Abstract
A variety of hormones have been shown to play a role in affective disorders. Reproductive steroids are particularly informative in our efforts to understand the pathophysiology of affective dysregulation for several reasons: i) Reproductive endocrine-related mood disorders (premenstrual dysphoric disorder, perinatal depression, perimenopausal depression) are wonderful clinical models for investigating the mechanisms by which affective state changes occur; ii) Reproductive steroids regulate virtually every system that has been implicated as disturbed in the ontogeny of affective disorders; iii) Despite the absence of a reproductive endocrinopathy a triggering role in the affective disturbance of reproductive mood disorders has been shown clearly for changes in reproductive steroids. The existing data, therefore, support a differential sensitivity to reproductive steroids in reproductive mood disorders such that an abnormal affective state is precipitated by normal changes in reproductive steroids. The therapeutic implications of these findings for affective illness are discussed.
Se ha demostrado que diversas hormonas desempeñan un papel en los trastornos afectivos. Los esteroides sexuales han aportado importante información en nuestros esfuerzos por comprender la fisiopatología de la desregulación afectiva por varias razones: 1) Los trastornos del estado de ánimo relacionados con el sistema endocrino reproductivo (trastorno disfórico premenstrual, depresión perinatal, depresión perimenopáusica) son excelentes modelos clínicos para investigar los mecanismos por los cuales se producen cambios en el estado afectivo, 2) Los esteroides sexuales regulan virtualmente todos los sistemas que se han involucrado en las alteraciones de la ontogenia de los trastornos afectivos y 3) A pesar de la ausencia de una endocrinopatía reproductiva, se ha demostrado claramente que los cambios en los esteroides sexuales tienen un papel desencadenante en la alteración afectiva de los trastornos del estado de ánimo reproductivo. Por lo tanto, los datos existentes apoyan una sensibilidad diferencial a los esteroides sexuales en los trastornos del estado de ánimo reproductivo, de manera que los cambios normales en los esteroides sexuales precipitan un estado afectivo anormal. Se discuten las consecuencias terapéuticas de estos hallazgos para la enfermedad afectiva.
Il a été prouvé que certaines hormones jouent un rôle dans les troubles de l'humeur. Les hormones sexuelles nous ont particulièrement instruits dans notre démarche pour comprendre la physiopathologie de la dysrégulation de l'humeur pour plusieurs raisons : 1) les troubles de l'humeur liés au système endocrinien sexuel (trouble dysphorique prémenstruel, dépression périnatale, dépression périménopausique) sont de parfaits modèles cliniques pour la recherche des mécanismes sous-jacents aux changements d'humeur ; 2) les stéroïdes sexuels régulent pratiquement tout système ayant été identifié comme perturbé dans l'ontogenèse des troubles de l'humeur ; 3) Même en l'absence de pathologie endocrinienne, les variations de taux de stéroïdes sexuels ont clairement démontré le rôle déclencheur des perturbations liées à la reproduction dans les troubles de l'humeur. Les données existantes sont donc en faveur d'une sensibilité différentielle aux stéroïdes sexuels dans les troubles de l'humeur liés à la reproduction telle qu'un état émotionnel anormal est déclenché par des variations normales de stéroïdes sexuels. Nous discuterons des implications thérapeutiques de ces résultats pour les troubles de l'humeur.
Introduction
The idea of using hormones to treat affective illness is by no means novel. In 1849, Berthold suggested that animal organs (testes) contained substances that could, upon exogenous administration (through transplant), dramatically alter physiology (including behavior).Citation1The field of organotherapy was subsequently born with the publication of a paper in The Lancet in 1889 by Claude Edouard Brown Sequard.Citation2This paper reported that the subcutaneous self-administration (by the author) of extracts of ground-up dog and guinea pig testes reversed many of the effects of aging and had a profoundly salutary effect on energy and mood (all of which was likely a substantial placebo response). Although most of the reports from this field over the next 30 years were subsequently refuted (as most, but not all, organ extracts are inactive when orally administered), the benefits associated with the administration of thyroid extractCitation3 and adrenal extractCitation4 for their respected deficiency syndromes (myxedema and Addison's disease) were substantiated and gave rise to the modern field of endocrinology.
