Abstract
One of the most sexually dimorphic aspects of metabolic regulation is the bidirectional modulation of glucose and energy homeostasis by testosterone in males and females. Testosterone deficiency predisposes men to metabolic dysfunction, with excess adiposity, insulin resistance, and type 2 diabetes, whereas androgen excess predisposes women to insulin resistance, adiposity, and type 2 diabetes. This review discusses how testosterone acts in the central nervous system, and especially the hypothalamus, to promote metabolic homeostasis or dysfunction in a sexually dimorphic manner. We compare the organizational actions of testosterone, which program the hypothalamic control of metabolic homeostasis during development, and the activational actions of testosterone, which affect metabolic function after puberty. We also discuss how the metabolic effect of testosterone is centrally mediated via the androgen receptor.
Uno de los aspectos con mayor dimorfismo sexual de la regulación metabólica es la modulación bidireccional de la glucosa y la homeostasis energética por testosterona en hombres y mujeres. El déficit de testosterona predispone al hombre a una disfunción metabólica con exceso de adiposidad, resistencia a la insulina y diabetes tipo 2, mientras que el exceso de andrógeno predispone a la mujer a resistencia a la insulina, adiposidad y diabetes tipo 2. Esta revisión analiza cómo actúa la testosterona en el sistema nervioso central y especialmente en el hipotálamo, para promover la homeostasis o disfunción metabólica de una manera dimórfica sexual. Se comparan las funciones organizadoras de la testosterona, las cuales programan el control hipotalámico de la homeostasis metabólica durante el desarrollo, y sus acciones activadoras que afectan la función metabólica después de la pubertad. También se discute cómo el efecto metabólico de la testosterona está mediado centralmente a través del receptor androgénico.
L'un des aspects les plus sexuellement dimorphes de la régulation métabolique est la modulation bidirectionnelle de l'homéostasie du glucose et de l'énergie par la testostérone chez les hommes et les femmes. L'insuffisance en testostérone prédispose les hommes à un dysfonctionnement métabolique, avec une adiposité excessive, une résistance à l'insuline et un diabète de type 2, tandis qu'un excès d'androgènes prédispose les femmes à une résistance à l'insuline, une adiposité et un diabète de type 2. Cet article analyse la façon dont la testostérone agit dans le système nerveux central et en particulier dans l'hypothalamus pour favoriser la dysfonction ou l'homéostasie métaboliques d'une façon sexuellement dimorphe. Nous comparons le rôle d'organisation de la testostérone, programmant le contrôle hypothalamique de l'homéostasie métabolique pendant le développement et son rôle d'activation, agissant sur la fonction métabolique après la puberté. Nous analysons aussi la médiation centrale de l'effet métabolique de la testostérone via le récepteur des androgènes.
Introduction
The role of sex and gender in disease is a fundamental issue in medicine. Elucidating the molecular, biochemical, and physiological determinants of these biological sex differences is a necessary step toward precision, gender-specific medicine. There are fundamental aspects of the control of metabolic homeostasis that are regulated differently in males and females and that probably influence both the development of diabetes and obesity, as well as the response to pharmacological intervention.Citation1 Perhaps one of the most sexually dimorphic aspects of metabolic regulation is the bidirectional modulation of glucose and energy homeostasis by the male hormone testosterone in males and females.Citation2 Indeed, testosterone deficiency predisposes men to obesity, metabolic syndrome, and type 2 diabetes (T2D).Citation2-Citation5 In contrast, testosterone excess predisposes women to obesity and T2D.Citation2,Citation5,Citation6 Considering that men with androgen deficiency and women with androgen excess represent a large segment of the population, it is vital to understand the determinants of sex differences in testosterone's effects on metabolism.Citation3,Citation4,Citation6 This review will discuss how androgens act in the central nervous system (CNS) to impact metabolism in a sexually dimorphic manner in males and females.
Causes of sex differences in physiology
Perinatal programming by testosterone
Extensive evidence relates sexually dimorphic aspects of physiology to brain masculinization by the testicular perinatal testosterone surges in males.Citation7-Citation10 Hypothalamic structure and function are modified by testosterone, leading to sex differences in reproductive behavior and physiology.Citation10
The organizational-activational hypothesis of hormone action in the nervous system has gathered substantial attention since the publication of the seminal paper by Phoenix et al.Citation11,Citation12 These authors demonstrated that prenatal testosterone exposure in female rodents reduced the ability of estrogen administered in adulthood to elicit female mating behavior. This paper set the framework for the organizational-activational hypothesis, which states that: (i) organizing actions of testosterone occur during a critical period of development in which testosterone acts on the brain to cause permanent changes that persist in the absence of hormone, in effect programming the brain to be masculinized (and defeminized); and (ii) activational actions of testosterone occur after puberty when the hormone acts transiently and reversibly on circuitry that has already been established.Citation12,Citation13
The idea that there is a critical period in which testosterone exerts organizing effects is key to the organizational-activational hypothesis.Citation12,Citation13 Testosterone exposure must occur during this critical period for the brain to be masculinized. In human and nonhuman primate fetuses, the testicular testosterone surge occurs during the second trimester of pregnancy, which coincides with the development of the hypothalamus, the brain region that regulates both reproduction and metabolism.Citation14,Citation15 In rodents, the testicular testosterone surge occurs in the first week of neonatal life, which coincides with the development of the hypothalamus.Citation16,Citation17 During this critical period, testicular testosterone is capable of masculinizing the brain in males, and exogenous testosterone is capable of masculinizing the brain in females.Citation7,Citation10
In the brain, testosterone can be converted via aromatase to estradiol, which exerts its actions via estrogen receptors (ERs), or via 5α-reductase to dihydrotestosterone (DHT), which exerts its effects via androgen receptors (ARs).Citation18 Theoretically, either of these hormones could be responsible for the effects of testosterone on brain masculinization because both of these enzymes are expressed in the developing brain.Citation18 However, neonatal estradiol exposure is sufficient to masculinize the brain and elicit male-typical behavior in rodents.Citation7 Moreover, male mice with CNS-specific AR deficiency retain male behavior, but the frequency is reduced.Citation19,Citation20 This suggests that the brain is organized to perform male behavior in the absence of AR but that AR is required to activate male behavior.
