Abstract
Neural oscillations at low- and high-frequency ranges are a fundamental feature of large-scale networks. Recent evidence has indicated that schizophrenia is associated with abnormal amplitude and synchrony of oscillatory activity, in particular, at high (beta/gamma) frequencies. These abnormalities are observed during task-related and spontaneous neuronal activity which may be important for understanding the pathophysiology of the syndrome. In this paper, we shall review the current evidence for impaired beta/gamma-band oscillations and their involvement in cognitive functions and certain symptoms of the disorder. In the first part, we will provide an update on neural oscillations during normal brain functions and discuss underlying mechanisms. This will be followed by a review of studies that have examined high-frequency oscillatory activity in schizophrenia and discuss evidence that relates abnormalities of oscillatory activity to disturbed excitatory/inhibitory (E/I) balance. Finally, we shall identify critical issues for future research in this area.
Las oscilaciones neurales en los rangos de baja y alta frecuencia constituyen una característica fundamental de las redes a gran escala. La evidencía reciente ha indicado que la esquizofrenia está asociada con la amplitud anormal y la sincronía de la actividad oscilatoria, en particular, a altas frecuencias (beta/gama). Estas alteraciones se observan tanto durante la actividad neuronal espontánea como en la relacionada con tareas, to que puede ser importante para la comprensión de la fisiopatología del síndrome. En este artículo, se revisa la evidencia actual del deterioro de las oscilaciones de las bandas beta/gama y su participatión en las funciones cognitivas y algunos síntomas de este trastorno. En la primera parte, se entrega una actualización sobre las oscilaciones neuronales durante las funciones normales del cerebro y se discuten los mecanismos subyacentes. A continuación se revisan los estudios que han examinado la actividad oscilatoria de alta frecuencia en la esquizofrenia y se discute la evidencia que relaciona las alieraciones de la actividad oscilatoria con el deterioro del balance excitatorio/inhibitorio (E/I). Finalmente se identifican los temas críticos para el futuro de la investigación en esta área.
Les oscillations neuronales de basse et de haute fréquences sont une caractéristique fondamentale des réseaux de grande échelle. D'après des données récentes, l'amplitude et la synchronisation de l'activité oscillatoire sont anormales dans la schizophrénie, en particulier aux hautes fréquences (bêta/gamma). Ces anomalies sont observées lors de l'activité neuronale de repos et de travail, ce qui peut être important pour comprendre la physiopathologie de ce syndrome. Nous analysons dans cet article les preuves actuelles de l'altération des oscillations gamma/bêta et de leur rôle dans les fonctions cognitives et certains symptômes de la maladie. Dans la première partie, nous proposons une mise a jour sur les oscillations neuronales lors de l'activité normale du cerveau et nous en étudions les mécanismes. Puis nous examinons les études qui ont analysé l'activité oscillatoire à haute fréquence dans la schizophrénie et nous discutons les preuves reliant les anomalies de l'activité oscillatoire à la perturbation de l'équilibre excitation/inhibition (E/I). Enfin, nous identifions les points cruciaux de la recherche à venir dans ce domaine.
Neural oscillations in large-scale networks
The majority of cognitive and perceptual functions are based on the coordinated interactions of large numbers of neurons that are distributed within and across different specialized brain areas. A fundamental, yet unresolved, problem of modern neuroscience is how this coordination is achieved. One possibility is that neural oscillations at low- (theta, alpha) and high- (beta/gamma) frequency ranges facilitate the transient formation of large-scale networks that represent the neural correlates of a cognitive content or a motor program.Citation1,Citation2
In recent years, oscillatory activity and related synchronization phenomena have received a renewed interest in cognitive neuroscience. This is because of the evidence that synchronization and phase locking gate communication among neuronsCitation3 and thereby can support the dynamic configuration of functional networks.Citation2,Citation4,Citation5 While the first demonstrations of rhythmic activity were already obtained by investigators in the early 20th century,Citation6,Citation7 evidence for a potential function was only established many decades later.
An important link between oscillations and cortical computations was the discovery that oscillatory rhythms in the gamma range (30 to 80 Hz) establish precise synchronization of distributed neural responses. Gray and colleaguesCitation4 showed that action potentials generated by cortical cells align with the oscillatory rhythm in the gamma-band range. This has as a consequence that neurons participating in the same oscillatory rhythm synchronize their discharges with very high precision. Thus, high-frequency oscillations facilitate neuronal synchronization.
As a result of these discoveries, initial research focused on the relationship between gamma-band activity and perceptual processes (for a review see ref 8)Citation8. However, it soon became clear that context and goal-dependent synchronization of neural oscillations was not restricted to visual responses and the gamma-frequency band but also occurred at lower frequencies (beta, alpha, theta)Citation9,Citation10 and in a large number of brain structures in association with a wide range of cognitive and executive processes involving highly distributed processes in large-scale networksCitation1,Citation2 (Table I). More recently, these tight correlations between synchronized oscillations and higher cognitive functions prompted investigations of synchronization phenomena in pathological brain states.Citation11
Table I Neural oscillations in networks
Important and distinct variables of these dynamic processes are the power and frequency of oscillatoryactivity in local circuits and the long-range synchronization of these temporally structured activities across brain areasCitation2 (for an overview of core concepts of neuronal dynamics see Table II). Support for the need to distinguish between local oscillatory versus long-range synchronization processes comes from studies that have examined the frequencies at which neuronal ensembles oscillate. Local processes tend to be associated with high-frequency oscillations above 30 Hz, the gamma band, while long-range interactions tend to involve synchrony in lower frequency bands comprising theta (4 to 7 Hz), alpha (8 to 12 Hz), and beta (13 to 30 Hz) frequencies.Citation12,Citation13 One reason could be that larger networks cannot support synchronization with very high temporal precision as a result of long conduction times. This is because lower frequencies put fewer constraints on the precision of timing since the phases of increased and reduced excitability are longer.Citation14
Table II. Key concepts of neuronal dynamics.
