Abstract
Autism is a neurodevelopmental disorder whose diagnosis is based on three behavioral criteria: unusual reciprocal social interactions, deficits in communication, and stereotyped repetitive behaviors with restricted interests. A large number of de novo single gene mutations and chromosomal deletions are associated with autism spectrum disorders. Based on the strong genetic evidence, mice with targeted mutations in homologous genes have been generated as translational research tools. Mouse models of autism have revealed behavioral and biological outcomes of mutations in risk genes. The field is now poised to employ the most robust phenotypes in the most replicable mouse models for preclinical screening of novel therapeutics.
El autismo es un trastorno del neurodesarrollo cuyo diagnóstico se basa en tres criterios conductuales: interacciones sociales recíprocas inusuales, déficit en la comunicación y conductas repetitivas estereotipadas con intereses disminuidos. Los trastornos del espectro autista están asociados con un gran número de mutaciones monogénicas de novo y deleciones cromosómicas. En base a la gran evidencia genética, se han generado ratones con mutaciones específicas en genes homólogos como herramientas de investigatión traslacional. Hay modelos de autismo en el ratón que han revelado resultados conductuales y biológicos que corresponden a mutaciones en genes de riesgo en genes de riesgo. Este campo ahora está preparado para emplear los fenotipos más potentes en los modelos de ratón más reproducibles para la evaluatión preclínica de nuevas terapéuticas.
L'autisme est un trouble du neurodéveloppement dont le diagnostic se fonde sur 3 critères comportementaux: des interactions sociales réciproques inhabituelles, des déficits de communication et des comportements répétitifs stéréotypés accompagnés d'intérêts restreints. Les troubles autistiques sont associés à de nombreuses mutations monogéniques de novo et à des deletions chromosomiques. Sur la base des arguments génétiques solides, des souris aux mutations ciblées sur des gènes homologues ont été élevées comme outil de recherche translationnelle. Les modèles murins d'autisme ont présenté des mutations biologiques et comportementales correspondant aux mutations des gènes à risque. Ce domaine de recherche est maintenant prêt à employer les phénotypes les plus fiables des modèles murins les plus reproductibles pour le dépistage préclinique de nouveaux traitements.
Genetic causes of autism spectrum disorder
Autism spectrum disorders were originally diagnosed by Kanner and Asperger in the 1930s.Citation1,Citation2 However, the diagnostic criteria were not codified until the 1994 Diagnostic and Statistical Manual of Mental Disorders (DSM).Citation3 Astonishingly high heritability of autism spectrum disorders, reaching 90% concordance for monozygotic twins, as compared with less than 10% concordance for dizygotic twins and siblings, along with a 4:1 male:female ratio of prevalence, quickly led to an major international search for genes causing autism. By assembling large numbers of simplex and familial cases, several research consortia have discovered single gene mutations, rare and common polymorphisms, and epigenetic modifications associated with autism.Citation4,Citation5 Copy number variants, including duplications of a sequence of genes within defined chromosomal loci, were reported to be relatively common in autism.Citation6-Citation9 Clearly, autism is not a single-gene disorder.
To parse the role of each of these many genetic abnormalities in the etiology and symptomology of autism spectrum disorders, and in other neurodevelopmental disorders in which autism is concomitantly diagnosed, homologous genetic mutations have been generated in experimental animals. Because the targeted gene mutation technology was perfected in the mouse, mice are currently used throughout biomedical research as the primary model organism for generating transgenic and knockout mouse models of human genetic disorders. Table I and the descriptions in this review illustrate a small portion of the wealth of available mouse models of autism. In addition, recent advances in generating knockout ratsCitation10 are leading to the development of mutant rat models of neurodevelopmental disorders.
Table I Autism-relevant behavoral phenotypes in selected mouse models with targeted mutations in associated with autism.Citation155-Citation169
Because concordance for autism spectrum disorder is not 100% between identical twins, whose genomes are presumably identical, environmental and epigenetic causes of autism spectrum disorders are also under investigation. Hypotheses about prenatal exposure to toxicological and immunological insults, and neuroanatomical lesions, have been modeled in mice, rats, and monkeys.Citation11-Citation18 The challenge now is to understand the consequences of each of these genetic and environmental perturbations, and their interactions. Animal models employing behavioral assays relevant to the specific symptoms offer excellent translational research tools to identify the biological mechanisms underlying the core features of autism spectrum disorder.
How do we model the behavioral symptoms of autism in mice?
As defined in the DSM-IV,Citation3 the diagnosis of autism requires the presence of at least six symptoms, including a minimum of two measures of qualitative impairment in social interaction, one symptom of qualitative impairment in communication, and one symptom of restricted and repetitive behaviour.Citation19,Citation20 The DSM-5 is expected to redefine Autism Spectrum Disorder into two symptom domains: (i) Social interaction and social communication deficits; (ii) Restricted, repetitive patterns of behavior, interests, or activities (http://www.dsm5.org/ProposedRevisions/Pages/proposedrevision.aspx?rid=94, January 2011). Associated symptoms that appear in subsets of individuals with autism include seizures, anxiety, intellectual impairment, hyperactivity, hyper-responsiveness and hyporesponsiveness to sensory stimuli, sleep disruption, and gastrointestinal distress.Citation20-Citation26
Given that the defining criteria for autism are behavioral, investigations employing mouse models require considerable insight into which specific behaviors in the mouse repertoire are sufficiently relevant to each category of the diagnostic symptoms of autism. Inclusion of behavioral assays relevant to associated symptoms further enhance the heuristic value of animal models of autism spectrum disorders. We and other behavioral neuroscientists have generated a comprehensive set of assays for social interaction, social communication, and repetitive behaviors in mice, to test hypotheses about the causes of autism.Citation27-Citation45 Social approach, reciprocal social interactions, olfactory communication, ultrasonic vocalizations, motor stereotypies such as circling and vertical jumping, repetitive behaviors such as self-grooming and digging, and perserveration in spatial tasks, are now in routine use for phenotyping mouse and rat models of autism and other neurodevelopmental disorders. Procedures for assaying behaviors relevant to associated symptoms of autism, including neurodevelopmental milestones, cognitive abilities, anxiety-like tendencies, seizures, motor dysfunctions, hyperactivity, responsiveness to sensory stimuli, and altered sleep patterns in mice have been adapted from the available behavioral neuroscience literature.Citation46
In each case, we begin with the human endophenotype. In what ways are mouse behaviors similar to a defining feature of autism? Luckily, Mus musculus is a social species. Laboratory mice display a social repertoire that includes approach to olfactory pheromones emitted by other mice, approach to familiar and new conspecifics, reciprocal social interactions, ultrasonic vocalizations, communal nesting, sexual and parenting behaviors, territorial scent marking, and aggressive behaviors.Citation47-Citation49 Standardized methods for scoring adult social approaches, reciprocal social interactions, nesting, sexual interactions, parental behaviors, and aggressive encounters are available in the behavioral neuroscience literature.Citation28,Citation50-Citation61