Although the available evidence clearly demonstrates that AR is not necessary for organizational effects on behavior in rodents, this is not the case in primates. Prenatal administration of DHT to female rhesus macaques during the critical period programs more male behavior in offspring.Citation21 Similarly, prenatal exposure to DHT programs less female sexual behavior in female macaques.Citation22 This is consistent with the observation of men with complete androgen insensitivity syndrome who have nonfunctional ARs but normal-to-high testosterone levels.Citation23 These individuals often identify as females and display female-typical behaviors. Also in contrast to rodents, estrogen appears not to play a role in the masculinization of behavior in humans because men with aromatase deficiency or ER mutation show normal male-typical behaviors and gender identity.Citation24 In females, the polycystic ovarian syndrome (PCOS) is an endocrine disorder characterized by androgen excess throughout life, including prenatally.Citation25,Citation26,Citation27 Lesbian women have a significantly higher prevalence of PCOS than heterosexual women, suggesting that prenatal androgen exposure programs male-typical sexual preferences.Citation28 Together, these results suggest that, unlike in rodents, estradiol is not necessary for the organizational effects of testosterone and that AR is responsible for brain masculinization in primates, including humans. The role of central AR in masculinizing the brain with respect to metabolism will be discussed below.
Sex hormones after puberty
Recent evidence suggests that puberty may constitute an additional critical period in which sex hormones can exert organizational effects on the brain.Citation29 However, discussion of such is beyond the scope of this review. After puberty, hormones exert purely activational effects. AR is clearly required for the activation of male-typical behaviors in rodents because male mice with CNS-specific AR deficiency exhibit a reduction in the frequency of these behaviors.Citation19,Citation20 In addition, testosterone can activate aggressive behavior in adult female mice, although not to the same levels as testosterone in castrated males.Citation30 This suggests that the AR is required for activation of male-typical behavior in both males and females. The role of central AR activation in metabolism in adults will be discussed below.
Sex chromosomes
In addition to activational and organizational effects of hormones, sex differences in physiology can result from differences in sex chromosomes. The most obvious is the sex-determining region Y (SRY) gene on the Y chromosome, which codes the testis-determining factor and is responsible for the development of the testis and the production of testosterone.Citation9 In addition, the gene for the AR is located on the X chromosome.Citation19,Citation20 Males with Klinefelter syndrome, who have an extra X chromosome, show excess abdominal adiposity and have an increased risk of T2D, suggesting that the additional X chromosome promotes metabolic dysfunction.Citation31,Citation32
In rodents, the “four core genotypes” model has been developed to differentiate the impact of gonadal sex and sex chromosomes on physiology. This model consists of XX and XY males and females. To generate these mice, the SRY gene was removed from the Y chromosome to generate XY females. To generate XX males, the SRY gene was inserted onto an autosomal chromosome. This model shows that XX animals, regardless of gonadal sex, have increased fasting insulin, increased insulin resistance, increased liver triglycerides, increased levels of fatty acid oxidation enzymes, and increased fat mass when exposed to a high-fat diet.Citation33 These results are in agreement with observational data from patients with Klinefelter syndrome, suggesting that the X chromosome may impair metabolic function. Interestingly, the same group showed that XY animals on a chow diet show increased fat mass and impaired glucose tolerance relative to XX animals.Citation34 These results suggest that the X chromosome may only contribute to impaired metabolism in conditions of nutrient excess.
Androgen developmental programming of the CNS and the resulting sex differences in metabolism
Sex differences in neural circuitry
Differences in neural circuitry between males and females are mainly an issue of quantity and not connectivity.Citation10 Males and females display the same connections between the same regions, but it is the strength of these connections that differs. Within the hypothalamus, the anteroventral periventricular nucleus (AVPV) sends descending projections to the arcuate nucleus (ARC), and these descending projections are more pronounced in females.Citation35 These neurons are responsible for controlling gonadotropin-releasing hormone release and, thus, luteinizing hormone release. The fact that these connections are more robust in the female brain makes sense functionally because females have a greater luteinizing hormone response to estradiol than males.Citation36 Conversely, descending projections within the hypothalamus from the medial preoptic nucleus (MPN) to the ventromedial hypothalamus (VMH), are more robust in males.Citation10 This pathway may be involved in the initiation of male sexual behavior.Citation10 The anterior olfactory bulb sends projections to the medial amygdala and bed nucleus of the striata terminalis (BNST), which both project to several hypothalamic nuclei. This pathway is involved in pheromone sensing and is more robust in males.Citation10 Projections from the BNST to the AVPV are also much more robust in males than females.Citation37 Although the role of these projections is unknown, the authors hypothesize that they may be involved in olfaction.Citation37 Apart from reproduction and sexual behavior, the hypothalamus is also a key area for the control of energy balance and glucose homeostasis.Citation38 Therefore, the striking sex differences in hypothalamic neural circuitry described above suggest that similar sex differences in the hypothalamic circuitry controlling glucose and energy homeostasis exist.