In addition, evidence is accumulating that networks oscillating at different frequencies can become associated by cross-frequency coupling.Citation15 Such interactions can take several forms and lead to correlated power/power fluctuations or phase-amplitude coupling.Citation16 In the latter case, the amplitude of a high-frequency oscillation is modulated by the phase of a slower rhythm. Thus, in a number of studies the power of gamma oscillations has been shown to be modulated by the phase of theta or alpha-band oscillations.Citation17,Citation18
Generation of high-frequency oscillations in large-scale networks
The formation of functional networks through synchronized oscillations at beta/gamma-band frequencies is critically depended upon the dynamics of excitatory and inhibitory networks (E/I-balance) that establish transient links between ensembles of neurons through the modulation of the level of neuronal responsiveness.Citation19 Recent insights into the cellular mechanisms underlying these dynamics and, more specifically, the generation of rhythms and the establishment of long-range synchrony, make it now possible to engage in a targeted search for pathophysiological mechanisms of diseases associated with abnormal neuronal dynamics such as schizophrenia (SCZ).
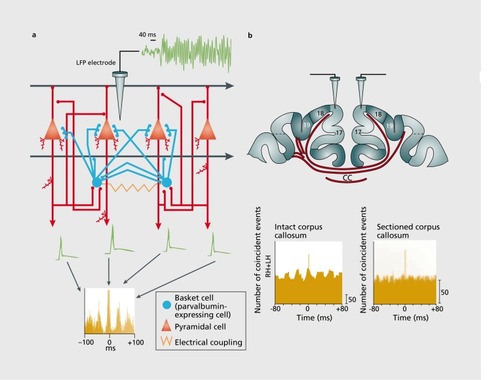
Previous experimental and theoretical work had already provided support for the notion that γ-aminobutyric acid (GABA)-ergic neurons play a pivotal role in the primary generation of high-frequency oscillations and their local synchronization,Citation20-Citation22 whereas glutamatergic inputs appear to control their strength, duration, and long-range synchronization.Citation23
GABAergic interneurons, especially those expressing the calcium binding protein parvalbumin (PV), play a particularly important role in the generation of high-frequency oscillations because of their fast-spiking characteristics and the short time constants of synaptic interactions mediated by these cells.Citation24 In a landmark paper, Sohal and colleaguesCitation22 probed the influence of up- and downregulation of PV interneurons on gamma-band oscillations in mice. Inhibition of PV interneurons led to an immediate suppression of 30- to 80-Hz oscillations while 10- to 30-Hz oscillations increased in power. In contrast, increasing PV interneuron mediated feedback inhibition by boosting principal cell activity enhanced gamma-band power.Citation25
Recent studies have also examined the specific role of glutamatergic inputs to PV interneurons for the generation of coordinated network activity. Carlen et alCitation26 examined the effect of deleting N-methyl-D-aspartate (NMDA) NR1 receptors on PV interneurons applying an optogenetic approach. Mice with a reduced expression of NR1 subunits were characterized by increased spontaneous 36- to 44-Hz activity in somatosensory cortex compared with control animals while showing reduced gamma -band activity during sensory stimulation. This change in neuronal dynamics was accompanied by dysfunctions in habituation, working memory, and associative learning. Optic stimulation of PV interneurons revealed diminished spike synchronization as well as increased spike latency and variance in spike timing.
Further evidence that 2-amino-3-(3-hydroxy-5-methylisoxazol-4-y) propanoic acid (AMPA) and NMDA receptor- mediated activation of PV interneurons is essential for the generation of high-frequency oscillatory activity, and its synchronization has been obtained in the hippocampus. Reduction of the GLuR-D receptor leads to a decrease of AMPA-mediated currents in PV interneurons and reduced power of oscillations in the 20- to 80-Hz range which is accompanied by a deficit in working memory.Citation27 In addition, selective ablation of the NMDA NR1 subunit in PV interneurons is associated with a significant reduction of power, stability, and rhythmicity of theta oscillations and an enhancement of gamma oscillations in CA1.Citation28
While the reciprocal connections between excitatory and inhibitory neurons determine the strength and duration of the oscillations and mediate local synchronization, long-range synchronization of spatially segregated cell groups has been attributed mainly to the action of excitatory pathways that target both excitatory and inhibitory neurons.Citation14,Citation29 Specifically, modeling and experimental evidence suggests that generation of long-range synchronization is dependent on AMPA-type glutamate receptor.Citation29
More recently, evidence has emerged that long-range inhibitory projections that originate from GABAergic cells and terminate selectively on inhibitory interneurons in the respective target areas could constitute an important substrate for inter-regional synchronization.Citation30 Given the pace-maker function of inhibitory networks, such direct coupling could provide a very efficient mechanism for the temporal coordination of distributed processes. In addition to GABAergic and glutamatergic circuit dynamics, modulatory systems play an important role in the gating of oscillations and synchrony. Thus, gamma oscillations and their synchronization depend critically on the activation of muscarinic acetylcholin-receptors.Citation31 Evidence is also available that dopamine and 5-HT modulate the prevalence of oscillations in different frequency bands.Citation32-Citation35
High-frequency oscillations in schizophrenia
Because of the close relations with underlying physiological parameters and evidence for the functional involvement of oscillatory networks in cognitive processes, there is increasing interest in the possibility that neural oscillations in SCZ may be informative for revealing the causes of cognitive deficits as well as establish potential links to the pathophysiology. Indeed, a large body of work has examined rhythmic activity during both spontaneous and task-related activity in SCZ patients with electroencephalography (EEG)/magnetoencephalography (MEG). Because of the prominent role of gamma-band activity in cognition during normal brain functioning, a particular focus has been on the investigation of high-frequency activity in patient populations.