Although the enthusiasm for organotherapy gradually disappeared in the early 20th century, the belief that one could treat behavioral disorders with “hormones” (a term coined in 1905)Citation5 was substantially bolstered following the isolation and characterization of estradiol in 1929. Werner (1934) performed a placebo-controlled trial of an estradiol preparation, “theelin,” in involutional melancholia and demonstrated theelin's superior efficacy.Citation6 During this period, many reproductive steroids were isolated and characterized including estrone, progesterone, and several androgens. Further, chemists identified modifications of the steroids that could alter their absorption (ie, acetylation) and potency (eg, addition of ethinyl group or removal of C-19 methyl groups), thus fueling a renewed interest in reproductive steroids as medicines. In the 1950s menopausal symptoms were treated with estrogen replacement therapy, and 1960 saw FDA approval of the first oral contraceptive (ie, Enovid). Hormone therapy “took off” (albeit with the trajectory of a rollercoaster rather than a rocket), and papers appeared describing the use of reproductive steroids in affective disorders related to reproduction: involutional melancholia, premenstrual syndrome, and postpartum depression. In this article, we will not comprehensively review hormonal therapy but rather will focus on reproductive steroids in women to attempt to answer the following questions: How do hormones work and what accounts for the variability in response to hormones? How do hormones affect the systems implicated in depression? How can new models of depression help us to understand the role of reproductive steroids in affective regulation? What is the evidence for a role of reproductive steroids in affective illness? What is the therapeutic role of reproductive steroids in the treatment of depression? The concepts that will be developed apply to men and women and can be summarized as follows: i) the effects of hormones on the brain are pleiotropic and highly context dependent; ii) the paucity of well-designed studies and methodologic shortcomings preclude certainty about the role of hormones in the treatment of affective disorders; iii) one can, nonetheless, define a role for hormones in reproductive endocrine-related mood disorders in women that help answer what is perhaps the critical question in psychiatry, namely why do people respond differently to ostensibly the same stimulus?
How do hormones work and what accounts for the variability in their actions?
The original meaning of hormone—a glandular secretion that is distributed by the bloodstream to distant sites—is, for our purposes, woefully inadequate. Hormones include small (peptides) and long proteins as well as steroids, which act at membrane and intracellular receptors as well as ion channels, act at time frames from seconds to hours, can be synthesized de novo in non-glandular tissues like the brain, and can be synthesized in neuronal terminals and released into synapses to effect post-synaptic cellular activation. To reduce the scope of our discussion to manageable dimensions, we will focus on one class of hormones, ovarian steroids. All steroid hormones (including non-ovarian steroids) are metabolized from cholesterol. Cholesterol is transported into the mitochondria by STAR (steroid acute regulatory hormone) and then metabolized to pregnenolone, which gives rise to all of the biologically active steroids through a series of enzymatic steps performed by a small group of enzymes with multiple actions along the steroid synthetic cascade. As such, the way in which a steroid is metabolized determines its action. Testosterone can be aromatized to estradiol, which then activates the estrogen signaling system, can be reduced to dihydrotestosterone, an androgen 2-10 fold more powerful than testosterone,Citation7 or can be metabolized to androsterone, a “neurosteroid” that can acutely regulate membrane neurotransmitter receptors. Classical steroid signaling involves intracytoplasmic receptors (although some principally reside in the nucleus), which are bound by the steroids after they diffuse through the cell membrane. The receptors are transcription factors, which recruit other proteins to form a complex that remodels chromatin (to make genes accessible for the messenger RNA (mRNA) transcribing enzyme, RNA polymerase) and recruits the transcription factors that help initiate transcription. Steroid receptors can also join with other transcription factors—a process called tethering—to initiate transcription of genes that do not have classical receptor binding sites—response elements—in the DNA. Through these combined mechanisms, steroids can regulate thousands of genes.Citation8 Additionally, steroid receptors exist at the membrane, from which they can initiate downstream signaling to activate enzymes, amplify the effect of activated receptors, or directly influence transcription.Citation9 Finally, steroids like estradiol regulate the activity of all three polymerases (ie, those generating mRNA, ribosomal RNA, and transfer RNA), thus allowing estradiol to prepare cells acutely for transcription and more chronically for translation (protein formation).Citation8
The variability of steroid actions is best conveyed by the array of protein partners with which they combine to achieve a biological outcome. For example, there are approximately 350 coregulatory proteins—both coactivators and coinhibitors—that bind to hormone bound steroid receptors to determine whether genes are turned on or off.Citation10 As described by Lonard and O'Malley,Citation11 these coregulators (TS) combine in groups, and the impact of each of the coregulators is determined by its chemical modification (eg, phosphorylation, methylation). With even only eight potential modifications of each coregulator and groups of six, there are 10Citation13 different potential functional outcomes of a hormone-bound receptor! Further, coregulators exist in a tissue-specific fashion, enabling estrogen-like compounds called SERMs (selective estrogen receptor modulators) to act like an estrogen agonist in some tissues and an antagonist in others, depending on the coregulator profile in the cells. Further contributing to the signaling variability, the effects of steroids are highly context dependent, with that context including prior exposure and genetic background.Citation12
How do hormones influence the systems implicated in depression?