Sex differences in AR expression in the brain
Many of the regions described above that show sexually dimorphic circuitry also express the AR, suggesting that AR may be involved in programming the sexual dimorphism of this circuitry. AR is expressed in the AVPV, ARC, and VMH of both males and females with similar levels of expression.Citation39-Citation41 AR is also expressed in the MPN and BNST, with expression levels being higher in males.Citation19,Citation39-Citation42 Other sites of AR expression include the lateral septum, medial amygdala, and premamillary nucleus.Citation39-Citation42 All of these nuclei are sexually dimorphic.Citation10 One study reported AR expression in the suprachiasmatic nucleus, paraventricular nucleus (PVN), lateral hypothalamus, and dorsomedial hypothalamus (DMH) in males, with no expression in females.Citation43 This is probably due to the poor quality of the AR antibody used. In the brain stem, there is also low AR expression in the parabrachial nucleus and the nucleus of the tractus solitarius (NTS) of males, both of which are involved in metabolism.Citation39,Citation44,Citation45 Perinatal exposure to testosterone or estradiol masculinizes the MPN and BNST by increasing AR expression in these nuclei.Citation20 Therefore, AR expression appears to result from the organizational effects of estradiol. However, DHT treatment in adult gonadectomized males and females restores AR expression to intact male levels.Citation40 Together, these results suggest that in rodents, estrogens can program AR expression, whereas androgens can activate AR expression.
In humans, there is a positive correlation between testosterone levels and gray matter volume in the parahippocampus, putamen, amygdala, and occipital and insular cortices.Citation46 Additionally, men tend to have larger amygdala volumes, whereas females tend to have larger hippocampal volumes.Citation47 Both of these regions show strong AR expression in rodents.Citation39
Thus, the association between sex differences in hypothalamic neurocircuitry and sex differences in hypothalamic AR expression suggests that androgen acting on AR in these hypothalamic areas may differentially affect metabolic function in males and females. Indeed, many of these regions that show sexually dimorphic expression of the AR are also involved in metabolic homeostasis, which will later be discussed in detail.Citation43
Sex differences in androgen perinatal CNS programming of metabolism
Several rodent, sheep, and primate models have been used to show that perinatal androgen exposure in females programs metabolic dysfunction with adiposity and insulin resistance in adult offspring.Citation48-Citation53 This is consistent with the observation that women with prenatal androgen excess, because of adrenal hyperplasia or virilizing tumors, develop central obesity and insulin resistance as adults despite normalization of androgen excess with treatment.Citation54,Citation55 However, it is unclear if these effects are due to androgen acting in the CNS or elsewhere. Nevertheless, there is extensive evidence that prenatal androgen excess alters metabolism via central actions. PCOS, for example, is characterized by hyperandrogenemia and ovarian dysfunction.Citation25 In a rat model of PCOS in which rats are exposed to androgen excess perinatally, an enhanced sympathetic activity is observed in adulthood that precedes the development of ovarian cysts, suggesting that increased sympathetic outflow was a result of androgen programming the CNS during the perinatal period.Citation56 This enhanced sympathetic activity could also contribute to the development of obesity.Citation57 Indeed, perinatal testosterone exposure in female mice increases fat mass in adulthood.Citation52 This is accompanied by increased norepinephrine turnover in white adipose tissue, a marker of increased sympathetic activity.Citation52 These results suggest that enhanced sympathetic activity caused by central testosterone action predisposes females to the development of obesity.Citation58 However, it is not known whether testosterone programmed sympathetic outflow via the AR after conversion to DHT or via ERs after conversion to estradiol. Indeed, in rodents, neonatal estradiol exposure increases adiposity in adult female offspring, whereas neonatal DHT exposure does not.Citation53 Instead, neonatal DHT exposure increases food intake in adult female offspring, which is accompanied by a decrease in hypothalamic expression of proopiomelanocortin (POMC), an anorexigenic peptide, and a decreased intensity of neuronal projections from POMC neurons within the ARC.Citation53 This is essentially a masculinization of the female hypothalamic melanocortin system regarding feeding behavior, as females exposed to DHT exhibit levels of food intake, POMC expression, and POMC neuron projections similar to those of littermate control males.Citation53 Thus, in mice, perinatal AR activation appears to be sufficient to program the hypothalamic melanocortin system toward male-like feeding behavior. In humans, females from opposite sex twin pairs exposed to prenatal testosterone from testis of a male co-twin also develop masculinized eating behaviors as adults.Citation59 However, in humans, it is unknown if this is mediated via AR or ER action. Thus, testosterone seems to play a role in the programming of the brain with respect to energyintake in both rodents and humans. However, in humans, it is unknown if this is mediated via AR or ER actions. The developmental effects of testosterone in females are summarized in ().
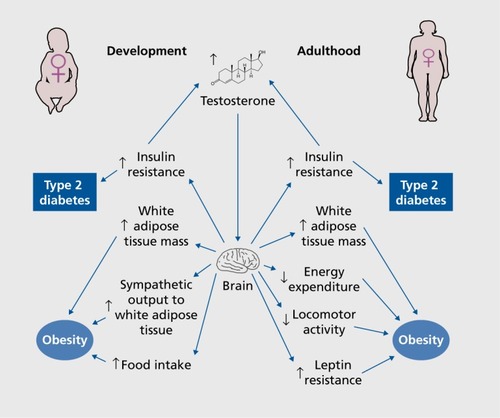
Interestingly, in male offspring, perinatal testosterone excess decreases food intake, the opposite of what is seen in littermate females.Citation60 Paradoxically, this decrease in food intake is accompanied by a compensatory, but inefficient, increase in the hypothalamic expression of the orexigenic peptides neuropeptide Y (NPY), agoutirelated peptide (AgRP), and orexin.Citation60 These mice also display a secondary decrease in energy expenditure that favors adiposity, which is probably the result of increased hypothalamic expression of the orexigenic peptides.Citation60-Citation62 This sex difference in the programming effect of neonatal testosterone in littermate male and female metabolic homeostasis underscores the potential for sex differences in metabolic diseases arising from the complements of sex-linked genes outside the testis determining gene SRYCitation60
Sex differences in metabolism due to androgen action in the CNS during adulthood
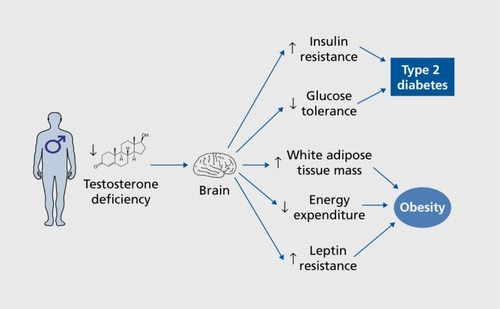
In men, testosterone deficiency is associated with increased visceral obesity, insulin resistance, and an increased risk of T2D.Citation1-Citation5,Citation63 Accordingly, male mice lacking AR develop obesity, insulin resistance, and glucose intolerance with aging.Citation64,Citation65 Testosterone deficiency is probably causative in the development of T2D, as men on androgen-deprivation therapy for the treatment of prostate cancer are more likely to develop T2D.Citation63,Citation66,Citation67 Evidence demonstrates that in males, testosterone maintains glucose homeostasis via action on AR in muscle, liver, and pancreatic β-cells.Citation1,Citation68 The question is whether testosterone acts in the male brain to maintain energy and glucose homeostasis and whether testosterone actions that favor metabolic homeostasis in males are mediated via AR or ERs. Testosterone action is probably mediated at least in part via the AR, as men that have AR variants with low transcriptional activity exhibit hyperinsulinemia and obesityCitation69 However, ERs are also involved in testosterone's metabolic effect in men, as treatment with an aromatase inhibitor blocked the ability of testosterone replacement to suppress adiposity in men.Citation70 More direct evidence for the role of the AR in metabolic homeostasis can be gathered from androgen-receptor knockout (ARKO) mouse models. However, in many of these models, AR is deleted developmentally, making it hard to differentiate the organizational effects from the activational effects of androgen.