First diagnostic category
We employ social assays that have been refined from standard tests in the behavioral neuroscience literature.Citation48,Citation49 These choices are designed to maximize relevance to the types of social deficits specific to autism, including playing alone with inanimate toys rather than engaging in social interactions, and inappropriate responses to social cues. To quantify tendencies to engage in reciprocal social interactions, each subject mouse is paired with a novel partner mouse, inside a testing arena that permits free interactions over a test session of 10 to 30 minutes' duration. Digital videocameras record the session for later scoring of multiple parameters of social interactions. Ratings are performed by investigators who are blind to the genotype or treatment of the subject mice. Parameters routinely scored include sniffing, following, physical contact, and allogrooming.Citation58,Citation62 Automated videotracking systems can accurately score some of the simpler elements of social interaclions.Citation34,Citation35,Citation44,Citation63,Citation64
Tendency to spend time with a novel mouse versus a novel nonsocial object is evaluated in a social approach apparatus (). This 3-chambered assay, which was developed by our team to provide a simple measure of general sociability,Citation30 is widely used as an initial, highthroughput test for social deficits in mouse models of autism.Citation27,Citation32,Citation34,Citation38,Citation41,Citation64-Citation70
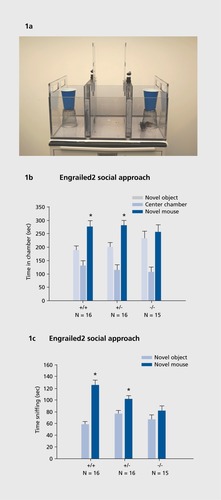
Second diagnostic category
Social communication in rodents is mostly through the emission and detection of olfactory pheromones, and perhaps to a lesser extent, the emission and detection of ultrasonic vocalizations.Citation40,Citation44,Citation55,Citation57,Citation59,Citation60,Citation71-Citation74 Olfactory communication is assayed by time spent sniffing olfactory stimuli from novel mice, and identification of novel versus familiar mice through olfactory cues.Citation36,Citation75,Citation76
Vocal communication is assayed by recording ultrasonic vocalizations emitted during social interactionsCitation77 (). Number of calls and their properties are subsequently scored by investigators. Software is available for quantifying some of the simpler parameters. Different patterns of ultrasonic vocalizations are emitted by separated pups, adult males interacting with estrus females or urine from estrus females, adult females interacting with each other, and adult male residents in response to an intruder.Citation72,Citation78
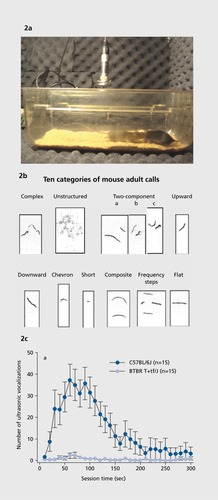
The anatomical and neurobiological substrates of olfactory and ultrasonic communication in mice do not precisely map onto the biological substrates of language and visual social communication in humans. In addition, considerably more work is needed to fully understand the communication value of ultrasonic vocalizations in mice.
At present, the existing tasks offer a reasonable start for discovering mechanistic similarities between species. Face validity of these mouse social interaction and communication tasks to the tendencies of people with autism to engage in less social approach and interaction, and to respond appropriately to complex social cues, remains inferential. We cannot know what a mouse is thinking, feeling, or intending, but only the quantifiable external expressions of those internai states.
Third diagnostic category
Mice with various genetic mutations exhibit spontaneous motor stereotypies such as circling and vertical jumping, and spontaneous repetitive behaviors such as long bouts of self-grooming and excessive digging in the litter (). Assays generally focus on the number of bouts of the behavior, or the cumulative time engaged in the behavior, during a defined test session of 10 minutes or longer.Citation40,Citation58,Citation65,Citation79-Citation81 Restricted interests, insistence on sameness, and special interests are more challenging diagnostic features of autism to model in mice. Perseveration of spatial habits, such as difficulty in learning a new location of a re inforcer in a T-maze or water maze after the initial learning of a first location, has been employed with some success in mouse models of autism.Citation82,Citation83
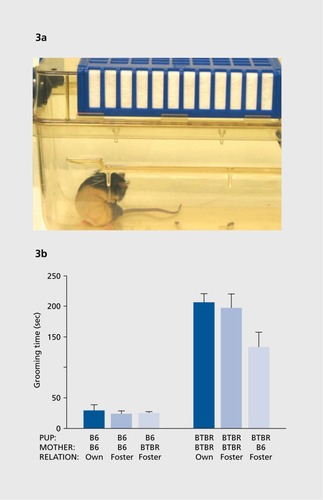
Associated symptoms
Established, standardized tests are available in the voluminous behavioral neuroscience literature for most of the associated symptoms of autism.Citation46,Citation84-Citation86 Neurodevelopmental milestones are scored from postnatal day 2 to postnatal day 18 on physical attributes such as body length and eye opening, and behavioral attributes such as responses to handling and the righting reflex. Spontaneous seizures are scored with a rating scale or EEG electroencephalography. Learning and memory tests for mice include Morris water maze spatial navigation tasks, contextual and cued fearconditioned freezing after exposure to an aversive footshock, and operant nose-poke and touch-screen reinforcement schedules.
Anxiety-related tests for rodents are primarily approach-avoidance conflicts. Mice are nocturnal. They prefer to be in dimly lit, enclosed environments. The current gold standard for anxiety-like tests for mice is the elevated plus-maze, which consists of two open and two enclosed arms, raised 1 meter from the floor, thus offering the choice between enclosed spaces and a high dropoff ledge.Citation85 The corroborating light-dark test consists of a two-compartment apparatus in which one chamber is dark and enclosed while the other chamber is open and brightly lit.Citation87,Citation88 Mice spend more time in the closed arms of the elevated plus-maze and more time in the dark compartment of the lighte↔dark apparatus. Excessive anxiety-like traits are interpreted when the preference is unusually high for the closed arms and for the dark compartment. Anxiolytic-like treatment responses are interpreted when mice venture out more frequently into the open arms of the elevated plus-maze and the brightly lit chamber of the light↔dark box.
Responses to sensory stimuli include acoustic startle, olfactory habituation and dishabituation to a series of non-social and social odors, and the hot plate and tail flick thermal tests. Hyperactivity is scored from automated parameters of locomotion in a novel open field. Unusual sleep patterns are scored by observations of the home cage during the daylight sleeping hours and during the nighttime active hours, and/or by EEG recordings. Optimal animal models should incorporate: (i) face validity, ie, close analogies to the defining features of the human syndrome; (ii) construct validity, ie, the biological dysfunction that causes the human disease, such as a gene mutation or anatomical abnormality; and (iii) predictive validity, ie, responsiveness to treatments that prevent or reverse symptoms in the human disease. The best animal models of autism and related developmental disorders will maximize face, construct, and predictive validities. At present this combination represents a very small subset of the model systems in use, particularly for neurodevelopmental disorders in which no effective therapeutics exist to test predictive validity of the animal model. The selected examples below are designed to illustrate the progress and promise of the mouse modeling approach in autism basic research and therapeutic development. Our goal is to systematically analyze the wealth of emerging animal models of neurodevelopmental disorders, understand the strengths and weaknesses of each, gain basic knowledge about phenotypic outcomes, and employ the best model systems for treatment discovery.