Given the protean effects of steroids, it should not be surprising to learn that virtually every system implicated in depression is modulated by estradiol. The following are just three examples (see refs 13 and 14 for more complete discussion):
1. Neurotransmitter “deficiency.” The synthetic and metabolic enzymes and receptors for all classical neurotransmitters are regulated by estradiol. Estradiol also primes neurons to respond more efficiently to stimuli, in part through regulation of the brain's major excitatory neurotransmitter, glutamate. Through the two major estrogen receptors, ER α and β, estradiol can activate metabotropic (indirectly linked to ion channels) glutamate receptors even in the absence of glutamate and increase the synaptic trafficking of at least one type of ionotropic (ion channel containing) glutamate receptor, the AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptor.Citation15 Estradiol also directly regulates the activity of calcium and potassium channels, thus acutely (in seconds to minutes) regulating neuronal excitation, inhibition and neurosecretory coupling.Citation16,Citation17 If depression is a consequence of excitatory:inhibitory imbalance,Citation18 estradiol is in a prime position to modulate that disturbance.
2. Neuroplasticity deficiency. Estradiol promotes neuronal survival, decreases oxidative stress, improves neuronal energetics by increasing mitochondrial respiratory efficiency, increases both dendritic spine density and synaptic plasticity, and increases cell survival in response to a variety of toxic insults (eg, hypoxia, inflammation, excess glutamate, decreased glucose).Citation19,Citation20 Levels of brain-derived neurotrophic factor (BDNF), a critical growth factor deficient in depression, is increased by estradiol, just as it is by antidepressants.Citation19
3. Network abnormality. Neural networks are structurally and dynamically connected brain regions whose coordinated activity enables effective and efficient responses to the environment. Examples of neural networks include the following: a) The Default Mode Network, which mediates internal-based thought (eg, day dreaming, reflecting) and permits recall of the past and imagining of the future;Citation21 b) Social Cognition Network, which enables one to “read” the intentions of others (theory of mind);Citation22 c) Reward Network, which permits the assignment of affective valence (positive or negative) to events, thoughts, and experiences and is critical for decision-making; d) Affective Regulation Network, which regulates the interplay between more cognitive (eg, dorsolateral prefrontal cortex [dlPFC]) and more “affective” (eg, limbic, amygdala) brain regions;Citation23 e) Central Executive Network, critical for mediating executive functions like cognitive appraisal.Citation24,Citation25 Evidence exists for dysfunction of each of these networks (and others) in depression.Citation23,Citation25 Similarly, evidence exists for the modulation of these networks by estradiol. For example imaging studies performed in women whose reproductive state was manipulated by administering the gonadotropin-releasing hormone agonist leuprolide (which suppresses ovarian steroid secretion) alone or in combination with estradiol or progesterone demonstrated that estradiol, but not progesterone, activated key regions of the DMN (medial PFC and posterior cingulate; Wei et al, unpublished data): both estradiol and progesterone supported the top-down modulation of the hippocampus by the dlPFC (affective regulation network), coordinated/coupled activity that was absent during hormone suppression.Citation26 In a recent animal study, McHenry et alCitation27 described a hypothalamic reward circuit that was powerfully regulated by estradiol. Both estrus cycle and exogenous estradiol resulted in a dramatic, in vivo upregulation of self-administered optogenetic stimulation to genetically defined medial preoptic neurons, with the estradiol-induced reward behavior linked to activation of the ventral tegmental area and phasic dopamine release in the “reward center” (nucleus accumbens).
How can new models of depression help us to understand the role of reproductive steroids in affective regulation?
