroflexus, which connects the habenula to the interpeduncular nuclei.Citation6 The development of novel molecules, such as A-85380, which label with high affinity the major brain a432 nicotinic receptors, opened up the possibility of measuring receptor distribution using positron emission tomography.Citation7-Citation9 Confirming the high degree of receptor expression in the thalamus, these studies were rapidly extended to pathological conditions.Citation5,Citation10,Citation11 Importantly, significant labeling was also observed in the white matter.Citation5 While providing further evidence about the importance of the nicotinic receptors they also highlight the need for more precise mapping of the receptor distribution with ligands of the receptor subtypes in normal or pathological conditions. Obtaining precise mapping of receptor distribution becomes indispensable for understanding the role of nAChRs in brain function. Progress toward this goal has, however, been hampered by the absence of selective antibodies, as shown by studies carried out in knockout animals.Citation12
The isolation of the genes encoding for the nicotinic receptors allowed their reconstitution in host systems and, consequently, their functional characterization. Experiments carried out in Xenopus oocytes or in cell lines expressing the human receptors confirmed that these ligand gated channels are permeable to cations, causing a depolarization of the cell when they are activated.Citation2,Citation13,Citation14 The ionic selectivity of nAChRs differs markedly in function of the receptor subtype. For example, while the muscle receptors display a very low permeability to the divalent calcium ions, the homomeric α7 nAChRs present a higher permeability to calcium than sodium.Citation15 Activation of α7 nAChRs was shown to increase the intracellular calcium concentration and, for receptors expressed presynaptically, indirectly causing neurotransmitter release.
Allowing for the first time the evaluation of the properties of human receptors in vitro, these studies also revealed some of their distinct properties. Nicotinic acetylcholine receptors are characterized by the fact that the amplitude of their response depends upon the membrane potential of the cell, causing a physiological effect only when the cell is hyperpolarized. This voltage dependence, or inward rectification, will contribute to neuron function in processes such as coincidence detection of different events.Citation13,Citation14 Another, very important, property of the nicotinic receptors is their high permeability to calcium. The most calcium-permeable subtype is the homomeric α7 receptor.Citation15,Citation16 The calcium influx caused by activation of the α7 nicotinic receptors is sufficient to trigger different cellular effects and was shown, in presynaptic boutons, to control or modulate the release of neurotransmitters.Citation2,Citation17-Citation21 Numerous examples have now been provided, confirming the physiological relevance of nicotinic receptors in controlling the synaptic transmission of synapses in which the signal is mediated by other neurotransmitters.
In natural conditions, activation of nicotinic acetylcholine receptors is caused by the release of acetylcholine. It was, however, shown that α7 receptors are also activated by high concentrations of choline and it was proposed that choline released by the breakdown of acetylcholine by acetylcholine esterase might play a role in controlling these receptors' activity.Citation4
Modulation at the receptor level