How do we discover therapeutics using mouse models?
The unmet medical need for effective treatments for neurodevelopmental disorders is striking. The number of reported cases of autism lias risen rapidly over the past décade.Citation89,Citation90 This rapid rise is largely a fonction of better diagnostic instruments and public awareness, allhough possible environmental causes and gene x environment interactions are under investigation.Citation91-Citation93
Personal and financial costs are high, to the affected individuals, their families, schools, and health care providers. At present the only effective interventions are intensive behavioral therapies.Citation26,Citation94 The only pharmacological treatments approved by the US Food and Drug Administration are risperidone and aripiprazole: Risperdol™ and Abilify.™ Their approved use is solely for the associated “irritability,” which includes aggression, self-injury, and tantrums.Citation95
A major revelation from the genetic association studies is that the most frequent mutations in autism are in genes that mediate the formation and maturation of synapses, particularly the postsynaptic densities, dendritic spines, and signaling mechanisms downstream from receptors mediating excitatory neurotransmission.Citation96-Citation100 Pharmacological agents that alter synaptic functions are already available to some extent, and next-generation compounds are under development.Citation101-Citation104 To evaluate the ability of novel drug treatments to reverse and/or prevent the symptoms of diseases, biomedical researchers often begin by testing exploratory compounds in appropriate animal models. Robust behavioral pnenotypes with face validity to autism, In mouse models with construct validity to autism spectrum disorders, hold great promise as predinical tools for discovering effective treatments for components of autism spectrum disorders.
Because rodents are similar to humans in many aspects of biochemistry, physiology, anatomy, and genetics, mice and rats are routinely employed in biomedical research as translational systems. Compounds that reverse behavioral and biological phenotypes in mouse models of autism offer leads which may be worth pursuing in human clinical trials. However, species differences exist in drug metabolism, alternate biochemical pathways, genetic variants, and toxicology. As in any field of biomedical research employing model systems, 100% predictive validity of efficacy and practicality in humans cannot be expected.
Keeping these caveats in mind, we design rigorous methods to evaluate proposed therapeutic interventions for autism spectrum disorders for their ability to reverse and/or prevent the major phenotypes in mouse models.Citation29,Citation43 Behavioral pharmacologists test acute and chronic drug treatments, across a dose range, at various time points after administration, and assay for the most robust autism-relevant behaviors, in the strongest mouse models. Examples of some of the successes are described in the next section.
Mouse models with high translational value
Cell surface adhesion glycoproteins
Cell surface adhesion glycoproteins are a primary mechanism through which connections of presynaptic axons and postsynaptic dendrites are elaborated in neuronal synapses.Citation97,Citation105 Mutations in cell surface protein genes have been reported with comparatively high frequency in neurodevelopmental disorders. Individuals with autism have been identified with mutations in NEUREXIN1, NEUROLIGIN3, NEUROLIGIN4, SHANK2, SHANKS, and CNTNAP2. For each of these rare mutations, a small number of individuals with the mutations who meet the diagnostic criteria for autism spectrum disorder has been identified.Citation106-Citation109 Mice with homologous mutations in these genes are available from several excellent molecular genetics laboratories and from The Jackson Laboratory repository.
Shank3 knockout mice
Shank3 knockout mice present a particularly fascinating example of the importance of the location of the mutation within the gene. The Shank3 gene includes an ankyrin repeat domain, a PDZ domain, and a Homer binding domain.Citation110-Citation112 Five distinct lines of Shank3 knockout mice with mutations at these various sites were generated and phenotyped in the past 2 years.Citation71,Citation81,Citation113,Citation114 Two lines of Shank3 knockouts containing the mutation at the ankyrin domain displayed impairments in excitatory neurotransmission and long-term potentiation, but were predominantly normal on standard measures of sociability, with only small genotype differences detected in ultrasonic vocalizations and repetitive behavior.Citation71,Citation81 Inserting the mutation at the Homer binding site resulted in mice with more social interactions, primarily in the form of aggression, along with mostly normal dendritic spines, reduced long-term potentiation, and enhanced long-term depression.Citation113 When the mutation was in the PDZ domain, Shank3 knockouts displayed much more severe phenotypes, including high spontaneous self-grooming resulting in skin lesions, impaired sociability, reduced corticostriatal excitatory transmission, longer dendritic spines, and lower density of dendritic spines, as compared with wild-type controls.Citation81 These divergent outcomes of mutations at differing sites within the same gene provide a unique opportunity to understand the binding partners and their downstream signaling actions that determine the severity of symptoms in humans. For example, deficits in mGluRS signaling have been reported after Shank3 knockdown in neuronal cultures.Citation115 Augmentation of mGluRS activity could be beneficial in cases of autism with SHANK3 mutations, and in individuals with Phelan-McDermid syndrome, an intellectual disability syndrome in which the SHANK3 mutation is central to the 22q13 chromosomal deletion.Citation108
Contactin associated protein 2 (Cntnap2)
Contactin associated protein 2 (Cntnap2), a member of the neurexin superfamily, plays a role in neuron-glia interactions and neuronal migration during early brain maturation. Mutations in CNTNAP2 are associated with autism in a small number of individuals, particularly with language disabilities.Citation107,Citation116 Cntnap2 knockout mice were generated to understand the actions of this protein on brain development and autism-relevant behaviors.Citation40 Seizures were detected in 9 out of 10 null mutants. Social behaviors were impaired on the 3-chambered task, during reciprocal interactions, and in home cage nesting. Repetitive self-grooming was elevated. Resistance to change was seen in the Morris water maze, in which the initial learning was normal but the Cntnap2 knockouts failed the reversal test when the escape platform location was changed. Less spontaneous alternation in a T-maze was seen in the null mutants, concomitant with moderate hyperactivity Reduced number of GABAergic interneurons and impaired migration of cortical projection neurons in this line of Cntnap2 mice underlie their seizures and some of their behavioral abnormalities. The Geschwind team proceeded to test risperidone, the antipsychotic approved by the US Food and Drug Administration for the treatment of irritability in autism. At 0.2 mg/kg IP daily for 7 days, a dose and regimen which did not affect locomotion in the wildtype controls, risperidone reduced the hyperactivity and repetitive selfgrooming in Cntnapl null mutant mice.Citation40 Social behaviors were unaffected by the treatment with risperidone, which is an atypical antipsychotic.
Single gene mutations, chromosomal deletions, and duplications cause a variety of neurodevelopmental disorders, including Fragile X, Rett, Angelman, PraderWilli, Smith-Lemli-Opitz, Timothy, Williams, and PhelanMcDermid syndromes, and tuberous sclerosis.Citation97,Citation108 A surprisingly large number of these de novo mutations code for signaling proteins that mediate the biochemical events downstream to postsynaptic neurotransmitter receptors. Interactome network analyses revealed convergences in genes that mediate transcriptional and splicing mechanisms that may be dysregulated in autism spectrum disorders.Citation117 Mutant mouse models of many of these syndromes have been generated.Citation43,Citation44,Citation114,Citation118-Citation122 While clinically distinct disorders caused by known single gene mutations suggest straightforward targets, as compared with complex disorders such as cases of autism in which the genetic substrates are unknown, increasing knowledge about the actions of downstream signaling proteins could identify pharmacological interventions which target key mechanistic sites in convergent biochemical cascades. Mice with homologous mutations are being employed as translational tools to evaluate convergent downstream target mechanisms, and to screen compounds that yield useful interventions at those sites.