One can argue that depression represents an “adaptive failure;” ie, a failure of integration or orchestration of neural networks that subserve the functions observed to be disturbed in depression. As such, depression represents a dynamic or “software” problem, not a “hardware” problem. Just as there is no single gene abnormality in depression, there is likely no single brain region “lesion” in this disorder, with the “locus” of dysfunction dynamically shifting across time. In line with this view, depression is not a collection of particular symptoms so much as it is a dysregulation of affective state. What is a state? A state can be defined as a transient, coherent, replicable, integrated, self-organized assemblage of thoughts, associations, affects, memories, perceptions, interpretations, etc.Citation28 States are programs for interpreting and interacting with our environment in an efficient fashion. For example, the cognitive/affective/perceptual/behavioral state that might accompany a picnic on a beautiful day would clearly not be in evidence if you were suddenly attacked by a bear. The advantage of the state model is several-fold. First, it overlays nicely on the network model, permitting both the formation and changes in state to be described in terms of component network functions and dynamic interactions between networks. Second, it allows us to focus on the kinetics of affective state change rather than simply the particular set of symptoms. Depression, then, can be thought of as the failure to be able to change affective state, which otherwise should be both transient and susceptible to either our environment or our efforts to modify our state. Indeed, Bunney et al suggested 50 years ago that depression might best be understood by studying the characteristics of the switch rather than the characteristics of the depressed state.Citation29 Consistent with this suggestion, premenstrual dysphoric disorder (PMDD) should be seen not as a collection of specific symptoms but as an affective state change that is choreographed by the menstrual cycle. Third, the state model is testable; ie, if one hypothesizes that reproductive steroids are informational molecules that “by design” generate behavioral states, then one should be able to alter the appearance of distressing affective states in reproductive endocrine-related mood disorders by manipulating the reproductive context in which the disorders appear (as will be described below).
What is the evidence for a role of reproductive steroids in affective illness?
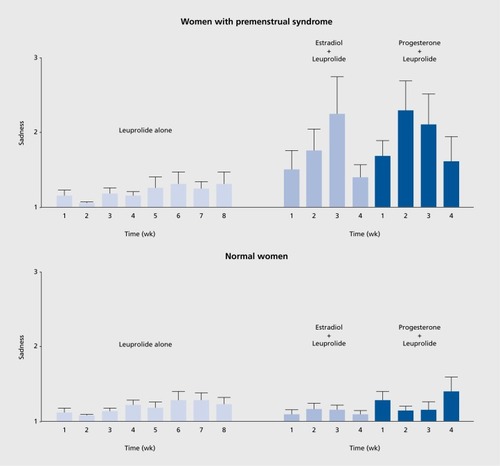
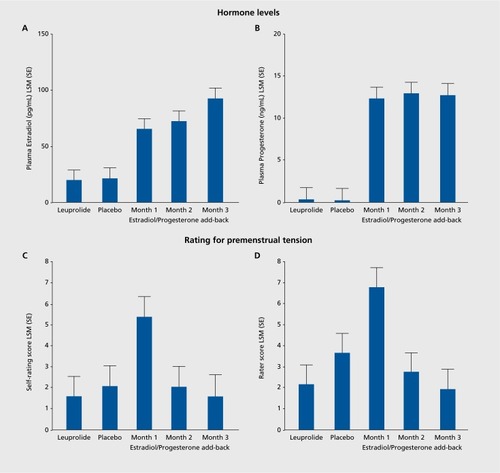
Conceptually, the most promising affective disorders in which to search for a role of reproductive steroids are those that are temporally linked to periods of reproductive endocrine change: perimenopausal depression (PMD), postpartum depression (PPD), and PMDD. None of these disorders is characterized by abnormal levels of reproductive (or any other) hormones, so clearly these are not endocrinopathies like hypothyroidism. Several studies suggest that PMD can be effectively treated with estradiol,Citation30,Citation31 but that says nothing about the role of estradiol in the precipitation of the affective state. Schmidt et al addressed this question by determining whether the blinded withdrawal of estrogen therapy in euthymic women with a history of PMD would precipitate a switch into a depressed state.Citation32 The blinded withdrawal of estradiol did indeed precipitate a dysphoric affective state within one week, before the appearance of withdrawal-related hot flushes. Notably, the same hormone manipulation procedure was completely without effect on affective state in women lacking a history of PMD. This study, therefore, suggested that withdrawal of estradiol would precipitate a depressed state, but only in those who were, for unclear reasons, susceptible (ie, those with a history of perimenopausal depression). A second endocrine manipulation paradigm created a scaled-down model of the puerperium in euthymic women with a history