Gamma band (30-100 Hz)
The overwhelming evidence points to a reduction of gamma-band oscillations during the execution of cognitive tasks in SCZ patients relative to controls .Citation36 Reductions in gamma-band amplitude have been demonstrated for a wide range of cognitive and perceptual paradigms, including working memory,Citation37 executive control,Citation38 and perceptual processing.Citation39,Citation40 There is preliminary evidence that the decrease in gamma-band spectral power is independent of medication status.Citation38
Recent studies have also examined the contribution of high (> 60 Hz) gamma-band oscillations to perceptual and cognitive deficits in schizophrenia. In a recent study by our groupCitation41 , impaired task performance during a perceptual organization task was accompanied by a widespread deficit in the power of gamma-band oscillations between 60 and 120 Hz. This deficit was associated with an effect size of d=1.26 which is in the range and above of effect sizes for event-related potentials (ERPs) that have been frequently investigated in SCZ, such as the Mismatch Match Negativity (MMN).Citation42 Similar results supporting the relevance of dysfunctions in oscillatory activity > 60 Hz have been reported by Tsuchimoto et alCitation43 and Hamm and colleaguesCitation44 who examined high gamma-band activity during an auditory steady state (ASS) paradigm.
Of particular importance is the evidence that not only the amplitude but also the synchronicity of gamma oscillations is reduced in SCZ patients.Citation45,Citation46 This is relevant because a large body of evidence suggests that the functional networks underlying perception, attention, and executive processes rely on dynamic coordination by phase locking of oscillatory activity originating in widely distributed cortical areas.Citation2,Citation5 Accordingly, reduced longrange phase synchronization could lead to a functional disconnection syndrome which has been proposed by several theorists to constitute a core impairment in SCZ.Citation47
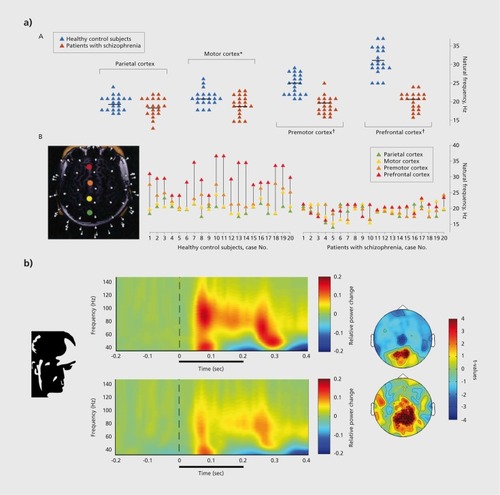
A potentially informative way of probing the ability of neural circuits to support the generation of high-frequency oscillations is the application of TMS in combination with EEG. Ferrarelli et alCitation48 applied transcranial magnetic stimulation (TMS) over four cortical areas and analysed stimulus-evoked EEG-activity for peak-frequency, synchrony as well as amplitude of neural oscillations (Figure 2). In controls, TMS pulses elicited robust activity in the 25- to 35-Hz frequency range over frontal electrodes while premotor, motor, and parietal cortex were characterized by beta-band activity. In SCZ patients, the peak frequency of evoked oscillations over frontal electrodes was characterized by a reduction of ~ 10 Hz compared with controls which correlated with both positive and negative symptoms as well as with neurocognitive impairments. In a previous study,Citation49 the same group demonstrated that TMS-elicited gamma-band oscillations propagated less beyond the area of stimulation in SCZ patients than in controls. One reason for this reduced spreading of activity could be impaired synchrony which should reduce propagation of neuronal activity.
Recent studies point to the possibility that the pattern of spontaneously occurring gamma-band oscillations may differ from that associated with cognitive processing and entrainment through TMS. Kikuchi et alCitation50 examined resting-state EEG data in medication-naive, first-episode patients with SCZ and healthy controls and found significantly elevated gamma-band power over frontal electrodes in patients. A similar finding was reported by Spencer et alCitation51 who showed significantly increased ~ 40 Hz baseline source power in chronic patients with schizophrenia. However, a study with MEG which investigated resting-state activity in chronic SCZ patients could not confirm this finding.Citation52
However, an important issue in regard to the interpretation of the elevated spontaneous high-frequency activity, and to task-related activity in general, is the question whether the changes during resting-state reflect an oscillatory process. An oscillation is characterized by a frequency-specific and narrow-banded modulation of spectral power,Citation53 while a broad band increase of high frequencies, at least in electrocorticography and perhaps also MEG recordings, is considered to reflect the sum of local synaptic events and action potentials and hence just the level of local cortical activation.Citation54
Beta-band oscillations in SCZ
Oscillations in the 13- to 30-Hz frequency range have been associated with the formation of widely distributed functional networks in the context of polymodal sensory processing, sensory-motor coordination, and the maintenance of posture during normal brain functioning.Citation55 More recently, Engel and FriesCitation56 have suggested that synchronized beta-band activity serves the maintenance of the actual sensorimotor or cognitive states.
In contrast to gamma-band activity, the role of beta-band oscillations has been less explored in SCZ. Uhlhaas et alCitation45 showed a pronounced impairment in long-range synchronization deficits in chronic SCZ patients during perceptual organization This is consistent with evidence highlighting the role of beta-band oscillations in establishing transient patterns of interactions across larger distances in oscillatory networks.Citation14
Further evidence for an involvement of disturbed betaband oscillations in cognitive deficits in SCZ was reported by Ford and colleagues.Citation57 The authors hypothesized that SCZ patients may fail to adequately predict the causes of sensory perception which could, for example, lead to self-generated speech acts being assigned to an external source as the result of a failure in the efference copy.Citation58 To investigate this hypothesis, Ford et al recorded EEG activity prior to self-generated speech vs a perception condition during which self-generated utterances were played back to the participants. Results showed that the phase-locking of beta-band oscillations was larger in the prespeech than in the prelistening interval. In SCZ patients, however, beta-band synchrony in the prespeech condition was reduced relative to controls and this reduction was particularly pronounced in patients with a history of auditory hallucinations. The authors suggest that the synchronized beta-band activity reflects a forward model which dampens auditory responsiveness to selfgenerated speech. In SCZ patients, this forward model is impaired and, as a result, self-generated speech acts may be experienced as an externally generated percept.
