Abstract
Observing another individual acting upon an object triggers cerebral activity well beyond the visual cortex of the observer in areas directly involved in planning and executing actions. This we will call action simulation. Importantly, the brain does not solely simulate the actions of others but also the sensations they feel, and their emotional responses. These simulation mechanisms are most active in individuals who report being very empathic. Simulation may indeed be instrumental for our understanding of the emotional and mental state of people in our sight, and may contribute heavily to the social interactions with our peers by providing a first-person perspective on their inner feelings. Simulation mechanisms are at work at an early stage of social development and might be defective in young individuals with autism spectrum disorders (ASD). However, the results to date regarding ASD are not clearcut, and an equal number of studies report positive and negative findings.
El observar la acción de otro individuo sobre un objeto gatilla actividad cerebral bastante más allá de la corteza visual del observador en áreas directamente involucradas en acciones de planeamiento y ejecución. Esto se denominará acción de simulación. Es importante considerar que el cerebro no sólo simula las acciones de otros, sino también las sensaciones que ellos sienten y sus respuestas emocionales. Estos mecanismos de simulación son más activos en individuos que refieren ser muy empáticos. Ciertamente la simulación puede constituir un instrumento para nuestra comprensión del estado mental y emocional de las personas ante nosotros, y puede contribuir potentemente a las interacciones sociales con nuestros pares al entregar una perspectiva en primera persona de sus sentimientos internos. Los mecanismos de simulación comienzan a operar en etapas precoces del desarrollo social y pueden ser defectuosos en sujetos jóvenes con trastornos del espectro autista (TEA). Sin embargo, los resultados a la fecha en relación con los TEA no son definitivos y existe igual número de estudios que entregan hallazgos tanto positivos como negativos.
Observer quelqu'un d'autre jouer avec un objet déclenche une activité cérébrale bien au-delà du cortex visuel de l'observateur, dans des aires directement impliquées dans des actions de planification et d'exécution. C'est ce que nous appellerons une simulation de l'action. Il faut noter que le cerveau ne simule pas seulement les agissements des autres mais aussi leurs sensations et leurs réponses émotionnelles. Ces mécanismes de simulation sont plus actifs chez les personnes qui disent être très empathiques. La simulation peut en effet jouer un rôle clé dans notre compréhension de l'état mental et émotionnel de personnes de notre entourage et peut fortement contribuer aux interactions sociales avec nos pairs en leur fournissant une perspective personnelle sur leurs sentiments intimes. Des mécanismes de simulation sont actifs à un stade précoce du développement social et peuvent être absents chez les jeunes autistes. Cependant, les résultats concernant les autistes ne sont pas nets à ce jour et il existe autant d'études positives que négatives.
Mirror neurons and shared circuits for action execution and observation
Mirror neurons were first discovered in the ventral premotor cortex of the monkey (area F5), a cortical region that was studied for its involvement in action preparation. They have the astonishing property of firing not only during action execution, but also as the monkey observes another individual performing a similar action, or just upon hearing the sound of the action.Citation1-Citation5 With the firing of these neurons, the monkey can be said to simulate the actions of its conspecifics in that it activates premotor neurons “as if” performing a similar action. Later on, neurons with the same property were also found in the inferior parietal cortex of the monkey.Citation6,Citation7
In humans, noninvasive brain imaging techniques have provided ample evidence that the premotor and parietal cortices are not only active during the planning and execution of actions, but also while someone is observing or listening to the action performed by someone else (). Citation8-Citation13 The presence of shared circuits for action execution and action perception is classically attributed to the functioning of mirror neurons.