of PPD.Citation33 In this case, it was the addback of high-dose reproductive steroids that precipitated the switch into the depressed state. Once again, however, the identical hormone manipulation paradigm in women without a history of PPD produced no affective state change at all. (These findings have recently been replicated in a larger group (Schiller et al, manuscript in preparation.) Finally, in PMDD, the experimental and blinded elimination of the mid-late luteal phase of the menstrual cycle did not influence the appearance of the PMDD state, which emerged in the experimentally created follicular phase, thus uncoupling PMDD from the endocrine events of the mid-late luteal phase.Citation34 In a subsequent study, ovarian suppression did prevent the appearance of the PMDD state, which was then precipitated under blinded conditions by the addition (in the context of ovarian suppression) of estradiol or progesterone.Citation35 As the same hormone manipulation was without effect on women without a history of PMDD, the findings suggested that the reproductive steroids did precipitate the PMDD state, but only in those who were, again, differentially sensitive to the effects of the steroids. As one can observe in , it was impossible to determine whether the change in steroid levels following their reintroduction was the offending stimulus or whether the hormones simply played a permissive role in the expression of an infradian zeitgeber (which would be consistent with the attenuation of the steroid-precipitated affective state despite continued hormone administration). As recently described (), a hormone manipulation study involving blinded administration of 12 weeks of estradiol and progesterone administration in the context of ovarian suppression demonstrated that it was the change (ie, increase) in hormones that triggered the depressed state, which then gradually attenuated without subsequent appearance throughout the remainder of the 12 weeks.Citation36 Together, these findings are most consistent with a role of reproductive steroids in regulating the appearance of a dysfunctional affective state (in a susceptible group) rather than simply “making patients symptomatic.”
Although the source of the susceptibility is currently unknown, the hormone manipulation paradigm described above (ovarian suppression + hormone addback) was exploited to identify a group of women with PMDD in whom the critical variable of hormone sensitivity was confirmed, as was its absence in the comparison group. Lymphoblastoid cell cultures were then created and transcriptomics performed (by RNAseq in the first group and by qRT-PCR in the replication sample).Citation37 Among the gene “families” identified as differentially expressed in the pathway analysis, the ESC/E(Z) family was selected for further study for the following reasons: i) it has only 13 member genes; ii) it is regulated by estradiol and progesterone; iii) it has been implicated in affective disorders; and iv) it is a major mediator of epigenesis, the process by which the environment can regulate, in an enduring fashion, the expression of genes. Almost all of the 13 genes in this family were upregulated in the cells from the women with PMDD (in both samples), four significantly so. Further, and most interestingly, the response of four of these genes to exposure to estradiol or progesterone in culture for 24 hours differed in patients and controls.Citation37 These data provide the first demonstration of a cellular model for the differential sensitivity to reproductive steroids that characterizes women with reproductive endocrine-related mood disorders. In the implication of a major epigenetic regulatory system, they also provide a plausible model for how a normal (internal) environmental signal (ie, reproductive steroids)—one critical for long-term potentiation and synaptic rewiringCitation38—might acquire the capacity to trigger a switch into a state characterized by network dyscoordination.
What is the therapeutic role of reproductive steroids in the treatment of depression?