Male mice with global AR deficiency exhibit late-onset obesity caused by decreased energy expenditure.Citation64 In addition, male mice with global AR deficiency exhibit resistance to centrally administered leptin, providing indirect evidence that brain AR may also be involved in ARKO-induced leptin resistance.Citation43 Indeed, leptin fails to activate signal transducer and activator of transcription 3 (STAT3) in ARC neurons of male ARKO mice and does not reduce food intake or body weight.Citation43 The authors suggest a number of hypothalamic sites that express both AR and the leptin receptor where androgen may be acting to cause leptin resistance. These sites include the ARC, VMH, DMH, PVN, lateral hypothalamus, premamillary nucleus, and suprachiasmatic nucleus.Citation43 Central loss of AR function is instrumental in this phenotype, as selective neuronal AR deficiency also causes late-onset obesity in male mice.Citation71
Furthermore, global AR deficiency in male mice produces glucose intolerance, insulin resistance, and increased lipogenesis.Citation65 CNS-specific AR deficiency produces insulin resistance, glucose intolerance, and obesity on a chow diet in aging male mice.Citation71 These mice exhibit hypothalamic insulin resistance with enhanced nuclear factor-κB activation leading to upregulation of protein-tyrosine phosphatase 1B (PTP1B), an inhibitor of insulin signaling.Citation71 This hypothalamic insulin resistance results in excess expression of the orexigenic peptide AgRP, which probably mediates the effects on obesity, insulin resistance, and glucose intolerance.Citation71 Clearly, hypothalamic AR action confers protection against obesity and diabetes in males, at least in part by maintaining insulin action in the hypothalamus. However, it is uncertain if the effects of AR in the brain are due to organizational or activational effects, as AR deletion may lead to developmental defects that are revealed in adulthood. The central effects of testosterone deficiency in men are summarized in ().
However, excess AR activation can also impair metabolism in males. For example, testosterone administration to castrated rats at physiological doses improves insulin sensitivity; high doses of testosterone do not.Citation72 Perhaps the relationship between androgen levels is parabolic, with both low levels and very high levels causing metabolic dysfunction. This curve would be shifted far to the right in males compared with females.
In women, testosterone excess increases the risk of developing T2D.Citation2,Citation5,Citation6,Citation73 Testosterone levels in women are positively correlated with insulin resistance and impairment of glucose tolerance.Citation6,Citation73,Citation74 PCOS is an endocrine disorder affecting 7% of women and characterized by androgen excess throughout life.Citation75 In the United States, 80% of women with PCOS are obese.Citation75 Many women with PCOS, including lean individuals, are insulin resistant and glucose intolerant.Citation74,Citation76-Citation78In fact, androgen levels in women with PCOS are positively correlated with insulin resistance.Citation74 Moreover, female to male transsexuals (with high testosterone levels) exhibit insulin resistance, suggesting a causal relationship between androgen excess and insulin resistance in women.Citation79 This also suggests that androgens exert activational effects to cause metabolic dysfunction in women, as androgens are administered during adulthood.Citation79 In addition, insulin resistance in hyperandrogenemic women can be partially reversed by antiandrogen treatment, suggesting a causal role of hyperandrogenemia on insulin resistance.Citation76
Studies in animal models suggest that androgen excess in these women impairs metabolism via both organizational and activational effects, as both perinatal and adult androgen excess induce metabolic dysfunction in animal models.Citation48-Citation53,Citation80
In female mice with chronic androgen excess during adulthood, leptin fails to reduce body weight, leading to obesity. This is paralleled by a failure of leptin to upregulate brown adipose tissue (BAT) expression of the thermogenic uncoupling protein 1 (UCP-1), which is associated with decreased energy expenditure.Citation80 These results suggest that there is a disruption in neural communication between the central leptin signal and BAT. In these mice, androgen excess decreased hypothalamic POMC messenger RNA expression. Interestingly, androgen excess increased POMC intensity in neuronal bodies of the ARC while simultaneously decreasing the intensity of POMC projections to the DMH, suggesting that axonal transport of POMC from the soma to the nerve terminals was impaired. As leptin action in the DMH increases sympathetic tone to BAT and increases thermogenesis, these results suggest that in mice, androgen excess decreases energy expenditure via an alteration of the melanocortin pathway to the DMH.Citation80,Citation81 In female mice, androgen excess produced no alterations in food intake or leptin's anorectic action.Citation80 Similar to the female mouse model of androgen excess, deletion of the leptin receptor from POMC neurons leads to decreased POMC expression and increased fat mass without altering food intake.Citation82 Moreover, female rats exposed to androgen excess during adulthood showed reduced locomotor activity, suggesting that decreased locomotor activity may also be contributing to the obesity seen in females exposed to chronic androgen excess.Citation83 The exact site of androgen action on AR that leads to this phenotype is unknown. A previous study found that only 3% of POMC neurons express AR,Citation84 although there is robust expression in other ARC neurons,Citation39-Citation40 suggesting that the alteration in POMC neurons is indirect.