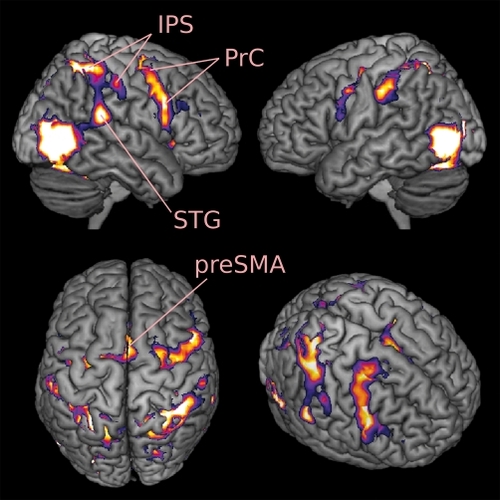
Shared circuits for somatosensation
Importantly, simulation is not restricted to cortices involved in motor planning: the somatosensory cortex also seems capable of vicarious activity.Citation14-Citation16 It is helpful to distinguish two forms of somatosensations: passive touch, where a body is touched by an object, and active touch, where an individual deliberately touches an object. For passive touch, evidence accumulates that while the first levels of cortical somatosensory processing (BA3) only responds when the participant experiences passive touch directly, the higher levels (BA1, 2, and SII) can also be activated vicariously by the mere sight of someone else being touched, with this vicarious activity being most robustly observed in SII.Citation17-Citation21 For active touch, BA3 is again only recruited while participants manipulate objects themselves, but BA2 seems to be the region most robustly recruited while viewing other individuals manipulate objects.Citation10,Citation15 During the observation of active touch, simulation in the motor system seems to go hand in hand with somatosensory simulation in the higher levels of the somatosensory system: BA2 and also sometimes SII ().
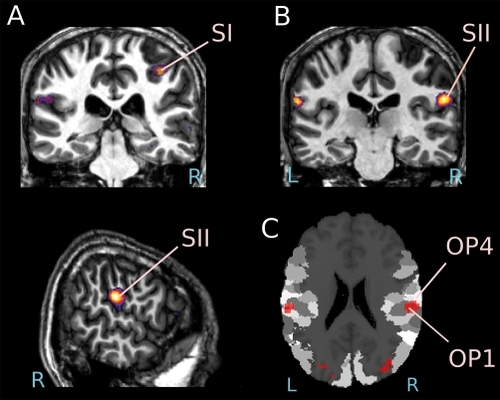
Pathological overactivation of the shared circuits for actions and tactile sensations
In most situations, one does not experience an actual sensation of touch upon seeing someone else being touched or touching an object. Likewise, one does not normally imitate every movement made by others. Somehow, the brain can compute a simulation in higherlevel areas (premotor and posterior parietal areas for actions, and SII and BA2 for somatosensations) without this simulation contaminating the primary motor cortex or the lower levels of somatosensory perception. In an analogy to computers, in which untrusted programs are “sandboxed,” ie, given limited access to resources to ensure that they will not cause damage, the brain seems to sandbox simulations of other people's actions and sensations to ensure that they can run safely, without causing unwanted body movements and misattributed sensations. There are instances, however, where this sandboxing mechanism loses its effectiveness.
Following brain injury, some patients show a spontaneous tendency to imitate an experimenter performing various gestures in front of them - scratching their forehead, clapping their hands, and so on.Citation22-Citation24 The patients keep imitating the behavior of the experimenter even after being explicitly told to stop doing so. This phenomenon affects as many as 4 out of 10 patients with frontal-lobe lesions, and virtually never occurs as a consequence of postrolandic brain lesion.Citation22 Infarct to the anterior cerebral artery resulting in medial frontal lesions seems to be a frequent cause. Imitation behaviors demonstrate the automatic aspect of simulation. Medial frontal lesions may impair the functioning of a gating system, resulting in the release of activity in the primary motor region.