The unfortunate answer to this question, despite all that we have learned about the dramatic role of reproductive steroids in the regulation of affective state, is that we simply don't know. In a 1997 meta-analysis, Zweifel and O'BrienCitation39 described a reasonably impressive effect size of 0.68 for the effect of hormone replacement therapy (HRT—estrogen plus a progestin) on depression. This suggested that HRT might be a promising treatment for or prophylaxis against depression. In a recent systematic review of studies examining the effects of HRT or estrogen therapy (ET) on mood in menopausal women since the 1997 meta-analysis, there was a remarkable paucity of well-designed, well-controlled randomized clinical trials (RCTs).Citation40 Just some among the myriad methodological problems are as follows: i) menopausal state was not defined or represented a mixture of perimenopausal and postmenopausal women, despite the markedly different physiologic characteristics of these two groups; ii) antidepressant effects were inferred from a group that was not depressed at baseline; iii) depressive symptoms from a rating scale were not distinguished from depression, a syndromal diagnosis; iv) important covariates (eg, past history of depression, presence of hot flushes) were not examined or reported; and v) risk of bias was moderate to high in almost all studies. Of the hundreds of studies reviewed, only 24 met criteria, and only five RCTs clearly examined depressed women! Based on three RCTs, one of the conclusions reached was that estradiol may have antidepressant efficacy in perimenopausal but not postmenopausal depressed women. This conclusion mirrors the “critical window” hypothesis by which (primarily) beneficial effects are attributed to hormone therapy initiated proximate to, but not distal from, the end of ovarian activity. This hypothesis, which finds both support and explanations in the animal literatureCitation41,Citation42 was adduced by someCitation43 to explain the surprising results of the Women's Health Initiative (WHI) Study.Citation44 This massive study was intended to test the effects of HRT on coronary heart disease (CHD) (primary outcome) and breast cancer (primary adverse outcome). Contrary to the 50% decrease in CHD with HRT seen in many observational studies, the WHI found a significant, 29% increase in CHD. The expected increase in breast cancer (found in observational studies with more than 4 years of HRT) was also found, and the study was terminated early in 2002. This study was heralded as the end of HRT, and indeed subsequent research on the effects of estradiol in humans almost disappeared. Criticisms of the WHI methodology appeared almost immediately,Citation45 and among the legion concerns (eg, use of medroxyprogesterone acetate, which has many adverse physiologic effects; poor health of large numbers of participants), one of the most prominent was the observation that the mean age of subjects was 63.3 and only 15% of subjects were within 5 years of the menopause.Citation46 In other words, the critical window was ignored by the very design of the WHI. In subsequent reanalysis of the WHI data, the adverse cardiovascular effects were not observed in those between the ages of 50 and 59 or in those receiving only estradiol,Citation47,Citation48 thus confirming earlier objections to the WHI.
The lost opportunity to investigate the psychotropic efficacy of estradiol in the wake of the WHI has no doubt limited the extent and reliability of the conclusions that can be drawn regarding the use of estradiol in affective disorders. Suggested conclusions are the following: i) In perimenopausal (not post-menopausal) women who are depressed and have other perimenopausal symptoms (eg, hot flushes, vaginal dryness), it is reasonable to initiate estradiol treatment before starting an antidepressant (as perimenopausal symptoms are a clearly agreed upon indication for estrogen therapy); ii) Perimenopausal depressed women who refuse to take (or are intolerant of) psychotropics may be tried on estradiol; iii) There is little theoretical or practical justification for using estrogen to treat depressed women who are more than several years post menopause; iv) Any woman started on estradiol should have a gynecologic and breast exam; additionally one should familiarize oneself with potential contraindications (eg, multiple family members with a history of breast cancer, family history of premenopausal or bilateral breast cancer, past history of breast cancer or thromboembolic disease including stroke); v) the effects of hormone therapy will depend on the type (ie, estrogen with or without progestin), dose, route, formulation, and duration; estradiol, particularly transdermal, is recommended, as it is least likely to be associated with thromboembolic phenomena and best recapitulates the premenopausal profile of estrogen metabolites. vi) Although one could consider the use of estradiol as an adjunct in perimenopausal women unresponsive to antidepressant therapy, available data are particularly exiguous and do not permit conclusion regarding the efficacy of this approach. To end on a somewhat more optimistic note, Gordon et al recently reported a prospective study of the prophylactic effects of 100 ug/day of transdermal estradiol compared with placebo over 1 year in 172 euthymic, perimenopausal women.Citation49 The women receiving estradiol were significantly less likely to experience clinically significant depressive symptoms during the study (CES-D>16, OR=2.5), with the mood benefits particularly prominent in proportion to the number of significantly stressful life events in the 6 months prior to study initiation (ie, the greater the number of stressful life events, the greater the prophylactic benefit of estradiol). These findings suggest that with time and additional study, the proper place for reproductive steroids in the treatment of affective state dysregulation may well be better defined.