This hypothesis is consistent with recent evidence that beta-band oscillations mediate mainly top-down activity, and hence are critically involved in the prediction of upcoming sensory events while gamma-band oscillations, at least in sensory cortices, are involved in feedforward signaling.Citation59 This distinction is supported by the differential laminar expression of beta and gamma-band oscillations, respectively.Citation60 In vitro and in vivo recordings show that gamma-band activity is prominently generated in superficial layers 2/3 of the cortex,Citation61 the main origin of feed forward connections, whereas beta oscillations are mainly found in infragranular layers,Citation62 from which feedback projections originate preferentially.
High-frequency oscillations and the neurobiology of schizophrenia
Significant progress has been made in the identification of the mechanisms generating high-frequency oscillations in local circuits, and this has generated in turn research on the effects of genetic, pharmacological, and developmental manipulations of high-frequency oscillations.Citation63 The results of these studies support the view that neural oscillations are ideally suited as a measure to establish links between genes, physiology, and behavior in SCZ, and eventually may contribute to the identification of pathophysiological mechanisms.
E/I balance parameters
Recent work has focused on the alteration of mechanisms that influence E/I-balance parameters as one possible cause for deficits in high-frequency oscillations. In this context it is noteworthy that the messenger RNA for the enzyme GAD 67 which synthesizes GABA is reduced in several cortical areas in SCZ patients.Citation64 Moreover, this decrease is accompanied by reduced expression of the GABA membrane transporter 1 (GAT1).Citation65 Further evidence for a dysfunction in GABAergic transmission comes from magnetic resonance spectroscopy (1H-MRS) studies which have shown abnormal GABA levels in SCZ patients,Citation66 especially at illness onset.
Another mechanism which is crucial for the generation of high-frequency oscillations and influences the E/Ibalance is the AMPA- and NMDA-receptor-mediated activation of PV interneuron. Dysfunctions of NMDA-receptor-mediated transmission in SCZ have been suggested by genetic linking studiesCitation67 as well as by the effects of pharmacological NMDA-receptor blockade on cortical dynamics and cognition. In healthy controls, ketamine, an antagonist of the NMDA receptor, elicits the full range of psychotic symptoms and impairments in cognitive processes.Citation68 Furthermore, blockade of NMDA receptors in animal models has been shown to induce aberrant high-frequency oscillations in extended cortical and subcortical networksCitation69 which is consistent with the preliminary evidence for an elevation of resting state high-frequency activity in EEG data from patient populations.Citation50
Anatomy
Abnormal cortico-cortical connections are a likely cause for the impaired long-range synchronization observed in SCZ patients. Studies involving lesions and developmental manipulations indicate that gamma-band activity and its synchronization are mediated by cortico-cortical connections. These long-range, predominantly excitatory pathways, not only link reciprocally cells situated in the same cortical area but also cells distributed across different areas and even across the two hemispheresCitation70 . Accordingly, abnormalities in the number and organization of anatomical connections should impair longrange synchronization. Early evidence from in vivo and post-mortem studies suggests that white matter volume and integrity are altered in patients with schizophrenia.Citation71 This evidence is further supported by the more recent findings that revealed alterations in the organization of the connectome in SCZ.Citation72
Genetics
SCZ is associated with a genetic predisposition and there is evidence that high-frequency oscillations also exhibit genetically determined fingerprints that are highly specific for individual subjects.Citation73 It is thus of particular interest that auditory evoked gamma-band activity is not only reduced in SCZ patients but also in first-degree relatives,Citation74 as well as in unaffected, monozygotic twins,Citation75 suggesting that high-frequency oscillations qualify as an endophenolype.
Following this line of reasoning, several animal models have been examined for the effects of risk genes on E/I balance parameters and changes in high-frequency oscillations. “Disrupted-In-Schizophrenia-1” (DISC1) is a gene whose chromosomal translocation is associated with an increased incidence of major mental disorders, including SCZ.Citation76 Flikida and colleaguesCitation77 generated a transgenic mouse with a dominant-negative (DN) truncated DISCI and examined several anatomical parameters. DN-DISC1 mice were characterized by a selective reduction of PV immunoreactivity, PV being a Ca++-scavenger with preferential location in fast-spiking GABAergic-neurons that play a major role in the generation of high frequency oscillations.
Another SCZ-susceptibility gene, Neuregulin-1 (NRG I), has been shown to increase the power of gamma-band oscillations in hippocampal slices.Citation78 This enhancement is mediated through the activation of ErbB4 receptors on PV interneurons. Finally, DTNBP1 is a gene that encodes the protein dystrobrevin-binding protein 1 (dysbindin-1) and has been found to be reduced in SCZ patients.Citation79 Reduced dysbindin-1 expression in mice caused reduced phasic inhibition of PV cells which in turn was associated with impaired auditory evoked gamma-band activity.Citation80
The relationship between genetic risk factors and long-range synchronization was examined in a study by Sigurdson et al.Citation81 The authors measured the synchronization between the hippocampus and the prefrontal cortex during a working memory (WM) task in Df(16)Al/mice which provide a genetic model for the microdeletion on human chromosome 22 (22qll.2).The 22qll.2 microdeletion is one of the strongest genetic risk factors for SCZ.Citation82 Df(16)A1/- mice were characterized byimpaired WM performance which was closely correlated with reduced phase-locking of theta-band oscillations between prefrontal and hippocampal cells, suggesting that the genetic risk for SCZ impacts directly on large-scale interactions which in turn could underlie the cognitive deficits associated with the disorder.