About 1% of individuals also seem to experience the tactile sensations of others as if they had happened to themselves.Citation25 One of these mirror-touch synesthetesCitation17 experienced touch upon seeing someone else being touched, but not when an object was touched. The feeling of touch was experienced on the same body part as that being touched on the other person. Functional MRI revealed a hyperactivation of the somatosensory cortices, the premotor cortex, and the anterior insula relative to controls during the observation of a video of someone being touched. Increased activity in the primary somatosensory cortex (SI) encompassing earlier stages of somatosensory perception may possibly provoke this phenomenon by which the feelings of others invade an area that would normally be reserved for the self. Participants with this form of synesthesia also report being more empathic.Citation26
Shared circuits for pain and disgust
The possible importance of shared circuits for understanding the emotions of others also became clear early on,Citation27 with several studies demonstrating that perceiving (or imagining) someone else in pain as well as witnessing disgust on the face of someone provokes an increase of activity in several brain areas involved in the first-person experience of these emotions. In one experiment, the participants viewed people taking a sip from a glass and being either disgusted, pleased, or neutral. Disgust observation was accompanied by a specific increase of activity in the anterior insular cortex,Citation28 an area shown to be strongly activated by the experience of disgust in the same participants. Moreover, another experiment using a similar paradigm found that the experience and the observation of strong gustatory pleasure can also trigger activity in a similar sector of the insula, suggesting that this region is not devoted only to the processing of negative emotions.Citation29 Using Granger causality analysis, this vicarious activity in the insula appears to be triggered by activity in the inferior frontal gyrus,Citation30 a region active both while viewing facial expressions and while performing similar expressions.Citation31,Citation32 This suggests that the insula performs an emotional simulation of what it would feel like to experience the positive or negative emotions of others, and that this simulation can be triggered by inputs from the region performing a motor simulation of the observed facial expressions. Multiple experiments have also demonstrated the involvement of the anterior cingulate cortex and the insula during pain observation. Increased activity is found in these regions when the participants are shown body parts in various painful situations,Citation33-Citation39 as well as when observing a painful facial expression,Citation40,Citation41 or just upon knowing that a loved one is experiencing pain.Citation42,Citation43 Furthermore, in at least two experiments, the level of activity in these regions was correlated to the intensity of the pain perceived, in accordance with the hypothesis of a role of simulation in understanding the feelings of others.Citation36,Citation41
Empathy and shared circuits
Unsurprisingly, the capacity to empathize with other individuals seem to have some relationships with the functioning of the shared circuits.Citation16,Citation27 Empathy, the ability to share other people's inner feelings, can be measured through a questionnaire where participants judge whether they are more or less likely to tremble when seeing the main character of a movie in a difficult situation, to take the point of view of someone else during a fight, and so on.Citation44
A number of researchers have now reported positive correlations between the strength of the response in simulation areas and the empathy scores of the participants. In one study conducted in our lab, the activation of the premotor cortex upon hearing the sound of actions was extremely strong in the most empathic participants and virtually inexistent in those participants with the lowest empathy scores.Citation9 Similarly, in the domain of emotions, there is evidence that the level of activity in the insula and the anterior cingulate cortex is augmented in empathic individuals witnessing disgust on a faceCitation29 or becoming aware that their partner is experiencing pain.Citation43 These results indicate that shared circuits may play a key role in social cognition by providing a first-person (vicarious) perspective on the feelings of others.Citation16,Citation27,Citation42,Citation45-Citation49 Does this imply that empathic individuals are likely to be overwhelmed by the feelings of others? It does not seem to be the rule. As the results of one study suggest, the inhibitory gating mechanism might also be more active in more empathic individuals.Citation40 Furthermore, independent cognitive factors are known to modulate our empathic responses. For instance, in male individuals who observe another person experiencing pain, simulation can be abolished if the person receiving pain had been unfair towards them in a game taking place before the experiment.Citation50
Shared circuits in autism
Given the apparent relevance of shared circuits for comprehending other's feelings from a first-person perspective, researchers started investigating the integrity of these circuits in autism spectrum disorders (ASD). The results, however, are not straightforward. Data concerning hand action observation show that, in some contexts at least, individuals with ASD activate their premotor cortex just as control individuals do.Citation51-Citation53 On the other hand, they do not experience difficulties with the imitation of goal-directed actions either,Citation54,Citation55 in contrast with what is commonly assumed in the literature on “mirror neurons and autism.”