Functional properties of the nicotinic cholinergic system are finely tuned by different mechanisms, including receptor phosphorylation and allosteric modulations. For example, it was shown that the level of phosphorylation of the α7 receptors controls the amplitude of the acetylcholine-evoked current without changing the response time course.Citation22 A first example of allosteric modulation of the α7 nicotinic receptors was provided with the observation of the effects caused by the anthelmintic drug ivermecticine.Citation23 Since then several molecules have been shown to modulate α7 receptor activity, with the most powerful effects caused by PNU120596.Citation24 Moreover, it was shown that α7 receptor activity is modulated by endogenous polypeptides.Citation25-Citation29 Modulation of receptor function is not restricted to α7 receptors, but has also been observed for heteromeric receptors. Divalent cations, such as calcium and zinc, can bind in the N-terminal extracellular domain of the receptors and modulate their activity. Exposure to zinc, in the µM range, potentiates the α4β2 or α4β4 receptor subtypes whereas it inhibits the α3β2 receptors, further exemplifying the pharmacological complexity associated with heteromeric receptor combinations.Citation30 Recalling that zinc is released during synaptic transmission, such modulation is supposed to provide an additional mechanism by which cells can regulate receptor function. Potentiation of the major brain α4β2 subtype by 17-β-estradiol provides still another example of nicotinic receptor modulation.Citation31
Importance of nAChRs for brain microcircuits
Since the discovery by S. Ramón Y Cajal of the structural organization of the cortical layers, it has become evident that brain function depends upon the activation of small neuronal networks, the activity of which is transmitted to the next layer of integration. The description of column activities in the visual cortex by D. Hubel and T Wiesel in 1959 confirmed the hypothesis formulated by Cajal. The cortical organization and columns converge on the pyramidal cells from layer 6 that project their axons toward other brain areas. Often seen as a computational unit with recurrent feedback, the pyramidal cell receives converging information from its apical tuft and from dendrites localized on its cell body. An important particularity of the pyramidal cell is the back propagation of the action potential from the cell body along the proximal dendrite.Citation32,Citation33 This process was shown to enhance or inhibit the signals transmitted by the apical tuft, and provides an exquisite mechanism for the integration of multiple inputs (). This mechanism depends on the firing rate of action potentials in the axon hillock, as well as synchrony of activities, and it was shown that back propagation can be disrupted by the activation of ion channels expressed along the principal dendrite.Citation34,Citation35
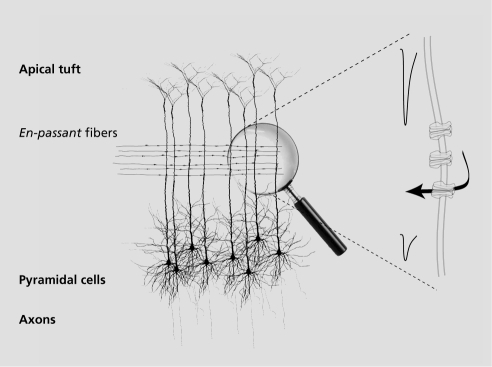
Nicotinic receptors are broadly expressed in the cortical area with some subtypes such as α7 receptors preponderantly expressed in presynaptic areas, whereas heteromeric receptors are expressed on cell bodies, main dendrites, axons, etc.Citation36-Citation38 Nicotine exposure has been shown to enhance attention and working memory by activating nicotinic receptors. Although our understanding of these effects remains limited, nicotine increases the threshold for synaptic spike-timingdependent-potentiation (STDP) in layer 5 of the prefrontal cortex of the mouse.Citation39 Systemic administration of nicotinic agonists such as SSR180711 or PNU-282987 has shown beneficial cognitive effects and reversal of amphetamine-induced deficits, thought to be mediated by acting at the microcircuit cortical level.Citation40,Citation41
Importantly, cholinergic projections that diffusely innervate the cortex are thought to make en-passant connections in the area of the principal dendrite of the pyramidal cells from layer 5 and 6.Citation42 Activation of these fibers causes the release, in a volumic manner, of acetylcholine that will diffuse and slowly activate receptors expressed on the principal dendrite. Opening of the nicotinic acetylcholine receptors reduces the membrane resistance and attenuates signals coming from the apical dendrites. This reduction of the influence of the apical tuft and external layer contributions modifies the integration and “computation” of the pyramidal cell. Altogether, acetylcholine can act at different levels ranging from synaptic release, signal transmission, and resting membrane potential to modulate microcircuit activity.