Tuberous sclerosis
Tuberous sclerosis, caused by a mutation in the Tsc1 or Tsc2 gene, is characterized by benign tubers in the cerebral cortex, seizures, a high incidence of intellectual impairment, and frequent comorbidity with autism.Citation123,Citation124 Tscl and Tsc2, which dimerize, are downstream targets of the PI3K/Akt postsynaptic signaling pathway elements that bind to mTORC2, and regulate mTORCl at a further downstream site.Citation125 mTOR, the mammalian target of rapamycin, is a serine/threonine kinase which regulates many facets of brain development and cytoskeletal organization.Citation126 Deletion of Tsc1 in knockout mice, hippocampal slices, or cortical cultures resulted in enlarged brains, large dysmorphic astrocytes, decreased myelination, reductions in γ-aminobutyric acid (GABA)-ergic interneurons in the cerebral cortex, and loss of mGluR-dependent long-term depression.Citation126,Citation127 Mice with mutations in Tsc2 display neuronal hypertrophy, reduced long-term potentiation in hippocampal slices, impaired hippocampally mediated fear conditioning, and impaired water maze learning.Citation128 Treatment with the mTOR inhibitor rapamycin for 5 days reversed the fear conditioning deficit and improved water maze learning, along with reducing brain weight and increasing survival.Citation128
This early demonstration of a pharmacological rescue of phenotypes in a mouse model of a neurodevelopmental disorder sparked optimism for treating disorders caused by perturbations in signal transduction.Citation129 In a separate mutant line, 4 weeks of treatment with rapamycin reduced the macroencephaly and increased the low social interaction in mice with a mutation in Pten, an upstream regulator of mTOR that is implicated in cancers, seizures, and autism.Citation38 Rapalogs, analogs of mTOR, are in clinical trials for cancers.Citation130 Rapalogs and compounds targeting PI3K and AktCitation131 present possibilities for therapeutic interventions in neurodevelopmental disorders with underlying mechanisms in the mTOR signaling pathway.
Fragile X syndrome
Fragile X syndrome is the most frequent genetic cause of intellectual disabilities. Constriction at the end of the X chromosome, termed a fragile site, is associated with a dramatic expansion of CGG triplet repeats, which transcriptionally silence the FMR1 gene.Citation132,Citation133 Fragile X mental retardation protein (FMRP) is highly expressed in the brain, where it negatively regulates the synthesis of a large number of downstream proteins.Citation134'Citation1 Mice with a mutation in Fmr1 display impairments in long-term potentiation, unusual social behaviors, and some unusual cognitive and anxiety-related behaviors.Citation135-Citation139
One functional consequence of the FMR1 mutation is upregulation of mGluRS receptors.Citation140 Bear and colleagues discovered that crossing mGluRS knockout mice with Fmrl knockout mice rescued the impaired longterm depression, elevated the dendritic spine densities in the hippocampus, and attenuated seizures.Citation141 Negative allosteric modulators of the mGluR5 receptor were therefore postulated as potential treatments for Fragile X Syndrome. Clinical trials are in progress to test this hypothesis.Citation142 Approximately 30% of individuals with Fragile X syndrome meet the diagnostic criteria for autism.Citation143 Considering this high comorbidity, we reasoned that a treatment effective in Fragile X Syndrome might act through a pathway convergent with other risk genes for autism. Citation144,Citation145 We discovered that the prototypic mGluR5 antagonist 6-methyl-2-(phenylethynyl) pyridine (MPEP), and GRN-529. a more selective negative allosteric modulators of the mGluRS receptor, reduced the high levels of repetitive self-grooming in BTBR mice,Citation79,Citation146 an inbred strain that displays robust social deficits, low vocalizations in social settings, and high repetitive self-grooming and digging.Citation42,Citation58,Citation60,Citation65,Citation78,Citation79,Citation147,Citation148 In addition, GRN-529 reduced the high levels of stereotyped jumping that characterize another inbred strain, C58/ICitation80,Citation146,Citation149 Further, MPEP reduced marble burying in Fmr1 knockout mice, reduced stereotypies in Swiss-Webster mice, and reduced repetitive self-grooming and marble burying in mice pretreated prenatally with valproic acid.Citation14,Citation150,Citation151 These reports lend credence to the notion that interventions acting through mGluR5 receptors could confer specific benefits for treating repetitive behaviors, a major component of the third diagnostic symptom of autism.
Conclusions
Promising early findings of therapeutic rescues in mouse models have energized the rational search for pharmacological treatments of autism spectrum disorder. While the optimal developmental period for pharmacological intervention remains to be determined, adults with autism will likely be recruited for the first clinical trials,Citation152 since the risks of adverse drug reactions are predicted to be greater in children. Challenges will include discovering the critical window during development and/or adulthood at which interventions are useful, dosages, and treatment regimens which minimize toxicity. We have taken the first step in a long journey.
We thank Dr Mu Yang for Figures 1a and 3a, and Dr Jennifer Brielmaier for Figure 1b. Dr Yang was a Research Fellow and Dr Brielmaier was a Postdoctoral Fellow in the author's Laboratory of Behavioral Neuroscience, National Institute of Mental Health Intramural Research Program, Bethesda, MD.