Perspectives for high-frequency oscillations in schizophrenia
The available evidence suggests that SCZ is associated with aberrant high-frequency oscillations which could potentially explain core features of the disorders, such as the pronounced impairments of cognitive functions. Importantly, available evidence also establishes close relations between alterations in E/I balance parameters and oscillatory activity. These novel data emphasize the close relations between genetics, signaling cascades — especially those involving inhibitory mechanisms and NMDA receptors — and abnormal brain dynamics. Because of the improved knowledge about the mechanisms generating high-frequency oscillations, this provides a valuable basis for hypothesis-driven analysis of the pathophysiological origins of SCZ that may eventually lead to novel and targeted treatments. Because it is perhaps the right time to engage in such an ambitious endeavor, we would like to discuss a number of important issues that we believe are worth to be considered in the context of such a research program.
Towards a systems-oriented understanding of neuronal dynamics
So far, electrophysiological studies in SCZ have largely focused on obtaining amplitude estimates of spectral power at the sensor level. While the fluctuation of gamma-band power is an important variable that reflects changes in the E/I balance, it nonetheless provides only limited insights into the dynamics of extended cortical circuits. Thus, future studies should employ novel measures that allow for the testing of time and frequency sensitive neuronal interactions between cortical regions. Preliminary results obtained with scalp-recorded EEG data have highlighted alterations in long-range synchronization at beta- and gamma-band frequencies.Citation45,Citation46 However, because of the methodological problems and low spatial resolution of these approaches, we suggest that this promising approach should be complemented by source-reconstruction of EEG and MEG data which allow better insights into the dynamics and organization of extended functional networks.
Further research into neural oscillations should also take into account the possibility that the impairments in highfrequency oscillations are related to alterations in low-frequency bands, in particular in the theta and alpha frequency ranges, which have been less explored so far. There is increasing evidence that neural oscillations exhibit cross-frequency coupling, suggesting that populations of neurons oscillating at different frequencies interact with each other, forming nested assemblies.Citation15 Such coupling has been proposed to be responsible for correlated amplitude fluctuations of oscillations in different frequencies and for the modulation of the amplitude of a fast oscillatory process by the phase of a low-frequency oscillation.Citation17,Citation18
The potential relevance of abnormal cross-frequency interactions has only been investigated recently. Spencer et alCitation83 reported a reduced modulation of gamma-band somatosensory evoked potentials (SSEPs) in the auditory cortex in schizophrenia through the phase of delta oscillations, while White et alCitation84 observed decreased interactions between alpha- and gamma-band activities during a somatosensory task. However, more recent results could not support impaired cross-frequency interactions between high and low frequency oscillations during auditory SSEPs.Citation85 Accordingly, this remains an important area for future research.
Neural oscillations as a biomarker
Increasing evidence suggests that alternations in highfrequency activity may not be specific to SCZ. Impairments in neural synchrony have been demonstrated in bipolar disorder because auditory steady-state responsesCitation86 as well as long-range coherenceCitation87 are significantly impaired, paralleling findings in patients with schizophrenia.Citation45,Citation88 This is consistent with a substantial overlap between the two syndromes with respect to biological vulnerability.Citation89 Yet, dysfunctional gamma-band activity may not extend to other disorders, such as personality or mood disorders.Citation90
We would like to note that the wide range of oscillation frequencies provides a rich parameter field that can likely be exploited to delineate disorder-specific neuronal dynamics. If successful, such frequency-specific markers could then be used to identify the underlying physiological mechanisms and perhaps be used to assign patients to novel disease categories. Fingerprints of neuronal dynamics, such as alterations in the frequency, temporal precision, phase locking, and topology of neuronal oscillations, both during processing and resting state, may provide novel criteria for differential diagnoses. Resting-state activity may be particularly suited for this purpose because it has been shown to be highly structured,Citation91 genetically determined,Citation92 and to most likely reflect the coherent activation of functional networks that maintain representations of internal states.Citation93
Implications for treatment and prevention
The data reviewed may already have implications for a targeted search of novel treatments and preventive efforts. In view of the converging evidence for disturbed E/I balance and the resulting changes in high-frequency oscillations that are caused by alterations in GABAergic and glutamatergic neurotransmission, it might be rewarding to search for drug targets that restore E/I balance. Evidence on the efficacy of this approach is still sparse with some treatments showing modest benefitsCitation94 while others failed to improve, for example, cognition in patients with schizophrenia.Citation95
Treatment strategies should also consider that circuit dynamics may undergo changes during the course of the disorder. Accordingly, different interventions may be required at different phases.Citation96 Proton magnetic resonance spectroscopy (1-H MRS) has revealed, for example, that GABA and glutamate concentrations are increased in unmedicated, first-episode patients but reduced in chronically medicated patients,Citation66 suggesting that E/I balance shifts during the course of the illness. Another possibility for therapeutic interventions is suggested by the protracted developmental trajectory of brain dynamics that undergoes marked changes in late adolescence. The manifestation of schizophrenia during the transition from late adolescence to adulthood is preceded by an extended period of mild psychotic symptoms and cognitive dysfunctionsCitation97,Citation98 and improvement in therapeutic success will very likely involve early interventions that should ideally be initiated prior to the full manifestation of the clinical symptoms.
Finally, one might conceive of interventions that modulate brain dynamics by biofeedback and electrical stimulation. There is increasing evidence that transcranial magnetic and transcranial direct current stimulation (TMS/tDCS) can be applied as tools to modulate neuronal oscillations and large-scale synchrony in a frequency specific way. Polania et alCitation99 showed that tDCS at theta-frequency can facilitate frontoparietal synchrony, and Engelhard et alCitation100 showed that monkeys can be trained to selectively enhance gamma-band oscillations in the motor cortex if they are rewarded for power increases of local-field potential oscillations recorded from this area. The potential of these novel approaches for the remediation of cognitive deficits needs to be investigated further.
Selected abbreviations and acronyms
| AMPA | = | 2-amino-3-(3-hydroxy-5-methyl-isoxazol-4-y) propanoic acid |
| E/l balance | = | excitatory/inhibitory balance |
| GABA | = | γ-aminobutyric acid |
| MEG | = | magnetoencephalography |
| NMDA | = | N-methyl-D-aspartate |
| PV | = | parvalbumin |
| SCZ | = | schizophrenia |
| TMS | = | transcranial magnetic stimulation |
This work was supported by the Max Planck Society and the LOEWE Grant “Neuronale Koordination Forschungsschwerpunkt Frankfurt”.