Androgen excess in females also alters glucose homeostasis. Administration of DHT to adult female mice leads to insulin resistance and glucose intolerance.Citation2 This effect is not observed in β-cell ARKO mice, suggesting that it is β-cell AR that is responsible for the metabolic dysfunction induced by androgen excess.Citation2,Citation85,Citation86 Our lab is currently exploring if neuronal AR also contributes to androgen excess-induced metabolic dysfunction. The central effects of testosterone excess in adult females are summarized in Figure 1.
Interestingly, androgen signaling could be important for proper metabolic function in females. Female global ARKO mice display normal metabolic phenotypes on a chow diet, yet they develop insulin resistance, glucose intolerance, and obesity when on a high-fat diet relative to normal mice on a high-fat diet.Citation87 However, the authors did not explore if this obesity was due to increased food intake or decreased energy expenditure. It is also unclear if the phenotype is due to AR in the CNS and if the effects of androgen are organizational or activational. Our lab is currently exploring if these effects are mediated by central AR.
Potential sites of androgen action in CNS on glucose and energy homeostasis
There are several regions of the brain that regulate glucose and energy homeostasis and also express AR.Citation39-Citation42 Only the most likely regions for androgen action on metabolism will be discussed here.
Women with PCOS exhibit increased gonadotropin-releasing hormone (GnRII) pulsatility, suggesting impairment of hypothalamic GnRH neurons.Citation88 It is proposed that androgen activation of AR in ARC y-aminobutyric acid (GABA)ergic neurons upstream of GnRII neurons downregulates the progesterone receptor, whose activity leads to suppression of GnRH pulsatility.Citation88 Perhaps AR action in these ARC neurons also contributes to metabolic dysfunction. The studies described above suggest that the ARC is a probable site at which androgen is acting to impact metabolism.Citation43,Citation53,Citation60,Citation71 Indeed, in mice, impairment of GABAergic signaling within the ARC reduces energy expenditure by reducing thermogenesis without altering food intake, a phenotype similar to that of the model of adult androgen excess in female mice.Citation80,Citation89 Additionally, androgen could target the ARC in females to induce leptin resistance and hepatic insulin resistance in female mice. Indeed, several mouse models implicate insulin or leptin receptors in POMC neurons of the ARC in hepatic glucose production and the prevention of hepatic insulin resistance.Citation90-Citation93
The VMH is another site of AR expression that could mediate androgen action on metabolic regulation.Citation39,Citation40 Early studies showed that lesions of the VMH, which also encompassed part of the ARC, produced hyperinsulinemia that was blocked by vagotomy, suggesting that the VMII is involved in control of glucose homeostasis via autonomic output.Citation94,Citation95 More recent studies have revealed that pharmacological and genetic manipulations of the VMH in mice alter insulin sensitivity.Citation96,Citation97 In contrast to the ARC, the VMII influences insulin sensitivity by controlling glucose uptake in skeletal muscle.Citation97,Citation98 In addition, the VMH lesions discussed above also produced obesity independent of food intake.Citation95 Other early studies showed that electrical stimulation of the VMH enhanced BAT thermogenesis, suggesting that the VMH regulates energy expenditure.Citation99 In fact, VMH-specific deletion of forkhead box O1 (FOXO1) reduced fat mass by increasing energy expenditure, supporting the conclusion that the VMH is also involved in regulating energy expenditure and fat mass.Citation97
As discussed above, the DMH is a key nucleus that regulates BAT thermogenesis and energy expenditure and could be a target of androgen action.Citation81,Citation100 Indeed, in female mice exposed to chronic androgen excess, we see reduced intensity of POMC fibers in the DMII and a reduced decrease in body weight in response to the melanocortin receptor agonist, melanotan II.Citation80
Conclusion
The studies reviewed here demonstrate that testosterone acts in the CNS to differentially impact glucose homeostasis and energy balance in males and females. In males, loss of central AR action (as can be observed during testosterone deficiency) decreases energy expenditure and predisposes to adiposity and insulin resistance. In females, androgen excess during the perinatal period or adulthood (as can be observed in PCOS) negatively impacts metabolic homeostasis. Perinatal androgen excess most likely involves central AR, as we observe alterations in the hypothalamic melanocortin system. Nevertheless, more studies are needed to determine the exact hypothalamic sites and mechanisms of androgen action that promote metabolic homeostasis or metabolic dysfunction during male androgen deficiency and female androgen excess. Additional studies are also needed to characterize the role of AR in female metabolic homeostasis.
Selected abbreviations and acronyms
| AR | = | androgen receptor |
| ARC | = | arcuate nucleus |
| CNS | = | central nervous system. |
| DHT | = | dihydrotestosterone |
| DMH | = | dorsomedial hypothalamus |
| ER | = | estrogen receptor |
| PCOS | = | polycystic ovarian syndrome |
| POMC | = | proopiomelanocortin |
| T2D | = | type 2 diabetes |
| VMH | = | ventromedial hypothalamus |
Conflict of Interest: The authors declare no conflict of interest.