REFERENCES
- KannerL.Child psychiatry; mental deficiency.Am J Psychiatry.194610252052221016902
- AspergerH.Die “Autistischen Psychopathen” im kindesalter.Archiv fur Psychiatrie und Nervenkrankheiten.194411776136
- American Psychiatric Association.Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association.1994
- El-FishawyP.StateMW.The genetics of autism: key issues, recent findings, and clinical implications.Psychiatr Clin North Am.2010338310520159341
- CookEH.JrSchererSW.Copy-number variations associated with neuropsychiatric conditions.Nature.200845591992318923514
- SebatJ.LakshmiB.MalhotraD.et al.Strong association of de novo copy number mutations with autism.Science.200731644544917363630
- SandersSJ.Ercan-SencicekAG.HusV.et al.Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism.Neuron.20117086388521658581
- LevyD.RonemusM.YamromB.et al.Rare de novo and transmitted copy-number variation in autistic spectrum disorders.Neuron.20117088689721658582
- GirirajanS.BrkanacZ.CoeBP.et al.Relative burden of large CNVs on a range of neurodevelopmental phenotypes.PLoS Genet.20117e100233422102821
- CuiX.JiD.FisherDA.WuY.BrinerDM.WeinsteinEJ.Targeted integration in rat and mouse embryos with zinc-finger nucleases.Nat Biotechnol.201129646721151125
- BaumanMD.ToscanoJE.BabineauBA.MasonWA.AmaralDG.Emergence of stereotypies in juvenile monkeys (Macaca mulatta) with neonatal amygdala or hippocampus lesions.Behav Neurosci.20081221005101518823158
- SmithSE.LiJ.GarbettK.MirnicsK.PattersonPH.Maternal immune activation alters fetal brain development through interleukin-6.J Neurosci.200727106951070217913903
- HeoY.ZhangY.GaoD.MillerVM.LawrenceDA.Aberrant immune responses in a mouse with behavioral disorders.PLoS One.20116e2091221799730
- MehtaMV.GandalMJ.SiegelSJ.mGluRS-antagonist mediated reversal of elevated stereotyped, repetitive behaviors in the VPA model of autism.PLoS One.20116e2607722016815
- BermanRF.PessahIN.MoutonPR.MavD.HarryJ.Low-level neonatal thimerosal exposure: further evaluation of altered neurotoxic potential in SJL mice.Toxicol Sci.200810129430917977901
- BaumanMD.LavenexP.MasonWA.CapitanioJP.AmaralDG.The development of social behavior following neonatal amygdala lesions in rhesus monkeys.J Cogn Neurosci.2004161388141115509386
- MartinLA.AshwoodP.BraunschweigD.CabanlitM.Van de WaterJ.AmaralDG.Stereotypies and hyperactivity in rhesus monkeys exposed to IgG from mothers of children with autism.Brain Behav Immun.20082280681618262386
- MalkovaL.MishkinM.SuomiSJ.BachevalierJ.Long-term effects of neonatal medial temporal ablations on socioemotional behavior in monkeys (Macaca mulatta).Behav Neurosci.201012474276021133531
- LordC.PetkovaE.HusV.et al.A multisite study of the clinical diagnosis of different autism spectrum disorders.Arch Gen Psychiatry.20126930631322065253
- LordC.CookEH.LeventhalBL.AmaralDG.Autism spectrum disorders.Neuron.20002835536311144346
- TuchmanR.MosheSL.RapinI.Convulsing toward the pathophysiology of autism.Brain Dev.2009319510319006654
- RogersSJ.OzonoffS.Annotation: what do we know about sensory dysfunction in autism? A critical review of the empirical evidence.J Child Psychol Psychiatry.2005461255126816313426
- LandaRJ.Diagnosis of autism spectrum disorders in the first 3 years of life.Nat Clin Pract Neurol.2008413814718253102
- VolkFR.StateM.KlinA.Autism and autism spectrum disorders: diagnostic issues for the coming decade.J Child Psychol Psychiatry.20095010811519220594
- AmaralD.DawsonG.Geschwind.DH.Autism Spectrum Disorders. New York, NY: Oxford University Press.2011
- DawsonG.BurnerK.Behavioral interventions in children and adolescents with autism spectrum disorder: a review of recent findings.Curr Opin Pediatr.20112361662022037220
- NakataniJ.TamadaK.HatanakaF.et al.Abnormal behavior in a chromosome-engineered mouse model for human 15q11-13 duplication seen in autism.Cell.20091371235124619563756
- YangM.SilvermanJL.CrawleyJN.Automated three-chambered social approach task for mice.Curr Protoc Neurosci.2011Chapter 8: Unit 8.2621732314
- CrawleyJN.Designing mouse behavioral tasks relevant to autistic-like behaviors.Ment Retard Dev Disabil Res Rev.20041024825815666335
- NadlerJJ.MoySS.DoldG.et al.Automated apparatus for quantitation of social approach behaviors in mice.Genes Brain Behav.2004330331415344923
- BrodkinES.BALB/c mice: low sociability and other phenotypes that be relevant to autism.Behav Brain Res.2007176536516890300
- DeLoreyTM.SahbaieP.HashemiE.HomanicsGE.ClarkJD.Gabrb3 gene deficient mice exhibit impaired social and exploratory behaviors, deficits in non-selective attention and hypoplasia of cerebellar vermal lobules: a potential model of autism spectrum disorder.Behav Brain Res.200818720722017983671
- RyanBC.YoungNB.MoySS.CrawleyJN.Olfactory cues are sufficient to elicit social approach behaviors but not social transmission of food preference in C57BL/6J mice.Behav Brain Res.200819323524218586054
- PageDT.KutiOJ.PrestiaC.SurM.Haploinsufficiency for Pten and Serotonin transporter cooperatively influences brain size and social behavior.Proc Natl Acad Sci U S A.20091061989199419208814
- RadyushkinK.HammerschmidtK.BoretiusS.et al.Neuroligin-3-deficient mice: model of a monogenic heritable form of autism with an olfactory deficit.Genes Brain Behav.2009841642519243448
- YangM.CrawleyJN.Simple behavioral assessment of mouse olfaction.Curr Protoc Neurosci.2009Chapter 8: Unit 82419575474
- SilvaAJ.EhningerD.Adult reversal of cognitive phenotypes in neurodevelopmental disorders.J Neurodev Disord.2009115015719812701
- ZhouJ.BlundellJ.OgawaS.et al.Pharmacological inhibition of mTORC1 suppresses anatomical, cellular, and behavioral abnormalities in neural-specific Pten knock-out mice.J Neurosci.2009291773178319211884
- EthertonM.FoldyC.SharmaM.et al.Autism-linked neuroligin-3 R451C mutation differentially alters hippocampal and cortical synaptic function.Proc Natl Acad Sci U S A.201116; 1081376413769
- PenagarikanoO.AbrahamsBS.HermanEl.et al.Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits.Cell.201114723524621962519
- SilvermanJ.TurnerS.BarkanC.et al.Sociability and motor functions in Shank1 mutant mice.Brain Res.2011138012013720868654
- PobbeRL.PearsonBL.DefensorEB.BolivarVJ.BlanchardDC.BlanchardRJ.Expression of social behaviors of C57BL/6J versus BTBR inbred mouse strains in the visible burrow system.Behav Brain Res.201021444344920600340
- SilvermanJL.YangM.LordC.CrawleyJN.Behavioural phenotyping assays for mouse models of autism.Nat Rev Neurosci.20101149050220559336
- SmithSE.ZhouYD.ZhangG.JinZ.StoppelDC.AndersonMP.Increased gene dosage of Ube3a results in autism traits and decreased glutamate synaptic transmission in mice.Sci Transl Med.20113103ra197
- SilvermanJ.TurnerS.BarkanC.et al.Sociability and motor functions in Shankl mutant mice.Brain Res.2011138012013720868654
- CrawleyJN.What's Wrong With My Mouse? Behavioral Phenotyping of Transgenic and Knockout Mice.20072nd ed. Hoboken, NJ: John Wiley & Sons, Inc.
- GrantE.MacintoshJ.A comparison of the social postures of some common laboratory rodents.Behaviour.196321246259
- CarterCS.WilliamsJR.WittDM.InselTR.Oxytocin and social bonding.Ann N Y Acad Sci.19926522042111626829
- TerranovaML.LaviolaG.Scoring of Social Interactions and Play in Mice During Adolescence.2005Vol 13. Hoboken, NJ: John Wiley & Sons, Inc.