Importance of nAChRs in brain macrocircuits
Communication between brain areas and between hemispheres relies on the conduction of the action potential along the axons. Brain axons can be myelinated or unmyelinated, with the latter displaying the slowest conduction velocity. Importantly, however, conduction velocity of myelinated axons is limited by the diameter of the fibers and ranges between 3.5 m/s in the visual cortex to at most 29 m/s in the thalamocortical pathway.Citation43-Citation45 This implies that for a distance of about 10 cm the propagation time ranges between 3.5 and 28 ms which is not negligible. Further complexity when considering the velocity of conduction is added when taking into account the different parameters dictating this velocity. Two determinant parameters are: (i) the physical properties of the axon with its diameter and space constant (the distance at which an electrical signal is attenuated by a factor of 2); and (ii) the membrane properties at the nodes of Ranvier. A wider expression of sodium channels in the node of Ranvier yields a larger inward current during the action potential and a faster velocity of conduction. This is well illustrated by the slowing of the conduction velocity observed during local anesthesiaCitation46 by a compound such as lidocaine. Conduction velocity at the node of Ranvier also depends on the presence of additional ion channels. Activation of channels causing an increase in the membrane conductance reduces the efficiency of the sodium channel effects. Activation of channels causing a depolarization of the node of Ranvier progressively inactivates the sodium channels, yielding a reduction in the sodium current and of the amplitude of the action potential. As it was shown that nAChRs are expressed at axonal level and are present in human white matter,Citation2,Citation5,Citation6 activation of these cationic receptors is expected to modulate conduction velocity. In agreement with this hypothesis, exposure to cholinergic drugs was shown to reduce conduction velocity of the habenulointerpeduncular pathway.Citation47 This might be of clinical relevance since lesions of this pathway in rats produce anxiety and hyperlocomotor activity.Citation48
Brain circuit dysfunction
In spite of the major progress made in brain imaging, our knowledge about the timing of propagation and relevance of brain activities remains limited. While the use of fMRI has increased our knowledge about brain areas involved in a given set of functions, these techniques have a temporal resolution that is orders of magnitudes too slow with respect to the actual brain processes. Electroencephalography with a large number of scalp electrodes, or in some cases, with deep brain electrodes, provides a much better time resolution but its usefulness remains limited by the issue of spatial resolution.
Data obtained by fMRI have shown that even brain functions that are thought to be simple require the contribution of activities from several areas. As communication between these areas must propagate along the small axon fibers from cortical neurons, modulation of the conduction velocity is expected to change the synchronicity of signals.
Although our knowledge of the exact computational tasks of specific brain areas, such as the hippocampus and the entorhinal cortex, or the prefrontal cortex and the thalamus, is limited, it is clear that a change in synchrony can lead to a different outcome of the function. Taking for example the interpretation of language and phonation that are known to involve the Wernicke and Broca areas, these distinct cortical structures are heavily connected by the arcuate fasciculus. Alteration of arcuate fasciculus function can lead in the extreme to the type of aphasia known as conduction aphasia. The importance of the arcuate fasciculus clearly illustrates the relevance of conduction of brain activity between brain areas. An even higher degree of complexity is easily understood when imaging the brain areas involved in the recognition of a voice calling a given name, and the need to recognize our own name before reacting and answering “yes I am here.”
Several psychiatric disorders ranging from schizophrenia and chronic depression to post-traumatic stress disorder have been associated with white-matter defects.Citation49 While some of these conditions may correlate with abnormalities in the development of brain connectivity, others are probably associated with temporary or successively permanent impairment of the white matter functionality. Proper timing of electrical activity in different areas of the brain is certainly imperative for the overall function of the brain. For example it can be foreseen that associative functions rely on the proper timing of electrical activities from many distinct brain areas.
Disruption of synchrony between single cells in the hippocampal and prefrontal cortex in an animal model was recently shown to alter acquisition tasks that are thought to be altered in schizophrenia.Citation50 Moreover, in humans, using complex electroencephalographic techniques, it was shown that successful memory formation is predicted by a tight coordination of spike timing and theta oscillation (4 to 10 Hz).Citation51 While confirming the need for accurate timing of signal transmission these data highlight the potential modulatory role that acetylcholine can play by adjusting the velocity conduction between brain areas, and therefore the time correlation between signals from different origins.