REFERENCES
- SingerW.Neuronal synchrony: a versatile code for the definition of relations?Neuron.199924496511112510677026
- VarelaF.LachauxJP.RodriguezE.MartinerieJ.The brainweb: phase synchronization and large-scale integration.Nat Rev Neurosci.2001222923911283746
- WomelsdorfT.SchoffelenJM.OostenveldR.et alModulation of neuronal interactions through neuronal synchronization.Science.20073161609161217569862
- GrayCM.KonigP.EngelAK.SingerW.Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties.Nature.19893383343372922061
- FriesP.A mechanism for cognitive dynamics: neuronal communication through neuronal coherence.Trends Cogn Sci.2005947448016150631
- AdrianED.The electrical activity of the mammalian olfactory bulb.Electroencephalography Clin Neurophysiol.19502377388
- BergerH.Ueber das Elektroencephalogramm des Menschen.Archiv fuer Psychiatrie und Nervenkrankheiten.192987527570
- UhlhaasPJ.PipaG.LimaB.et alNeural synchrony in cortical networks: history, concept and current status.Front Integr Neurosci.200931719668703
- BasarE.Basar-ErogluC.KarakasS.SchurmannM.Oscillatory brain theory: a new trend in neuroscience.IEEE Eng Med Biol Mag.199918566610337564
- BuzsakiG.DraguhnA.Neuronal oscillations in cortical networks.Science.20043041926192915218136
- UhlhaasPJ.SingerW.Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology.Neuron.20065215516817015233
- von SteinA.SarntheinJ.Different frequencies for different scales of cortical integration: from local gamma to long range alpha/theta synchronization.Int J Psychophysiol.20003830131311102669
- SiegelM.DonnerTH.EngelAK.Spectral fingerprints of large-scale neuronal interactions.Nat Rev Neurosci.20121312113422233726
- KopellN.ErmentroutGB.WhittingtonMA.TraubRD.Gamma rhythms and beta rhythms have different synchronization properties.Proc Natl Acad Sci U S A.2000971867187210677548
- JensenO.ColginLL.Cross-frequency coupling between neuronal oscillations.Trends Cogn Sci.20071126726917548233
- PalvaJM.PalvaS.KailaK.Phase synchrony among neuronal oscillations in the human cortex.J Neurosci.2005253962397215829648
- CanoltyRT.EdwardsE.DalalSS.et alHigh gamma power is phaselocked to theta oscillations in human neocortex.Science.20063131626162816973878
- OsipovaD.HermesD.JensenO.Gamma power is phase-locked to posterior alpha activity.PLoS One.20083e399019098986
- HaiderB.McCormickDA.Rapid neocortical dynamics: cellular and network mechanisms.Neuron.20096217118919409263
- CobbSR.BuhlEH.HalasyK.PaulsenO.SomogyiP.Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons.Nature.199537875787477292
- WangXJ.BuzsakiG.Gamma oscillation by synaptic inhibition in a hippocampal interneuronal network model.J Neurosci.199616640264138815919
- SohalVS.ZhangF.YizharO.DeisserothK.Parvalbumin neurons and gamma rhythms enhance cortical circuit performance.Nature.200945969870219396159
- WhittingtonMA.TraubRD.JefferysJG.Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation.Nature.19953736126157854418
- BartosM.VidaI.JonasP.Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks.Nat Rev Neurosci.20078455617180162
- CardinJA.CarlenM.MeletisK.et alDriving fast-spiking cells induces gamma rhythm and controls sensory responses.Nature.200945966366719396156
- CarlenM.MeletisK.SiegleJH.et alA critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior.Mol Psychiatry.20121753754821468034
- FuchsEC.ZivkovicAR.CunninghamMO.et alRecruitment of paralbumin-positive interneurons determines hippocampal function and associated behavior.Neuron.20075359160417296559
- KorotkovaT.FuchsEC.PonomarenkoA.von EngelhardtJ.MonyerH.NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory.Neuron.20106855756921040854
- FuchsEC.DohenyH.FaulknerH.et alGenetically altered AMPA-type glutamate receptor kinetics in interneurons disrupt long-range synchrony of gamma oscillation.Proc Natl Acad Sci U S A.2001983571357611248119
- MelzerS.MichaelM.CaputiA.et alLong-range-projecting GABAergic neurons modulate inhibition in hippocampus and entorhinal cortex.Science.20123351506151022442486
- RodriguezR.KallenbachU.SingerW.MunkMH.Short- and long-term effects of cholinergic modulation on gamma oscillations and response synchronization in the visual cortex.J Neurosci.200424103691037815548651
- KrauseM.JiaY.Serotonergic modulation of carbachol-induced rhythmic activity in hippocampal slices.Neuropharmacology.20054838139015721170
- WojtowiczAM.van den BoomL.ChakrabartyA.et alMonoamines block kainate- and carbachol-induced gamma-oscillations but augment stimulus-induced gamma-oscillations in rat hippocampus in vitro.Hippocampus.20091927328819173289
- DzirasaK.RamseyAJ.TakahashiDY.et alHyperdopaminergia and NMDA receptor hypofunction disrupt neural phase signaling.J Neurosci.2009298215822419553461
- DemiralpT.HerrmannCS.ErdalME.et alDRD4 and DAT1 polymorphisms modulate human gamma band responses.Cereb Cortex.2007171007101916751296
- UhlhaasPJ.SingerW.Abnormal neural oscillations and synchrony in schizophrenia.Nat Rev Neurosci.20101110011320087360
- HaenschelC.BittnerRA.WaltzJ.et alCortical oscillatory activity is critical for working memory as revealed by deficits in early-onset schizophrenia.J Neurosci.2009299481948919641111
- MinzenbergMJ.FirlAJ.YoonJH.GomesGC.ReinkingC.CarterCS.Gamma oscillatory power is impaired during cognitive control independent of medication status in first-episode schizophrenia.Neuropsychopharmacology.2010352590259920827271
- FordJM.RoachBJ.FaustmanWO.MathalonDH.Out-of-synch and outof-sorts: dysfunction of motor-sensory communication in schizophrenia.Biol Psychiatry.20086373674317981264
- HiranoS.HiranoY.MaekawaT.et alAbnormal neural oscillatory activity to speech sounds in schizophrenia: a magnetoencephalography study.J Neurosci.2008284897490318463243
- GrütznerG.WibralM.SunS.et alDeficits in high-frequency (> 60 Hz) gamma oscillations during visual processing in schizophrenia.Front Hum Neurosci. In press.