- HoferMA.ShairHN.Ultrasonic vocalization, laryngeal braking, and thermogenesis in rat pups: a reappraisal.Behav Neurosci.19931073543628484900
- MiczekKA.MaxsonSC.FishEW.FaccidomoS.Aggressive behavioral phenotypes in mice.Behav Brain Res.200112516718111682108
- WinslowJT.HearnEF.FergusonJ.YoungLJ.MatzukMM.InselTR.Infant vocalization, adult aggression, and fear behavior of an oxytocin null mutant mouse.Horm Behav.20003714515510753584
- WrennCC.HarrisAP.SaavedraMC.CrawleyJN.Social transmission of food preference in mice: methodology and application to galanin-overexpressing transgenic mice.Behav Neurosci.2003117213112619904
- WessonDW.KellerM.DouhardQ.BaumMJ.BakkerJ.Enhanced urinary odor discrimination in female aromatase knockout (ArKO) mice.Horm Behav.20064958058616448653
- PankseppJB.JochmanKA.KimJU.et al.Affiliative behavior, ultrasonic communication and social reward are influenced by genetic variation in adolescent mice.PLoS One.20072e35117406675
- WersingerSR.CaldwellHK.MartinezL.GoldP.HuSB.YoungWS.3rd. Vasopressin 1a receptor knockout mice have a subtle olfactory deficit but normal aggression.Genes Brain Behav.2007654055117083331
- WangH.LiangS.BurgdorfJ.WessJ.YeomansJ.Ultrasonic vocalizations induced by sex and amphetamine in M2, M4, M5 muscarinic and D2 dopamine receptor knockout mice.PLoS One.20083e189318382674
- McFarlaneHG.KusekGK.YangM.PhoenixJL.BolivarVJ.CrawleyJN.Autism-like behavioral phenotypes in BTBR T+tf/J mice.Genes Brain Behav.2008715216317559418
- ScattoniML.GandhySU.RicceriL.CrawleyJN.Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism.PLoS One.20083e306718728777
- ScattoniM.RicceriL.CrawleyJ.Unusual repertoire of vocalizations in adult BTBR T+tf/J mice during three types of social encounters.Genes Brain Behav.201110445620618443
- ArakawaH.ArakawaK.BlanchardDC.BlanchardRJ.A new test paradigm for social recognition evidenced by urinary scent marking behavior in C57BL/6J mice.Behav Brain Res.20081909710418359521
- YangM.ClarkeAM.CrawleyJN.Postnatal lesion evidence against a primary role for the corpus callosum in mouse sociability.Eur J Neurosci.2009291663167719419429
- AhernTH.ModiME.BurkettJP.YoungLJ.Evaluation of two automated metrics for analyzing partner preference tests.J Neurosci Methods.200918218018819539647
- MatsuoN.TandaK.NakanishiK.et al.Comprehensive behavioral phenotyping of ryanodine receptor type 3 (RyR3) knockout mice: decreased social contact duration in two social interaction tests.Front Behav Neurosci.20093319503748
- YangM.ZhodzishskyV.CrawleyJN.Social deficits in BTBR T+tf/J mice are unchanged by cross-fostering with C57BL/6J mothers.Int J Dev Neurosci.20072551552117980995
- CrawleyJN.Mouse behavioral assays relevant to the symptoms of autism.Brain Pathol.20071744845917919130
- CrawleyJN.ChenT.PuriA.et al.Social approach behaviors in oxytocin knockout mice: comparison of two independent lines tested in different laboratory environments.Neuropeptides.20074114516317420046
- YangM.ScattoniML.ZhodzishskyV.et al.Social approach behaviors are similar on conventional versus reverse lighting cycles, and in replications across cohorts, in BTBR T+ tf/J, C57BL/6J, and vasopressin receptor IB mutant mice.Front Behav Neurosci.20071118958184
- ChadmanKK.GongS.ScattoniML.et al.Minimal aberrant behavioral phenotypes of neuroligin-3 R451C knockin mice.Autism Res.2008114715819360662
- JamainS.RadyushkinK.HammerschmidtK.et al.Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism.Proc Natl Acad Sci U S A.20081051710171518227507
- BozdagiO.SakuraiT.PapapetrouD.et al.Haploinsufficiency of the autism-associated Shank3 gene leads to deficits in synaptic function, social interaction, and social communication.Mol Autism.201011521167025
- HolyTE.GuoZ.Ultrasonic songs of male mice.PLoS Biol.20053e38616248680
- EyE.LeBlondCS.BourgeronT.Behavioral profiles of mice carrying synaptic gene mutations associated with autism spectrum disorders.Autism Res.2011451621328568
- YoungDM.SchenkAK.YangSB.YNLYAltered ultrasonic vocalizations in a tuberous sclerosis mouse model of autism.Proc Natl Acad Sci U S A.2010107110741107920534473
- BakkerJ.HondaS.HaradaN.BalthazartJ.Sexual partner preference requires a functional aromatase (cyp19) gene in male mice.Horm Behav.20024215817112367569
- McGrawLA.YoungLJ.The prairie vole: an emerging model organism for understanding the social brain.Trends Neurosci.20103310310920005580
- ScattoniML.CrawleyJ.RicceriL.Ultrasonic vocalizations: a tool for behavioural phenotyping of mouse models of neurodevelopmental disorders.Neurosci Biobehav Rev.20093350851518771687
- WohrM.RoulletFl.CrawleyJN.Reduced scent marking and ultrasonic vocalizations in the BTBR T+tf/J mouse model of autism.Genes Brain Behav.20123265256541
- SilvermanJL.ToluSS.BarkanCL.CrawleyJN.Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluRS antagonist MPEP.Neuropsychopharmacology.20103597698920032969
- RyanBC.YoungNB.CrawleyJN.BodfishJW.MoySS.Social deficits, stereotypy and early emergence of repetitive behavior in the C58/J inbred mouse strain.Behav Brain Res.201020817818819941908
- PecaJ.FelicianoC.TingJT.et al.Shank3 mutant mice display autisticlike behaviours and striatal dysfunction.Nature.201147243744221423165
- MoySS.NadlerJJ.YoungNB.et al.Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains.Behav Brain Res.200717642016971002
- ArnodeoDA.JonesJH.SweeneyJA.RagozzinoME.Differences in BTBR T+tf/J and C57BL/6J mice on probabilistic reversal learning and stereotyped behaviors.Behav Brain Res.2012227647222056750
- LuckiI.The spectrum of behaviors influenced by serotonin.Biol Psychiatry.1998441511629693387
- CryanJF.HolmesA.The ascent of mouse: advances in modelling human depression and anxiety.Nat Rev Drug Discov.2005477579016138108
- FoxW.Reflex-ontogeny and behavioural development of the mouse.Animal Behavior.196913234241
- CrawleyJN.Exploratory behavior models of anxiety in mice.Neurosci Biobehav Rev.Spring 1985937442858080
- CrawleyJ.GoodwinFK.Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines.Pharmacol Biochem Behav.1980131671706106204
- RiceC.et al.Prevalence of autism spectrum disorders - Autism and Developmental Disabilities Monitoring Network, United States, 2006.MMWR Surveill Summ.200958120
- FombonneE.Epidemiology of pervasive developmental disorders.Pediatr Res.20096559159819218885