Nicotinic receptors and brain dysfunction
Examining the correlation between genetic alterations and brain dysfunctions can provide a further understanding of the role of a given receptor set. Analysis of genetically transmissible forms of epilepsy revealed that a particular form of nocturnal epilepsy was associated with a variation in CHRNA4, the gene encoding for the α4 subunit of the nicotinic acetylcholine receptors.Citation52 This initial finding was soon followed by the description of other mutations in this gene and the contribution of nicotinic acetylcholine receptors was confirmed with the identification of additional mutations in CHRNB2, the gene encoding for the α2 subunit that is indispensable to produce functional receptors (reviewed in ref 53). The autosomal dominant nature of the mutation, together with the characteristics of these epilepsies, which are nocturnal and have a frontal lobe origin, lead to the nomenclature of this syndrome as “autosomal dominant nocturnal frontal lobe epilepsy” or ADNFLE. Importantly, all mutations identified so far yield a gain of function of the α4β2 nicotinic acetylcholine receptors. This suggests that inhibition of these receptors is expected to reduce the severity of the symptoms. Indeed, epilepsies can be controlled by low concentrations of the antiepileptic drug carbamazepine (CBZ) in many patients. In some families this treatment remains, however, inefficacious. Studies of control and mutated nicotinic acetylcholine receptors corresponding to these different families revealed in most cases a high sensitivity to CBZ, which inhibits the α4α2 nicotinic acetylcholine receptors, whereas for other mutations sensitivity to CBZ was reduced.Citation54-Citation56
A more detailed examination of the patient conditions in different families presenting ADNFLE revealed that some mutations are also associated with significant cognitive impairments.Citation53
Labeling studies performed in monkey and rat brain have revealed significant differences in the level of expression of specific subunits. For example it was shown that the α2 subunit is largely expressed in the prefrontal cortex of the monkey brain, whereas its level of expression is much lower in the rat.Citation57 The discovery of a mutation in the CHRNA2, the gene encoding for the α2 subunit of the nicotinic acetylcholine receptor, and its association with one form of genetically transmissible epilepsy opened a new chapter in our understanding of the link between the cholinergic system and neurological diseases.Citation58,Citation59
These data are in good agreement with the hypothesis of the crucial role played by the cholinergic system in cognitive functions. A progressive decline in cholinergic tone has been proposed to correlate with the cognitive impairments observed in patients with Alzheimer disease. To compensate for the loss of cholinergic tone, cholinesterase inhibitors has been introduced in clinical practice and, more recently, nicotinic agonists have been studied in clinical trials. These clinical trials represent the first step toward the design of additional studies aimed at further reducing or compensating for the effects of low acetylcholine levels in the brain.
Conclusions
Natural alkaloids, such as nicotine, can have multiple effects on our body, and these have been recognized since the earliest times. A long series of discoveries was, however, necessary to obtain enough information about the effects that such natural alkaloid have on central nervous system function. The identification of the genes encoding for neuronal nicotinic acetylcholine receptors marked a turning point in our approaches to the functional properties of the brain, and led to the characterization of receptors that are activated by low concentrations of nicotine. These receptors, which are permeable to cations, cause multiple effects depending upon their cellular and subcellular localization. Analyses of the receptor distribution and functions at the microcircuit level indicate that these receptor can modulate the release of neurotransmitters, affecting the signal integration and processing that is taking place at the cortical level. Receptors expressed in the white matter have been shown to modulate the velocity of propagation of the action potential and thereby modify the timing of activity between brain areas. In view of the critical role played by the synchronization between different brain areas in cognitive tasks and learning, it appears that control of the velocity of action potential transmission is determinant for high-level brain functions. Genetic analysis and associations observed between mutations in nicotinic acetylcholine receptor genes and neurological disorders has confirmed the relevance of nicotinic receptors in humans, and pave the way for future pharmacogenomic studies.