REFERENCES
- Mauvais-JarvisF.Sex differences in metabolic homeostasis, diabetes, and obesity.Biol Sex Differ.201561426339468
- NavarroG.AllardC.XuW.Mauvais-JarvisF.The role of androgens in metabolism, obesity, and diabetes in males and females.Obesity.201523471371925755205
- ZitzmannM.FaberS.NieschlagE.Association of specific symptoms and metabolic risks with serum testosterone in older men.J Clin Endocrinol Metab.200691114335434316926258
- ZitzmannM.Testosterone deficiency, insulin resistance and the metabolic syndrome.Nat Rev Endocrinol.200951267368119859074
- DingEL.SongY.MakikVS.LiuS.Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systemic review and meta-analysis.JAMA.2006295111288129916537739
- LegroRS.KunselmanAR.DodsonWC.DunaifA.Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women.J Clin Endocrinol Metab.19998411651699920077
- ArnoldAP.GorskiRA.Gonadal steroid induction of structural sex differences in the central nervous system.Annu Rev Neurosci.198474134426370082
- MacLuskyNJ.NaftolinF.Sexual differentiation of the central nervous system.Science.19812114488129413026163211
- MorrisJA.JordanCL.BreedloveSM.Sexual differentiation of the vertebrate nervous system.Nat Neurosci.20047101034103915452574
- SimerlyRB.Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain.Annu Rev Neurosci.200225150753612052919
- PhoenixCH.GoyRW.GerallAA.YoungWC.Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig.Endocrinology.195965336938214432658
- WallenK.The organizational hypothesis: reflections on the 50th anniversary of the publication of Phoenix, Goy, Gerall, and Young (1959).Horm Behav.200955556156519446072
- ArnoldAP.The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues.Horm Behav.200955557057819446073
- AbramovichDR.Human sexual differentiation-in utero influences.J Obstet Gynaecol Br Commonw.19748164484534407079
- KoutcherovY.MaiJK.AshwellKW.PaxinosG.Organization of human hypothalamus in fetal development.J Comp Neurol.2002446430132411954031
- CorbierP.EdwardsDA.RoffiJ.The neonatal testosterone surge: a comparative study.Arch Int Physiol Biochim Biophys.199210021271311379488
- BouretSG.DraperSJ.SimerlyRB.Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice.J Neurosci.200424112797280515028773
- CelottiF.MelcangiRC.MartiniL.The 5 alpha-reductase in the brain: molecular aspects and relation to brain function.Front Neuroendocrinol.19921321632151468601
- RaskinK.de GendtK.DuittozA.et alConditional inactivation of androgen receptor gene in the nervous system: effects on male behavioral and neuroendocrine responses.J Neurosci.200929144461447019357272
- JunttiSA.TollkuhnJ.WuMV.et alThe androgen receptor governs the execution, but not programming, of male sexual and territorial behaviors.Neuron.201066226027220435002
- ThorntonJ.ZehrJL.LooseMD.Effects of prenatal androgens on rhesus monkeys: a model system to explore the organizational hypothesis in primates.Horm Behav.200955563364519446080
- ThorntonJ.GoyRW.Female-typical sexual behavior of rhesus and defeminization by androgens given prenatally.Horm Behav.19862021291473721408
- Imperato-McGinleyJ.PetersonRE.GautierT.et alHormonal evaluation of a large kindred with complete androgen insensitivity: evidence for secondary 5a-reductase deficiency.J Clin Endocrinol Metab.19825459319416801078
- GrumbachMM.AuchusRJ.Estrogen: consequences and implications of human mutations in synthesis and action.J Clin Endocrinol Metab.199984124677469410599737
- EhrmannDA.Polycystic ovary syndrome.N Engl J Med.2005352121223123615788499
- AbbottDH.BarnettDK.BrunsCM.DumesicDA.Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome?Hum Reprod Update.200511435737415941725
- XitaN.TsatsoulisA.Review: fetal programming of polycystic ovary syndrome by androgen excess: evidence from experimental, clinical, and genetic association studies.J Clin Endocrinol Metab.20069151660166616522691
- AgrawalR.SharmaS.BekirJ.et alPrevalence of polycystic ovaries and polycystic ovary syndrome in lesbian women compared with heterosexual women.Fertil Steril.20048251352135715533359
- RomeoRD.Puberty: a period of both organizational and activational effects of steroid hormones on neurobehavioural development.J Neuroendocrinol.200315121185119214636181
- BarkleyMS.GoldmanBD.Testosterone-induced aggression in adult female mice.Horm Behav.1977917684561021
- BojesenA.KristensenK.BirkebaekNH.et alThe metabolic syndrome is frequent in Klinefelter's syndrome and is associated with abdominal obesity and hypogonadism.Diabetes Care.20062971591159816801584
- BojesenA.HostC.GravholtCH.Klinefelter's syndrome, type 2 diabetes and the metabolic syndrome: the impact of body composition.Mol Hum Reprod.201016639640120231162
- ChenX.McCluskyR.ChenJ.et alThe number of X chromosomes causes sex differences in adiposity in mice.PLoS Genet.201285e1 002709
- ChenX.McCluskyR.ItohY.ReueK.ArnoldAP.X and Y chromosome complement influence adiposity and metabolism in mice.Endocrinology.201315431092110423397033
- GuGB.SimerlyRB.Projections of thesexuallydimorphicanteroventral periventricular nucleus in the female rat.J Comp Neurol.199738411421649214545
- YamajiT.DierschkeDJ.HotchkissJ.BhattacharyaAN.SurveAH.KnobilE.Estrogen induction of LH release in the rhesus monkey.Endocrinology.1971894103410414999142