- UmbrichtD.KrljesS.Mismatch negativity in schizophrenia: a metaanalysis.Schizophr Res.20057612315927795
- TsuchimotoR.KanbaS.HiranoS.et alReduced high and low frequency gamma synchronization in patients with chronic schizophrenia.Schizophr Res.20111339910521849245
- HammJP.GilmoreCS.PicchettiNA.SponheimSR.ClementzBA.Abnormalities of neuronal oscillations and temporal integration to lowand high-frequency auditory stimulation in schizophrenia.Biol Psychiatry.20116998999621216392
- UhlhaasPJ.LindenDE.SingerW.et alDysfunctional long-range coordination of neural activity during Gestalt perception in schizophrenia.J Neurosci.2006268168817516885230
- SpencerKM.NestorPG.NiznikiewiczMA.SalisburyDF.ShentonME.McCarleyRW.Abnormal neural synchrony in schizophrenia.J Neurosci.2003237407741112917376
- StephanKE.FristonKJ.FrithCD.Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring.Schizophr Bull.20093550952719155345
- FerrarelliF.SarassoS.GullerY.et alReduced natural oscillatory frequency of frontal thalamocortical circuits in schizophrenia.Arch Gen Psychiatry.20126976677422474071
- FerrarelliF.MassiminiM.PetersonMJ.et alReduced evoked gamma oscillations in the frontal cortex in schizophrenia patients: a TMS/EEG study.Am J Psychiatry.2008165996100518483133
- KikuchiM.HashimotoT.NagasawaT.et alFrontal areas contribute to reduced global coordination of resting-state gamma activities in drug-naive patients with schizophrenia .Schizophr Res.201113018719421696922
- SpencerKM.Baseline gamma power during auditory steady-state stimulation in schizophrenia.Front Hum Neurosci.2012519022319485
- RutterL.CarverFW.HolroydT.et alMagnetoencephalographic gamma power reduction in patients with schizophrenia during resting condition.Hum Brain Mapp.2009303254326419288463
- BuzsakiG.WangXJ.Mechanisms of gamma oscillations.Annu Rev Neurosci.20123520322522443509
- UhlhaasPJ.PipaG.NeuenschwanderS.WibralM.SingerW.A new look at gamma? High- (> 60 Hz) gamma-band activity in cortical networks: function, mechanisms and impairment.Prog Biophys Mol Biol.2011105142821034768
- PfurtschellerG.NeuperC.AndrewC.EdlingerG.Foot and hand area mu rhythms.Int J Psychophysiol.1997261211359202999
- EngelAK.FriesP.Beta-band oscillations-signalling the status quo?Curr Opin Neurobiol.20102015616520359884
- FordJM.RoachBJ.FaustmanWO.MathalonDH.Synch before you speak: auditory hallucinations in schizophrenia.Am J Psychiatry.200716445846617329471
- FeinbergI.Efference copy and corollary discharge: implications for thinking and its disorders.Schizophr Bull.19784636640734369
- ArnalLH.GiraudAL.Cortical oscillations and sensory predictions.Trends Cogn Sci.20121639039822682813
- KramerMA.RoopunAK.CarracedoLM.TraubRD.WhittingtonMA.KopellNJ.Rhythm generation through period concatenation in rat somatosensory cortex.PLoS Comput Biol.20084e100016918773075
- BuffaloEA.FriesP.LandmanR.BuschmanTJ.DesimoneR.Laminar differences in gamma and alpha coherence in the ventral stream.Proc Natl Acad Sci U S A. 520111081126211267
- RoopunAK.KramerMA.CarracedoLM.et alPeriod concatenation underlies interactions between gamma and beta rhythms in neocortex.Front Cell Neurosci.20082118946516
- WhittingtonMA.RoopunAK.TraubRD.DaviesCH.Circuits and brain rhythms in schizophrenia: a wealth of convergent targets.Curr Opin Pharmacol.20111150851421555247
- CurleyAA.ArionD.VolkDW.et alCortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: clinical, protein, and cell typespecific features.Am J Psychiatry.201116892192921632647
- VolkD.AustinM.PierriJ.SampsonA.LewisD.GABA transporter-1 mRNA in the prefrontal cortex in schizophrenia: decreased expression in a subset of neurons.Am J Psychiatry.200115825626511156808
- KegelesLS.MaoX.StanfordAD.et alElevated prefrontal cortex gamma-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy.Arch Gen Psychiatry.20126944945922213769
- KirovG.PocklingtonAJ.HolmansP.et alDe novo CNV analysis implicates specific abnormalities of postsynaptic signalling complexes in the pathogenesis of schizophrenia.Mol Psychiatry .20121714215322083728
- KrystalJH.KarperLP.SeibylJP.et alSubanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses.Arch Gen Psychiatry.1994511992148122957
- PinaultD.N-methyl d-aspartate receptor antagonists ketamine and MK-801 induce wake-related aberrant gamma oscillations in the rat neocortex.Biol Psychiatry.20086373073518022604
- EngelAK.KonigP.KreiterAK.SingerW.Interhemispheric synchronization of oscillatory neuronal responses in cat visual cortex.Science.1991252117711792031188
- KubickiM.McCarleyR.WestinCF.et alA review of diffusion tensor imaging studies in schizophrenia.J Psychiatr Res.200741153016023676
- FornitoA.ZaleskyA.PantelisC.BullmoreET.Schizophrenia, neuroimaging and connectomics.Neuroimage.20121091278812793