REFERENCES
- CoffinOT.RadenDJ.Nicotine and beta-beta-dichlorethyl ether insecticide. No. 2,459,138.J Pat Off Soc. 1949108459
- DaniJA.BertrandD.Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system.Annu Rev Pharmacol Toxicol. 20074769972917009926
- CouturierS.BertrandD.MatterJM.et alA neuronal nicotinic acetylcholine receptor subunit (alpha 7) is developmentally regulated and forms a homo-oligomeric channel blocked by alpha-BTX.Neuron. 199058478561702646
- AlbuquerqueEX.PereiraEF.AlkondonM.RogersSW.Mammalian nicotinic acetylcholine receptors: from structure to function.Physiol Rev. 2009897312019126755
- DingYS.FowlerJS.LoganJ.et al6-[18F]Fluoro-A-85380, a new PET tracer for the nicotinic acetylcholine receptor: studies in the human brain and in vivo demonstration of specific binding in white matter.Synapse. 20045318418915236351
- PerryDC.XiaoY.NguyenHN.MusachioJL.Dávila-GarcíaMI.KellarKJ.Measuring nicotinic receptors with characteristics of alpha4beta2, alpha3beta2 and alpha3beta4 subtypes in rat tissues by autoradiography.J Neurochem. 20028246848112153472
- RueterLE.Donnelly-RobertsDL.CurzonP.BriggsCA.AndersonDJ.BitnerRS.A-85380: a pharmacological probe for the preclinical and clinical investigation of the alphabeta neuronal nicotinic acetylcholine receptor.CNS Drug Rev. 20061210011216958984
- KimesAS.HortiAG.LondonED.et al2-[18F]F-A-85380: PET imaging of brain nicotinic acetylcholine receptors and whole body distribution in humans.FASEB J. 2003171331133312759330
- SullivanJP.Donnelly-RobertsD.BriggsCA.et alA-85380 [3-(2(S)-azetidinylmethoxy) pyridine]: in vitro pharmacological properties of a novel, high affinity alpha 4 beta 2 nicotinic acetylcholine receptor ligand.Neuropharmacology. 1996357257348887981
- PicardF.BruelD.ServentD.et alAlteration of the in vivo nicotinic receptor density in ADNFLE patients: a PET study.Brain. 20061292047206016815873
- CollobySJ.PerryEK.PakrasiS.et alNicotinic 123I-5IA-85380 single photon emission computed tomography as a predictor of cognitive progression n in Alzheimer's disease and dementia with Lewy bodies.Am J Geriatr Psychiatry. 201018869020094022
- JonesIW.WonnacottS.Why doesn't nicotinic ACh receptor immunoreactivity knock out?Trends Neurosci. 20052834334515979499
- GopalakrishnanM.BuissonB.ToumaE.et alStable expression and pharmacological properties of the human alpha 7 nicotinic acetylcholine receptor.Eur J Pharmacol. 19952902372467589218
- BuissonB.GopalakrishnanM.ArnericSP.SullivanJP.BertrandD.Human alpha4beta2 neuronal nicotinic acetylcholine receptor in HEK 293 cells: a patch-clamp study.J Neurosci. 199616788078918987816
- BertrandD.GalziJL.Devillers-ThiéryA.BertrandS.ChangeuxJP.Mutations at two distinct sites within the channel domain M2 alter calcium permeability of neuronal alpha 7 nicotinic receptor.Proc Natl Acad Sci U S A. 199390697169757688468
- SéguélaP.WadicheJ.Dineley-MillerK.DaniJA.PatrickJW.Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium.J Neurosci. 1993135966047678857
- WanaverbecqN.SemyanovA.PavlovI.WalkerMC.KullmannDM.Cholinergic axons modulate GABAergic signaling among hippocampal interneurons via postsynaptic alpha7 nicotinic receptors.J Neurosci. 2007275683569317522313
- KiyosawaA.KatsurabayashiS.AkaikeN.PangZP.Nicotine facilitates glycine release in the rat spinal dorsal horn.J Physiol. 200153610111011579160
- WonnacottS.Gates and filters: unveiling the physiological roles of nicotinic acetylcholine receptors in dopaminergic transmission.Br J Pharmacol. 2008153(suppl 1)S2S418246098