- HuttonLA.GuG.SimerlyRB.Development of a sexually dimorphic projection from the bed nuclei of the stria terminalistotheanteroventral periventricular nucleus in the rat.J Neurosci.1998188300330139526017
- GaoQ.HorvathTL.Neurobiology of feeding and energy expenditure.Annu Rev Neurosci.20073036739817506645
- SimerlyRB.ChangC.MuramatsuM.SwansonLW.Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study.J Comp Neurol.1990294176952324335
- BrockO.De MeesC.BakkerJ.Hypothalamic expression of oestrogen receptor a and androgen receptor is sex-, age-and region-dependent in mice.J Neuroendocrinol.201527426427625599767
- LuSF.McKennaSE.Cologer-CliffordA.NauEA.SimonNG.Androgen receptor in mouse brain: sex differences and similarities in autoregulation.Endocrinology.19981394159416019528939
- ShahNM.PisapiaDJ.ManiatisS.MendelsohnMM.NemesA.AxelR.Visualizing sexual dimorphism in the brain.Neuron.200443331331915294140
- FanW.YanaseT.NishiY.et alFunctional potentiation of leptin-signal transducer and activator of transcription 3 signaling by the androgen receptor.Endocrinology.2008149126028603618703637
- CamposCA.BowenAJ.SchwartzMW.PalmiterRD.Parabrachial CGRP neurons control meal termination.Cell Metab.201623581182027166945
- DallaportaM.HimtniT.PerrinJ.OrsiniJC.Solitary tract nucleus sensitivityto moderate changes in glucose level.Neuroreport.199910122657266010574387
- LentiniE.KasaharaM.ArverS.SavicI.Sex differences in the human brain and the impact of sex chromosomes and sex hormones.Cereb Cortex.201323102322233622891037
- NeufangS.SpechtK.HausmannM.et alSex differences and the impact of steroid hormones on the developing human brain.Cereb Cortex.200919246447318550597
- EisnerJR.DumesicDA.KemnitzJW.ColmanRJ.AbbottDH.Increased adiposity in female rhesus monkeys exposed to androgen excess during early gestation.Obes Res.200311227928612582225
- NilssonC.NiklassonM.ErikssonE.BjorntorpP.HolmangA.Imprinting of female offspring with testosterone results in insulin resistance and changes in body fat distribution at adult age in rats.J Clin Invest.1998101174789421468
- AlexandersonC.ErikssonE.Stener-VictorinE.et alPostnatal testosterone exposure results in insulin resistance, enlarged mesenteric adipocytes, and an atherogenic lipid profile in adult female rats: comparisons with estradiol and dihydrotestosterone.Endocrinology.2007148115369537617656458
- AbbottDH.TarantalAF.DumesicDA.Fetal, infant, adolescent and adult phenotypes of polycystic ovary syndrome in prenatally androgenized female rhesus monkeys.Am J Primatol.200971977678419367587
- NoharaK.WaraichRS.LiuS.et alDevelopmental androgen excess programs sympathetic tone and adipose tissue dysfunction and predisposes to a cardiometabolic syndrome in female mice.Am J Physiol Endocrinol Metab.201330412E1321E133023612996
- NoharaK.ZhangY.WaraichRS.et alEarly-life exposure to testosterone programs the hypothalamic melanocortin system.Endocrinology.201115241661166921303958
- HagueWM.AdamsJ.RoddaC.et alThe prevalence of polycystic ovaries in patients with congenital adrenal hyperplasia and their close relatives.Clin Endocrinol (Oxf).19903345015102225492
- BarnesRB.RosenfieldRL.EhrmannDA.et alOvarian hyperandrogynism as a result of congenital adrenal virilizing disorders: evidence for perinatal masculinization of neuroendocrine function in women.J Clin Endocrinol Metab.1994795132813337962325
- LaraHE.FerruzJL.LuzaS.BustamanteDA.BorgesY.OjedaSR.Activation of ovarian sympathetic nerves in polycystic ovary syndrome.Endocrinology.19931336269026957902268
- LansdownA.ReesDA.The sympathetic nervous system in polycystic ovary syndrome: a novel therapeutic target?Clin Endocrinol (Oxf).201277679180122882204
- Mauvais-JarvisF.Developmental androgenization programs metabolic dysfunction in adult mice: clinical implications.Adipocyte.20143215115424719790
- CulbertKM.BreedloveSM.BurtSA.KlumpKL.Prenatal hormone exposure and risk for eating disorders: a comparison of opposite-sex and same-sex twins.Arch Gen Psychiatry.200865332933618316679
- NoharaK.LiuS.MeyersMS.et alDevelopmental androgen excess disrupts reproduction and energy homeostasis in adult male mice.J Endocrinol.2013219325926824084835
- CowleyMA.PronchukN.FanW.DinulescuDM.ColmersWF.ConeRD.Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat.Neuron.199924115516310677034
- WangJ.OsakaT.InoueS.Orexin-A-sensitive site for energy expenditure localized in the arcuate nucleus of the hypothalamus.Brain Res.2003971112813412691845
- OhJY.Barrett-ConnorE.WedickNM.WingardDL.Rancho Bernardo Study: endogenous sex hormones and the development of type 2 diabetes in older men and women.Diabetes Care.2002251556011772901
- FanWQ.YanaseT.NomuraM.et alAndrogen receptor null male mice develop late-onset obesity caused by decreased energy expenditure and lipolytic activity but show normal insulin sensitivity with high adiponectin secretion.Diabetes.20055441000100815793238
- LinH.XuQ.YehS.WangR.SparksJD.Insulin and leptin resistance with hyperleptinemia in mice lacking androgen receptor.Diabetes.20055461717172515919793
- KeatingNL.O'MalleyAJ.FreedlandSJ.SmithMR.Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer.J Natl Cancer Inst.20101021394619996060
- Mauvais-JarvisF.Androgen-deprivation therapy and pancreatic -cell dysfunction in men.Diabetes Complications.2016303389390