The study of the cerebral network involved in the perception of facial expressions may have provided a somewhat clearer picture. Table I summarizes the results of six studies that compared individuals with ASD and controls during the processing of facial expressions, and that report whole-brain analyses. In the first experiment, children of 12±2 years of age observed and imitated facial expressions.Citation56 Area BA44 in the ventral premotor cortex was less active in participants with ASD, and the activation at this level was negatively correlated with symptom severity. Two subsequent investigations with children and adolescents produced similar findings in tasks where the participants had to match upright and inverted faces,Citation57 or had to recognize themselves in a set of morphed pictures.Citation58
Table I. Six FMRI studies investigating face processing in participants with autism spectrum disorders (ASD) and typically developing (TD) individuals, and providing whole brain results. ASD, participants with autism spectrum disorder; TD, typically developing individuals; BA44/45, inferior frontal gyrus pars opercularis/triangularis; PrC, Precentral gyrus/sulcus; MPFC, medial prefrontal cortex; OFC, orbito-frontal cortex; vPMC, ventral premotor cortex; PoC, post-central gyrus/sulcus; STS, superior temporal sulcus; IFG, inferior frontal gyrus; *, Talairach coordinates
The results with adults appear quite different. Two out of three studies did not find any group difference in premotor areas.Citation59,Citation60 A single study with autistic adults documented a hypoactivation in this region.Citation61 This study included only 9 ASD and 7 typically developing (TD) participants, and there were twice as many females in the TD group. Since females tend to be more empathic,Citation62 and therefore simulate more than males,Citation29 the difference between groups may well be the consequence of the difference in sex ratio. In summary, the available data suggests that the simulation of facial expression in the premotor cortex is reduced in young children with autism, but this no longer seems to be the case in adults. This conclusion is supported by a recent study showing that facial mimicry tends to improve with age in autism, with older children showing more occurrences of congruent muscular response to happy faces.Citation63
Concluding remarks
Brain imaging research shows that part of the network supporting action execution is activated during action observation. This appears to be the same for other networks supporting the basic sensations of touch and pain, or the emotions of disgust and pleasure. To some extent, the cortex experiences the feelings of others as if it was its own, and this information, along with other more cognitive processes, may help the observer understand the state of mind of others. The simulation in one's own brain of the actions and feelings of others is apparently increased in more empathic individuals. Whether individuals with autism hypoactivate the shared networks for actions and sensations is still hotly debated. Our review suggests, however, that motor simulation of facial expressions may be dysfunctional in young individuals with ASD.
REFERENCES
- di PellegrinoG.FadigaL.FogassiL.GalleseV.RizzolattiG.Understanding motor events: a neurophysiological study.Exp Br Res.199291176180
- GalleseV.FadigaL.FogassiL.RizzolattiG.Action recognition in the premotor cortex.Brain.1996119(Pt 2)5936098800951
- KeysersC.KohlerE.UmiltàMA.NanettiL.FogassiL.GalleseV.Audiovisual mirror neurons and action recognition.Exp Br Res.2003153628636
- KohlerE.KeysersC.UmiltàMA.FogassiL.GalleseV.RizzolattiG.Hearing sounds, understanding actions: action representation in mirror neurons.Science.200229784684812161656
- RizzolattiG.CraigheroL.The mirror-neuron system.Annu Rev Neurosci.20042716919215217330
- FogassiL.FerrariPF.GesierichB.RozziS.ChersiF.RizzolattiG.Parietal lobe: from action organization to intention understanding.Science.200530866266715860620
- RozziS.FerrariPF.BoniniL.RizzolattiG.FogassiL.Functional organization of inferior parietal lobule convexity in the macaque monkey: electrophysiological characterization of motor, sensory and mirror responses and their correlation with cytoarchitectonic areas.Eur J Neurosci.2008281569158818691325