- CharmanT.PicklesA.ChandlerS.et al.Commentary: Effects of diagnostic thresholds and research vs service and administrative diagnosis on autism prevalence.Int J Epidemiol.20093812341238author reply 12431234.19737794
- RiceC.NicholasJ.BaioJ.et al.Changes in autism spectrum disorder prevalence in 4 areas of the United States.Disabil Health J.2010318620121122784
- DurkinMS.MaennerMJ.MeaneyFJ.et al.Socioeconomic inequality in the prevalence of autism spectrum disorder: evidence from a U.S. cross-sectional study.PLoS One.20105e1155120634960
- ZwaigenbaumL.BrysonS.LordC.et al.Clinical assessment and management of toddlers with suspected autism spectrum disorder: insights from studies of high-risk infants.Pediatrics.20091231383139119403506
- WinkLK.EricksonCA.McDougleCJ.Pharmacologic treatment of behavioral symptoms associated with autism and other pervasive developmental disorders.Curr Treat Options Neurol.20101252953820848330
- BourgeronT.A synaptic trek to autism.Curr Opin Neurobiol.20091923123419545994
- BetancurC.SakuraiT.BuxbaumJD.The emerging role of synaptic celladhesion pathways in the pathogenesis of autism spectrum disorders.Trends Neurosci.20093240241219541375
- BuxbaumJD.Multiple rare variants in the etiology of autism spectrum disorders.Dialogues Clin Neurosci.200911354319432386
- PenzesP.CahillME.JonesKA.VanLeeuwenJE.WoolfreyKM.Dendritic spine pathology in neuropsychiatrie disorders.Nat Neurosci.20111428529321346746
- HutslerJJ.ZhangH.Increased dendritic spine densities on cortical projection neurons in autism spectrum disorders.Brain Res.20101309839419896929
- BallardTM.WoolleyML.PrinssenE.HuwylerJ.PorterR.SpoorenW.The effect of the mGluS receptor antagonist MPEP in rodent tests of anxiety and cognition: a comparison.Psychopharmacology (Berl).200517921822915739074
- WangLW.Berry-KravisE.HagermanRJ.Fragile X: leading the way for targeted treatments in autism.Neurotherapeutics.2010726427420643379
- LynchG.PalmerLC.GallCM.The likelihood of cognitive enhancement.Pharmacol Biochem Behav.20119911612921215768
- BrennandKJ.GageFH.Concise review: the promise of human induced pluripotent stem cell-based studies of schizophrenia.Stem Cells.2011291915192222009633
- SudhofTC.Neuroligins and neurexins link synaptic function to cognitive disease.Nature.200845590391118923512
- CaseyJP.MagalhaesT.ConroyJM.et al.A novel approach of homozygous haplotype sharing identifies candidate genes in autism spectrum disorder.Hum Genet.201213156557921996756
- AlarconM.AbrahamsBS.StoneJL.et al.Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene.Am J Hum Genet.20088215015918179893
- SarasuaSM.DwivediA.BoccutoL.et al.Association between deletion size and important phenotypes expands the genomic region of interest in Phelan-McDermid syndrome (22q13 deletion syndrome).J Med Genet.20114876176621984749
- JamainS.QuachH.BetancurC.et al.Mutations of theX-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism.Nat Genet.200334272912669065
- TakeuchiM.HataY.HiraoK.ToyodaA.IrieM.TakaiY.SAPAPs. A family of PSD-95/SAP90-associated proteins localized at postsynaptic density.J Biol Chem.199727211943119519115257
- ShengM.KimE.The Shank family of scaffold proteins.J Cell Sci.2000113(Pt 11)1851185610806096
- HayashiMK.TangC.VerpelliC.et al.The postsynaptic density proteins Horner and Shank form a polymeric network structure.Cell.200913715917119345194
- BangashMA.ParkJM.MelnikovaT.et al.Enhanced polyubiquitination of Shank3 and NMDA receptor in a mouse model of autism.Cell.201114575877221565394
- WangX.McCoyPA.RodriguizRM.et al.Synaptic dysfunction and abnormal behaviors in mice lacking major isoforms of Shank3.Hum Mol Genet.2011203093310821558424
- VerpelliC.DvoretskovaE.VicidominiC.et al.Importance of shank3 in regulating metabotropic glutamate receptor 5 (mGluRS) expression and signaling at synapses.J Biol Chem.2011286348393485021795692
- WhalleyHC.O'ConnellG.SussmannJE.et al.Genetic variation in CNTNAP2 alters brain function during linguistic processing in healthy individuals.Am J Med Genet B Neuropsychiatr Genet.2011156694194821987501
- VoineaguI.WangX.JohnstonP.et al.Transcriptomic analysis of autistic brain reveals convergent molecular pathology.Nature.201147438038421614001
- HayashiML.RaoBS.SeoJS.et al.Inhibition of p21-activated kinase rescues symptoms of fragile X syndrome in mice.Proc Natl Acad Sci USA.2007104114891149417592139
- ZoghbiHY.Rett syndrome: what do we know for sure?Nat Neurosci.20091223924019238181
- MorettiP.BouwknechtJA.TeagueR.PaylorR.ZoghbiHY.Abnormalities of social interactions and home-cage behavior in a mouse model of Rett syndrome.Hum Mol Genet.20051420522015548546
- OgierM.WangH.HongE.WangQ.GreenbergME.KatzDM.Brainderived neurotrophic factor expression and respiratory function improve after ampakine treatment in a mouse model of Rett syndrome.J Neurosci.200727109121091717913925
- LauterbornJC.RexCS.KraE.et al.Brain-derived neurotrophic factor rescues synaptic plasticity in a mouse model of fragile X syndrome.J Neurosci.200727106851069417913902
- DattaAN.HahnCD.SahinM.Clinical presentation and diagnosis of tuberous sclerosis complex in infancy.J Child Neurol.20082326827318230839
- CusmaiR.MoaveroR.BombardieriR.VigevanoF.CuratoloP.Long-term neurological outcome in children with early-onset epilepsy associated with tuberous sclerosis.Epilepsy Behav.20112273573922142783
- HuangJ.ManningBD.A complex interplay between Akt, TSC2 and the two mTOR complexes.Biochem Soc Trans.200937(Pt 1)21722219143635
- CarsonRP.Van NielenDL.WinzenburgerPA.EssKC.Neuronal and glia abnormalities in Tsc1-deficient forebrain and partial rescue by rapamycin.Neurobiol Dis.20124536938021907282
- KhwajaOS.SahinM.Translational research: Rett syndrome and tuberous sclerosis complex.Curr Opin Pediatr.20112363363921970827
- EhningerD.HanS.ShilyanskyC.et al.Reversal of learning deficits in a Tsc2+/- mouse model of tuberous sclerosis.Nat Med.20081484384818568033
- EhningerD.LiW.FoxK.StrykerMP.SilvaAJ.Reversing neurodevelopmental disorders in adults.Neuron.20086095096019109903
- SchatzJH.Targeting the PI3K/AKT/mTOR pathway in non-Hodgkin's lymphoma: results, biology, and development strategies.Curr Oncol Rep.20111339840621755275