- NavarroG.XuW.JacobsonDA.et alExtranuclear actions of the androgen receptor enhance glucose-stimulated insulin secretion in the male.Cell Metab.201623583785127133133
- ZitzmannM.GromollJ.von EckardsteinA.NieschlagE.The CAG repeat polymorphism in the androgen receptor gene modulates body fat mass and serum concentrations of leptin and insulin in men.Diabetologia.2003461313912637980
- FinkelsteinJS.LeeH.Burnett-BowieSA.et alGonadal steroids and body composition, strength, and sexual function in men.N Engl J Med.2013369111011102224024838
- YuIC.LinHY.LiuNC.et alNeuronal androgen receptor regulates insulin sensitivity via suppression of hypothalamic NF-B-mediated PTP1B expression.Diabetes.201362241142323139353
- HolmangA.BjorntorpP.The effects of testosterone on insulin sensitivity in male rats.Acta Physiol Scand.199214645055101492567
- Page-WilsonG.GoulartAC.RexrodeKM.Interrelation between sex hormones and plasma sex hormone-binding globulin and hemoglobin A1c in healthy postmenopausal women.Metab Syndr Relat Disord.20097324925419344226
- SahinS.ErogluM.SelcukS.et alIntrinsic factors rather than vitamin D deficiency are related to insulin resistance in lean women with polycystic ovary syndrome.Eur Rev Med Pharmacol Sci.2014182851285625339479
- SamS.Obesity and polycystic ovarian syndrome.Obes Manag.200732697320436797
- MoghettiP.TosiF.CastelloR.et alThe insulin resistance in women with hyperandrogenism is partially reversed by antiandrogen treatment: evidence that androgens impair insulin action in women.J Clin Endocrinol Metab.19968139529608772557
- DunaifA.SegalKR.FutterweitW.DobrjanskyA.Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome.Diabetes.1989389116511742670645
- EhrmannD.BarnesR.RosenfieldR.CavaghanMK.ImperialJ.Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome.Diabetes Care.199922114114610333916
- PoldermanKH.GoorenLJ.AsschemanH.BakkerA.HeineRJ.Induction of insulin resistance by androgens and estrogens.J Clin Endocrinol Metab.19947912652718027240
- NoharaK.LaqueA.AllardC.MunzbergH.Mauvais-JarvisF.Central mechanisms of adiposity in adult female mice with androgen excess.Obesity.20142261477148424639082
- EnrioriPJ.SinnayahP.SimondsSE.RudazCG.CowleyMA.Leptin action in the dorsomedial hypothalamus increases sympathetic tone to brown adipose tissue in spite of systemic leptin resistance.J Neurosci.20113134121891219721865462
- BalthasarN.CoppariR.McMinnJ.et alLeptin receptor signaling in POMC neurons is required for normal body weight homeostasis.Neuron.200442698399115207242
- FengY.ShaoR.WeijdegardB.et alEffects of androgen and leptin on behavioral and cellular responses in female rats.Horm Behav.201160442743821819988
- FodorM.Delemarre-van de WaalHA.Are POMC neurons targets for sex steroids in the arcuate nucleus of the rat?Neuroreport.200112183989399111742225
- NavarroG.Suhuan LiuP.De GendtK.VerhoevenG.Mauvais-JarvisF.Importance of the beta-cell androgen receptor in type 2 diabetes.Endocr Rev.201132OR23OR22
- MorfordJ.NavarroG.AllardC.Mauvais-JarvisF.Excess androgen receptor activation in beta-cells produces hyperinsulinemia, insulin resistance, and secondary beta-cell failure in female mice.Diabetes.201665suppl 1A482
- FagmanJB.WilhelmsonAS.MottaBM.et alThe androgen receptor confers protection against diet-induced atherosclerosis, obesity, and dyslipidemia in female mice.FASEB J.20152941540155025550469
- MooreAM.CampbellRE.The neuroendocrine genesis of polycystic ovary syndrome: a role for arcuate nucleus GABA neurons.J Steroid Biochem Mol Biol.201516010611726455490
- KongD.TongQ.YeC.et alGABAergic RIP-Cre neurons in the arcuate nucleus selectively regulate energy expenditure.Cell.2012151364565723101631
- HuoL.GamberK.GreeleyS.et alLeptin-dependent control of glucose balance and locomotor activity by POMC neurons.Cell Metab.20099653754719490908
- BerglundED.ViannaCR.DonatoJ.et alDirect leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice.J Clin Invest.201212231000100922326958
- HillJW.EliasCF.FukudaM.et alDirect insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility.Cell Metab.201011428629720374961
- HillJW.XuY.PreitnerF.et alPhosphatidyl inositol 3-kinase signaling in hypothalamic proopiomelanocortin neurons contributes to the regulation of glucose homeostasis.Endocrinology.2009150114874488219819947
- BerthoudHR.JeanrenaudB.Acute hyperinsulinemia and its reversal by vagotomy after lesions of the ventromedial hypothalamus in anesthetized rats.Endocrinology.19791051146151446404
- CoxJE.PowleyTL.Intragastric pair feeding fails to prevent VMH obesity or hyperinsulinemia.Am J Physiol.19812405E566E5726786107
- LuoS.LuoJ.Cincotta aH.Chronic ventromedial hypothalamic infusion of norepinephrine and serotonin promotes insulin resistance and glucose intolerance.Neuroendocrinology.199970646046510657739
- KimKW.DonatoJ.BerglundED.et alFOXO1 in the ventromedial hypothalamus regulates energy balance.J Clin invest.201212272578258922653058
- RamadoriG.FujikawaT.AndersonJ.et alSIRT1 deacetylase in SF1 neurons protects against metabolic imbalance.Cell Metab.201114330131221907137
- PerkinsMN.RothwellNJ.StockMJ.StoneTW.Activation of brown adipose tissue thermogenesis by the ventromedial hypothalamus.Nature.198128957964014027464907
- aretskaiaMV.ZaretskyDV.ShekharA.DiMiccoJA.Chemical stimulation of the dorsomedial hypothalamus evokes non-shivering thermogenesis in anesthetized rats.Brain Res.20029281-211312511844478