- ChongTT-J.CunningtonR.WilliamsMA.KanwisherN.MattingleyJB.fMRI adaptation reveals mirror neurons in human inferior parietal cortex.Curr Biol.2008181576158018948009
- GazzolaV.Aziz-ZadehL.KeysersC.Empathy and the somatotopic auditory mirror system in humans.Curr Biol.2006161824182916979560
- GazzolaV.KeysersC.The observation and execution of actions share motor and somatosensory voxels in all tested subjects: single-subject analyses of unsmoothed fMRI data.Cereb Cortex.2009191239125519020203
- GazzolaV.RizzolattiG.WickerB.KeysersC.The anthropomorphic brain: the mirror neuron system responds to human and robotic actions.Neuroimage.2007351674168417395490
- HamiltonAFdC.GraftonST.Action outcomes are represented in human inferior frontoparietal cortex.Cereb Cortex.2008181160116817728264
- lacoboniM.WoodsRP.BrassM.BekkeringH.MazziottaJC.RizzolattiG.Cortical mechanisms of human imitation.Science.19992862526252810617472
- KeysersC.Mirror neurons.Curr Biol.200919R971R97319922849
- KeysersC.KaasJH.GazzolaV.Somatosensation in social perception.Nat Rev Neurosci.20101141742820445542
- SingerT.LammC.The social neuroscience of empathy.Ann N Y Acad Sci.20091156819619338504
- BlakemoreS-J.BristowD.BirdG.FrithC.WardJ.Somatosensory activations during the observation of touch and a case of vision-touch synaesthesia.Brain.2005128(Pt 7)1571158315817510
- BufalariI.AprileT.AvenantiA.Di RussoF.AgliotiSM.Empathy for pain and touch in the human somatosensory cortex.Cereb Cortex.2007172553256117205974
- EbischSJH.PerrucciMG.FerrettiA.Del GrattaC.RomaniGL.GalleseV.The sense of touch: embodied simulation in a visuotactile mirroring mechanism for observed animate or inanimate touch.J Cogn Neurosci.2008201611112318345991
- KeysersC.WickerB.GazzolaV.AntonJ-L.FogassiL.GalleseV.A touching sight: SII/PV activation during the observation and experience of touch.Neuron.20044233534615091347
- SchaeferM.XuB.FlorH.CohenLG.Effects of different viewing perspectives on somatosensory activations during observation of touch.Hum Brain Map.20093027222730
- De RenziE.CavalleriF.FacchiniS.Imitation and utilisation behaviour.J Neurol Neurosurg Psychiatr.1996613964008890779
- LhermitteF.'Utilization behaviour' and its relation to lesions of the frontal lobes.Brain.1983106(Pt 2)2372556850269
- LhermitteF.PillonB.SerdaruM.Human autonomy and the frontal lobes. Part I: Imitation and utilization behavior: a neuropsychological study of 75 patients.Ann Neurol.1986193263343707084
- BanissyMJ.Cohen KadoshR.MausGW.WalshV.WardJ.Prevalence, characteristics and a neurocognitive model of mirror-touch synaesthesia.Exp Br Res.2009198261272
- BanissyMJ.WardJ.Mirror-touch synesthesia is linked with empathy.Nat Neurosci.20071081581617572672
- BastiaansenJACJ.ThiouxM.KeysersC.Evidence for mirror systems in emotions.Philos Trans R Soc Lond, B, Biol Sci.20093642391240419620110
- WickerB.KeysersC.PlaillyJ.RoyetJP.GalleseV.RizzolattiG.Both of us disgusted in My insula: the common neural basis of seeing and feeling disgust.Neuron.20034065566414642287
- JabbiM.SwartM.KeysersC.Empathy for positive and negative emotions in the gustatory cortex.Neuroimage.2007341744175317175173
- JabbiM.KeysersC.Inferior frontal gyrus activity triggers anterior insula response to emotional facial expressions.Emotion (Washington, DC).20088775780
- CarrL.IacoboniM.DubeauM-C.MazziottaJC.LenziGL.Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas.Proc Natl Acad Sci U S A.20031005497550212682281
- van der GaagC.MinderaaRB.KeysersC.Facial expressions: what the mirror neuron system can and cannot tell us.Soc Neurosci.2007217922218633816
- ChengY.LinC-P.LiuH-L.et al.Expertise modulates the perception of pain in others.Curr Biol.2007171708171317900903
- CostantiniM.GalatiG.RomaniGL.AgliotiSM.Empathic neural reactivity to noxious stimuli delivered to body parts and non-corporeal objects.Eur J Neurosci.2008281222123018783380
- JacksonPL.BrunetE.MeltzoffAN.DecetyJ.Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain.Neuropsychologia.20064475276116140345
- JacksonPL.MeltzoffAN.DecetyJ.How do we perceive the pain of others? A window into the neural processes involved in empathy.Neuroimage.20052477177915652312