- ExleyR.CraggSJ.Presynaptic nicotinic receptors: a dynamic and diverse cholinergic filter of striatal dopamine neurotransmission.Br J Pharmacol. 2008153(suppl 1)S283S29718037926
- DickinsonJA.KewJN.WonnacottS.Presynaptic alpha 7- and beta 2-containing nicotinic acetylcholine receptors modulate excitatory amino acid release from rat prefrontal cortex nerve terminals via distinct cellular mechanisms.Mol Pharmacol. 20087434835918445710
- CharpantierE.WiesnerA.HuhKH.etal.alpha 7 neuronal nicotinic acetylcholine receptors are negatively regulated by tyrosine phosphorylation and Src-family kinases.J Neurosci. 2005259836984916251431
- KrauseRM.BuissonB.BertrandS.et alIvermectin: a positive allosteric effector of the alpha 7 neuronal nicotinic acetylcholine receptor.Mol Pharmacol. 1998532832949463487
- HurstRS.HajósM.RaggenbassM.et alA novel positive allosteric modulator of the alpha 7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization.J Neurosci. 2005254396440515858066
- ChimientiF.HoggRC.PlantardL.et alIdentification of SLURP-1 as an epidermal neuromodulator explains the clinical phenotype of Mal de Meleda.Hum Mol Genet. 2003123017302414506129
- MiwaJM.Ibanez-TallonI.CrabtreeGW.et alLynx1, an endogenous toxin-like modulator of nicotinic acetylcholine receptors in the mammalian CNS.Neuron. 19992310511410402197
- Ibañez-TallonI.MiwaJM.WangHL.et alNovel modulation of neuronal nicotinic acetylcholine receptors by association with the endogenous prototoxin lynx1.Neuron. 20023389390311906696
- MiwaJM.StevensTR.KingSL.et alThe prototoxin lynx1 acts on nicotinic acetylcholine receptors to balance neuronal activity and survival in vivo.Neuron. 20065158760016950157
- TekinayAB.NongY.MiwaJM.et alA role for LYNX2 in anxiety-related behavior.Proc Natl Acad Sci U S A. 20091064477448219246390
- HsiaoB.MihalakKB.RepickySE.et alDeterminants of zinc potentiation on the alpha 4 subunit of neuronal nicotinic receptors.Mol Pharmacol. 200669273616189299
- CurtisL.BuissonB.BertrandS.BertrandD.Potentiation of human alpha4beta2 neuronal nicotinic acetylcholine receptor by estradiol.Mol Pharmacol. 20026112713511752213
- LarkumME.ZhuJJ.SakmannB.Dendritic mechanisms underlying the coupling of the dendritic with the axonal action potential initiation zone of adult rat layer 5 pyramidal neurons.J Physiol. 200153344746611389204
- StuartGJ.SakmannB.Active propagation of somatic action potentials into neocortical pyramidal cell dendrites.Nature. 199436769728107777
- BergerT.LüscherHR.Timing and precision of spike initiation in layer V pyramidal cells of the rat somatosensory cortex.Cereb Cortex. 20031327428112571117
- BergerT.SennW.LüscherHR.Hyperpolarization-activated current Ih disconnects somatic and dendritic spike initiation zones in layer V pyramidal neurons.J Neurophysiol. 2003902428243712801902
- TribolletE.BertrandD.MargueratA.RaggenbassM.Comparative distribution of nicotinic receptor subtypes during development, adulthood and aging: an autoradiographic study in the rat brain.Neuroscience. 200412440542014980390
- JonesIW.WonnacottS.Precise localization of alpha7 nicotinic acetylcholine receptors on glutamatergic axon terminals in the rat ventral tegmental area.J Neurosci. 200424112441125215601930
- WeversA.Localisation of pre- and postsynaptic cholinergic markers in the human brain.Behav Brain Res. 2010In press.