- OgitaS.LorussoP.Targeting phosphatidylinositol 3 kinase (PI3K)-Akt beyond rapalogs.Target Oncol.2011610311721547565
- FuYH.KuhlDP.PizzutiA.et al.Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox.Cell.199167104710581760838
- PierettiM.ZhangFP.FuYH.et al.Absence of expression of the FMR-1 gene in fragile X syndrome.Cell.1991668178221878973
- BrownV.JinP.CemanS.et al.Microarray identification of FMRP-associated brain mRNAsand altered mRNA translational profiles in fragile X syndrome.Cell.200110747748711719188
- GantoisI.BakkerCE.ReyniersE.et al.Restoring the phenotype of fragile X syndrome: insight from the mouse model.Curr Mol Med.2001144745511899089
- MineurYS.HuynhLX.CrusioWE.Social behavior deficits in the Fmr1 mutant mouse.Behav Brain Res.200616817217516343653
- ChenLY.RexCS.BabayanAH.et al.Physiological activation of synaptic RaoPAK (p-21 activated kinase) signaling is defective in a mouse model of fragile X syndrome.J Neurosci.201030109771098420720104
- SpencerCM.GrahamDF.Yuva-PaylorLA.NelsonDL.PaylorR.Social behavior in Frnrl knockout mice carrying a human FMR1 transgene.Behav Neurosci.200812271071518513141
- BakerKB.WraySP.RitterR.MasonS.LanthornTH.SavelievaKV.Male and female Frnrl knockout mice on C57 albino background exhibit spatial learning and memory impairments. GenesBrain Behav.20109562574
- HuberKM.GallagherSM.WarrenST.BearMF.Altered synaptic plasticity in a mouse model of fragile X mental retardation.Proc Natl Acad Sci U S A.2002997746775012032354
- DolenG.OsterweilE.RaoBS.et al.Correction of fragile X syndrome in mice.Neuron.20075695596218093519
- KruegerDD.BearMF.Toward fulfilling the promise of molecular medicine in fragile X syndrome.Annu Rev Med.20116241142921090964
- HagermanR.HoemG.HagermanP.Fragile X and autism: Intertwined at the molecular level leading to targeted treatments.Mol Autism.201011220858229
- AuerbachBD.OsterweilEK.BearMF.Mutations causing syndromic autism define an axis of synaptic pathophysiology.Nature.2011480636822113615
- HagermanR.LauterbornJ.AuJ.Berry-KravisE.Fragile x syndrome and targeted treatment trials.Results Probl Cell Differ.20125429733522009360
- SilvermanJL.SmithDG.RizzoSJ.et al.Negative allosteric modulation of the mGluR5 receptor reduces repetitive behaviors and rescues social deficits in mouse models of autism.Sci Transi Med.20124131ra51
- BolivarVJ.WaltersSR.PhoenixJL.Assessing autism-like behavior in mice: variations in social interactions among inbred strains.Behav Brain Res.2007176212617097158
- PearsonBL.PobbeRL.DefensorEB.et al.Motor and cognitive stereotypies in the BTBR T+tf/J mouse model of autism. GenesBrain Behav.2011213446451
- MoySS.NadlerJJ.YoungNB.et al.Social approach and repetitive behavior in eleven inbred mouse strains.Behav Brain Res.200819111812918440079
- ThomasA.BurantA.BuiN.GrahamD.Yuva-PaylorLA.PaylorR.Marble burying reflects a repetitive and perseverative behavior more than noveltyinduced anxiety.Psychopharmacology (Berl).200920436137319189082
- BurketJA.HerndonAL.WinebargerEE.JacomeLF.DeutschSI.Complex effects of mGluRS antagonism on sociability and stereotypic behaviors in mice: possible implications for the pharmacotherapy of autism spectrum disorders.Brain Res Bull.20118615215821840381
- PivenJ.RabinsP.Autism spectrum disorders in older adults: toward defining a research agenda.J Am Geriatr Soc.2011592151215522091837
- ScattoniM.RicceriL.CrawleyJ.Unusual repertoire of vocalizations in adult BTBR T+tf/J mice during three types of social encounters.Genes Brain Behav.201110445620618443
- WohrM.RoulletFl.CrawleyJN.Reduced scent marking and ultrasonic vocalizations in the BTBR T+tf/J mouse model of autism.Genes Brain Behav.201110354320345893
- CarterMD.ShahCR.MullerCL.CrawleyJN.CarneiroAM.Veenstra-VanderweeleJ.Absence of preference for social novelty and increased grooming in integrin beta3 knockout mice: Initial studies and future directions.Autism Res.20114576721254450
- BlundellJ.BlaissCA.EthertonMR.et al.Neuroligin-1 deletion results in impaired spatial memory and increased repetitive behavior.J Neurosci.2010302115212920147539
- RadyushkinK.HammerschmidtK.BoretiusS.et al.Neuroligin-3-deficient mice: model of a monogenic heritable form of autism with an olfactory deficit.Genes Brain Behav.2009841642519243448
- JamainS.RadyushkinK.HammerschmidtK.et al.Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism.Proc Natl Acad Sci U S A.20081051710171518227507
- ScattoniML.GandhySU.RicceriL.CrawleyJN.Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism.PLoS One.20083e306718728777
- SilvermanJL.YangM.LordC.CrawleyJN.Behavioural phenotyping assays for mouse models of autism.Nat Rev Neurosci.20101149050220559336
- BlundellJ.TabuchiK.BolligerMF.et al.Increased anxiety-like behavior in mice lacking the inhibitory synapse cell adhesion molecule neuroligin 2.Genes Brain Behav.2009811412619016888
- MartinsGJ.PlachezC.PowellEM.Loss of embryonic MET signaling alters profiles of hippocampal interneurons.Dev Neurosci.20072914315817148957
- ShuW.ChoJY.JiangY.et al.Altered ultrasonic vocalization in mice with a disruption in the Foxp2 gene.Proc Natl Acad Sci U S A.20051029643964815983371
- FujitaE.TanabeY.ShiotaA.et al.Ultrasonic vocalization impairment of Foxp2 (R552H) knockin mice related to speech-language disorder and abnormality of Purkinje cells.Proc Natl Acad Sci U S A.20081053117312218287060
- EnardW.GehreS.HammerschmidtK.et al.A humanized version of Foxp2 affects cortico-basal ganglia circuits in mice.Cell.200913796197119490899
- BielskyIF.HuSB.SzegdaKL.WestphalH.YoungLJ.Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin Via receptor knockout mice.Neuropsychopharmacology.20042948349314647484
- PobbeRL.PearsonBL.DefensorEB.et al.Oxytocin receptor knockout mice display deficits in the expression of autism-related behaviors.Horm Behav.In press.
- SalaM.BraidaD.LentiniD.et al.Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: a neurobehavioral model of autism.Biol Psychiatry.20116987588221306704
- MacbethAH.SteppJE.LeeHJ.YoungWS.3rd.CaldwellHK.Normal maternal behavior, but increased pup mortality, in conditional oxytocin receptor knockout females.Behav Neurosci.201012467768520939667
- BrielmaierJ.MattesonPG.SilvermanJL.et al.Autism-relevant social abnormalities and cognitive deficits in engrailed-2 knockout mice.PLoS One.20127e4091422829897