- van PeltS.BoomsmaDl.FriesP.Magnetoencephalography in twins reveals a strong genetic determination of the peak frequency of visually induced gamma-band synchronization.J Neurosci.2012323388339222399760
- LeichtG.KarchS.KaramatskosE.et alAlterations of the early auditory evoked gamma-band response in first-degree relatives of patients with schizophrenia: hints to a new intermediate phenotype.J Psychiatr Res.20114569970521067772
- HallMH.TaylorG.ShamP.et alThe early auditory gamma-band response is heritable and a putative endophenotype of schizophrenia.Schizophr Bull.20113777878719946013
- St ClairD.BlackwoodD.MuirW.et alAssociation within a family of a balanced autosomal translocation with major mental illness.Lancet.199033613161973210
- HikidaT.Jaaro-PeledH.SeshadriS.et alDominant-negative DISCI transgenic mice display schizophrenia-associated phenotypes detected by measures translatable to humans.Proc Natl Acad Sci U S A.2007104145011450617675407
- FisahnA.NeddensJ.YanL.BuonannoA.Neuregulin-1 modulates hippocampal gamma oscillations: implications for schizophrenia.Cereb Cortex.20091961261818632742
- TangJ.LeGrosRP.LounevaN.et alDysbindin-1 in dorsolateral prefrontal cortex of schizophrenia cases is reduced in an isoform-specif ic manner unrelated to dysbindin-1 mRNA expression.Hum Mol Genet.2009183851386319617633
- CarlsonGC.TalbotK.HaleneTB.et alDysbindin-1 mutant mice implicate reduced fast-phasic inhibition as a final common disease mechanism in schizophrenia. ProcNatl Acad Sci U S A.2011108E962E970
- SigurdssonT.StarkKL.KarayiorgouM.GogosJA.GordonJA.Impaired hippocam pa l-pref rental synchrony in a genetic mouse model of schizophrenia.Nature.201046476376720360742
- KarayiorgouM.GogosJA.The molecular genetics of the 22q11-associated schizophrenia.Brain Res Mol Brain Res.20041329510415582150
- SpencerKM.NiznikiewiczMA.NestorPG.ShentonME.McCarleyRW.Left auditory cortex gamma synchronization and auditory hallucination symptoms in schizophrenia.BMC Neurosci.2009108519619324
- WhiteTP.JosephV.O'ReganE.HeadKE.FrancisST.LiddlePF.Alphagamma interactions are disturbed in schizophrenia: a fusion of electroencephalography and functional magnetic resonance imaging.Clin Neurophysioi.201012114271437
- KiriharaK.RisslingAJ.SwerdlowNR.BraffDL.LightGA.Hierarchical organization of gamma and theta oscillatory dynamics in schizophrenia.Biol Psychiatry.20127187388022361076
- O'DonnellBF.VohsJL.HetrickWP.CarrollCA.ShekharA.Auditory event-related potential abnormalities in bipolar disorder and schizophrenia.Int J Psychophysiol.200453455515172135
- OzerdemA.GuntekinB.SaatciE.TuncaZ.BasarE.Disturbance in long distance gamma coherence in bipolar disorder.Prog Neuropsychopharmacol Biol Psychiatry.20103486186520398717
- KwonJS.O'DonnellBF.WallensteinGV.et alGamma frequency-range abnormalities to auditory stimulation in schizophrenia.Arch Gen Psychiatry.1999561001100510565499
- HillSK.HarrisMS.HerbenerES.PavuluriM.SweeneyJA.Neurocognitive allied phenotypes for schizophrenia and bipolar disorder.Schizophr Bull.20083474375918448479
- LenzD.FischerS.SchadowJ.BogertsB.HerrmannCS.Altered evoked gamma-band responses as a neurophysiological marker of schizophrenia?Int J Psychophysiol.201179253120705107
- HippJF.HawellekDJ.CorbettaM.SiegelM.EngelAK.Large-scale cortical correlation structure of spontaneous oscillatory activity.Nat Neurosci.20121588489022561454
- Linkenkaer-HansenK.SmitDJ.BarkilA.et alGenetic contributions to long-range temporal correlations in ongoing oscillations.J Neurosci.200727138821388918077700
- DecoG.JirsaVK.McintoshAR.Emerging concepts for the dynamical organization of resting-state activity in the brain.Nat Rev Neurosci.201112435621170073
- Heresco-LevyU.ErmilovM.LichtenbergP.BarG.JavittDC.High-dose glycine added to olanzapine and risperidone for the treatment of schizophrenia.Biol Psychiatry.20045516517114732596
- BuchananRW.KeefeRS.LiebermanJA.et alA randomized clinical trial of MK-0777 for the treatment of cognitive impairments in people with schizophrenia.Biol Psychiatry.20116944244921145041
- WoodSJ.YungAR.McGorryPD.PantelisC.Neuroimaging and treatment evidence for clinical staging in psychotic disorders: from the at-risk mental state to chronic schizophrenia.Biol Psychiatry.20117061962521762875
- YungAR.McGorryPD.The initial prodrome in psychosis: descriptive and qualitative aspects.Aust NZ J Psychiatry.199630587599
- KlosterkotterJ.HellmichM.SteinmeyerEM.Schultze-LutterF.Diagnosing schizophrenia in the initial prodromal phase.Arch Gen Psychiatry.20015815816411177117
- PolaniaR.NitscheMA.KormanC.BatsikadzeG.PaulusW.The importance of timing in segregated theta phase-coupling for cognitive performance.Curr Biol.2012221314131822683259
- EngelhardB.OzeriN.IsraelZ.BergmanH.VaadiaE.Inducing gamma oscillations and precise spike synchrony by operant conditioning via brainmachine interface.Neuron.20137736137523352171