- CoueyJJ.MeredithRM.SpijkerS.et alDistributed network actions by nicotine increase the threshold for spike-timing-dependent plasticity in prefrontal cortex.Neuron. 200754738717408579
- BitonB.BergisOE.GalliF.et alSSR180711, a novel selective alpha7 nicotinic receptor partial agonist: (1) binding and functional profile.Neuropsychopharmacology. 20073211617019409
- HajósM.HurstRS.HoffmannWE.et alThe selective alpha7 nicotinic acetylcholine receptor agonist PNU-282987 [N-[(3R)-1-Azabicyclo[2.2.2]oct3-yl]-4-chlorobenzamide hydrochloride] enhances GABAergic synaptic activity in brain slices and restores auditory gating deficits in anesthetized rats.J Pharmacol Exp Ther. 20053121213122215523001
- DescarriesL.GisigerV.SteriadeM.Diffuse transmission by acetylcholine in the CNS.Prog Neurobiol. 1997536036259421837
- GirardP.HupéJM.BullierJ.Feedforward and feedback connections between areas V1 and V2 of the monkey have similar rapid conduction velocities.J Neurophysiol. 2001851328133111248002
- BojakI.LileyDT.Axonal velocity distributions in neural field equations.PLoS Comput Biol. 20106e100065320126532
- KimuraT.OzakiI.HashimotoI.Impulse propagation along thalamocortical fibers can be detected magnetically outside the human brain.J Neurosci. 200828125351253819020045
- GokinAP.PhilipB.StrichartzGR.Preferential block of small myelinated sensory and motor fibers by lidocaine: in vivo electrophysiology in the rat sciatic nerve.Anesthesiology. 2001951441145411748404
- BrownDA.DochertyRJ.HalliwellJV.The action of cholinomimetic substances on impulse conduction in the habenulointerpeduncular pathway of the rat in vitro.J Physiol. 19843531011096481621
- MurphyCA.DicamilloAM.HaunF.MurrayM.Lesion of the habenular efferent pathway produces anxiety and locomotor hyperactivity in rats: a comparison of the effects of neonatal and adult lesions.Behav Brain Res. 19968143528950000
- FieldsRD.White matter in learning, cognition and psychiatric disorders.Trends Neurosci. 20083136137018538868
- SigurdssonT.StarkKL.KarayiorgouM.GogosJA.GordonJA.Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia.Nature. 201046476376720360742
- RutishauserU.RossIB.MamelakAN.SchumanEM.Human memory strength is predicted by theta-frequency phase-locking of single neurons.Nature. 201046490390720336071
- SteinleinOK.MulleyJC.ProppingP.et alA missense mutation in the neuronal nicotinic acetylcholine receptor alpha 4 subunit is associated with autosomal dominant nocturnal frontal lobe epilepsy.Nat Genet. 1995112012037550350
- SteinleinOK.BertrandD.Nicotinic receptor channelopathies and epilepsy.Pflugers Arch . 200949049550320016990
- PicardF.BertrandS.SteinleinOK.BertrandD.Mutated nicotinic receptors responsible for autosomal dominant nocturnal frontal lobe epilepsy are more sensitive to carbamazepine.Epilepsia. 1999401198120910487182
- HoggRC.RaggenbassM.BertrandD.Nicotinic acetylcholine receptors: from structure to brain function.Rev Physiol Biochem Pharmacol. 200314714612783266
- ItierV.BertrandD.Mutations of the neuronal nicotinic acetylcholine receptors and their association with ADNFLE.Neurophysiol Clin. 2002329910712035491
- HanZY.Le NovèreN.ZoliM.HillJA.ChamptiauxN.ChangeuxJP.Localization of nAChR subunit mRNAs in the brain of Macaca mulatta.Eur J Neurosci. 2000123664367411029636
- HodaJC.WanischeckM.BertrandD.SteinleinOK.Pleiotropic functional effects of the first epilepsy-associated mutation in the human CHRNA2 gene.FEBS Lett.20095831599160419383498
- AridonP.MariniC.Di RestaC.et alIncreased sensitivity of the neuronal nicotinic receptor alpha 2 subunit causes familial epilepsy with nocturnal wandering and ictal fear.Am J Hum Genet. 20067934235016826524