- LammC.MeltzoffAN.DecetyJ.How do we empathize with someone who is not like us? A functional magnetic resonance imaging study.J Cogn Neurosci.20102236237619199417
- LammC.NusbaumHC.MeltzoffAN.DecetyJ.What are you feeling? Using functional magnetic resonance imaging to assess the modulation of sensory and affective responses during empathy for pain.PLoS ONE.20072e129218091986
- MorrisonI.PeelenMV.DowningPE.The sight of others' pain modulates motor processing in human cingulate cortex.Cereb Cortex.2007172214222217124286
- LammC.BatsonCD.DecetyJ.The neural substrate of human empathy: effects of perspective-taking and cognitive appraisal.J Cogn Neurosci.200719425817214562
- SaarelaMV.HlushchukY.WilliamsACdC.SchürmannM.KalsoE.HariR.The compassionate brain: humans detect intensity of pain from another's face.Cereb Cortex.20071723023716495434
- SingerT.The neuronal basis and ontogeny of empathy and mind reading: review of literature and implications for future research.Neurosci Biobehav Rev.20063085586316904182
- SingerT.SeymourB.O'DohertyJ.KaubeH.DolanRJ.FrithCD.Empathy for pain involves the affective but not sensory components of pain.Science.20043031157116214976305
- DavisMH.Measuring individual differences in empathy: Evidence for a multidimensional approach.J Person Soc Psychol.198344113126
- AdolphsR.How do we know the minds of others? Domain-specificity, simulation, and enactive social cognition.Brain Res.20061079253516507301
- GalleseV.KeysersC.RizzolattiG.A unifying view of the basis of social cognition.Trends Cogn Sci (Regul Ed).2004839640315350240
- IacoboniM.Imitation, empathy, and mirror neurons.Annu Rev Psychol.20096065367018793090
- KeysersC.GazzolaV.Towards a unifying neural theory of social cognition.Prog Brain Res.200615637940117015092
- KeysersC.GazzolaV.Expanding the mirror: vicarious activity for actions, emotions, and sensations.Curr Opin Neurobiol.20091966667119880311
- SingerT.SeymourB.O'DohertyJP.StephanKE.DolanRJ.FrithCD.Empathic neural responses are modulated by the perceived fairness of others.Nature.200643946646916421576
- AvikainenS.KulomäkiT.HariR.Normal movement reading in Asperger subjects.Neuroreport.1999103467347010619627
- BirdG.LeightonJ.PressC.HeyesC.Intact automatic imitation of human and robot actions in autism spectrum disorders.Proc Biol Sci.20072743027303117911053
- ObermanLM.RamachandranVS.PinedaJA.Modulation of mu suppression in children with autism spectrum disorders in response to familiar or unfamiliar stimuli: the mirror neuron hypothesis.Neuropsychologia.2008461558156518304590
- CarpenterM.PenningtonBF.RogersSJ.Understanding of others' intentions in children with autism.J Autism Dev Disord.20013158959911814270
- HamiltonAFdC.BrindleyRM.FrithU.Imitation and action understanding in autistic spectrum disorders: how valid is the hypothesis of a deficit in the mirror neuron system?Neuropsychologia.2007451859186817234218
- DaprettoM.DaviesMS.PfeiferJH.et al.Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders.Nat Neurosci.20069283016327784
- BookheimerSY.WangAT.ScottA.SigmanM.DaprettoM.Frontal contributions to face processing differences in autism: evidence from fMRI of inverted face processing.J Int Neuropsychol Soc.20081492293218954473
- UddinLQ.DaviesMS.ScottAA.et al.Neural basis of self and other representation in autism: an FMRI study of self-face recognition.PLoS ONE.20083e352618958161
- AshwinC.Baron-CohenS.WheelwrightS.O'RiordanM.BullmoreET.Differential activation of the amygdala and the 'social brain' during fearful face-processing in Asperger Syndrome.Neuropsychologia.20074521416806312
- PierceK.HaistF.SedaghatF.CourchesneE.The brain response to personally familiar faces in autism: findings of fusiform activity and beyond.Brain.2004127(Pt 12)2703271615319275
- HadjikhaniN.JosephRM.SnyderJ.Tager-FlusbergH.Abnormal activation of the social brain during face perception in autism.Hum Brain Map.200728441449
- Baron-CohenS.KnickmeyerRC.BelmonteMK.Sex differences in the brain: implications for explaining autism.Science.200531081982316272115
- BeallPM.MoodyEJ.McIntoshDN.HepburnSL.ReedCL.Rapid facial reactions to emotional facial expressions in typically developing children and children with autism spectrum disorder.J Exp Child Psychol.200810120622318561942