Conclusions and future directions
Undeniably, reproductive hormones can regulate mood, and their manipulation can, in specific instances, dramatically alter the course and expression of affective illness. Nonetheless, these hormonal effects are not universal and instead appear in subgroups of individuals who are differentially sensitive to the impact of reproductive steroids on the central nervous system. Going forward, it will be critical to identify the mediators of this differential sensitivity, facilitating the prediction of those who would respond to reproductive therapies and, of equal importance, those who might be at risk for adverse effects. Additionally, the very complexity of steroid actions that precludes simple inference about potential psychotropic mechanisms of action offers tremendous opportunity for “designer psychopharmacology,” whether through specific hormone preparations or receptor agonists/antagonists, steroidogenic enzyme inhibitors, or selective hormone receptor modulators (capitalizing on tissue-specific coregulators). Current uncertainties notwithstanding, the future for reproductive hormone therapy is deservedly bright.
The author has no conflict of interest to disclose.
REFERENCES
- ForbesTR.A. A. Berthold and the first endocrine experiment: Some speculations as to its origin.Bull Hist Med.194923263267
- BorellM.Brown-Séquard's organotherapy and its appearance in America at the end of the nineteenth century.Bull Hist Med.1976503309320791406
- MurrayGR.Note on the treatment of myxoedema by hypodermic injections of an extract of the thyroid gland of a sheep.BMJ. doi:10 1136/bmj. 2. 1606.796.189121606796797
- OliverG.SchäferEA.The physiological effects of extracts of the suprarenal capsules.J Physiol. doi:10 1113/jphysiol.1895. sp000564.1895183230276
- StarlingEH.The Croonian lectures on the chemical correlation of the functions of the body.Lancet.19051664275339341
- WernerAA.JohnsGA.HoctorEF.AultCC.KohlerLH.WeisMW.Involutional melancholia: probably etiology and treatment.JAMA.193410311316
- WrightAS.ThomasLN.DouglasRC.LazierCB.RittmasterRS.Relative potency of testosterone and dihydrotestosterone in preventing atrophy and apoptosis in the prostate of the castrated rat.J Clin Invest. doi:10 1172/JCI119074.1996981125582563
- HahN.KrausWL.Hormone-regulated transcriptomes: lessons learned from estrogen signaling pathways in breast cancer cells.Mol Cell Endocrinol. doi:10.1016/j.mce.2013.06.021.20143821652664
- SrivastavaDP.WoolfreyKM.PenzesP.Insights into rapid modulation of neuroplasticity by brain estrogens.Pharmacol Rev Pharmacol Rev. doi:10.1124/pr.111.005272.20136513181350
- LonardDM.KumarR.O'MalleyBW.Minireview: The SRC family of coactivators: an entrée to understanding a subset of polygenic diseases?Mol Endocrinol. doi:10.1210/me.2009-0276.2010242279285
- LonardDM.O'MalleyBW.Nuclear receptor coregulators: judges, juries, and executioners of cellular regulation.Mol Cell. doi:10.1016/j.molcel.2007.08.0122007275691700
- WardellSE.NelsonER.McDonnellDP.From empirical to mechanismbased discovery of clinically useful Selective Estrogen Receptor Modulators (SERMs).Steroids. doi:10.1016/j.steroids.2014.07.0132014903038
- RubinowDR.SchmidtPJ.Meltzer-BrodyS.HarshVL.Gonadal hormones and behavior in women: concentrations versus context. In: Pfaff DW, Arnold AP, Fahrbach SE, Etgen AM, Rubin RT, eds. Hormones, Brain and Behavior. San Diego: Academic Press; doi 10.1016/B978-008088783-8.00076-02010.
- RubinowDR.GirdlerSS.Hormones, heart disease, and health: Individualized medicine versus throwing the baby out with the bathwater.Depress Anxiety. doi:10.1002/da.208332011284282296
- SrivastavaDP.WatersEM.MermelsteinPG.KramarEA.ShorsTJ.LiuF.Rapid estrogen signaling in the brain: implications for the fine-tuning of neuronal circuitry.J Neurosci. doi:10.1523/JNEUROSCI.4097-11.2011201131451605616063
- ZupSL.MaddenAMK.Gonadal hormone modulation of intracellular calcium as a mechanism of neuroprotection.Front Neuroendocrinol. doi:10.1016/j.yfrne.2016.02.0032016424052
- ZhangL.SukharevaM.BarkerJL.et al.Direct binding of estradiol enhances Slack (sequence like a calcium-activated potassium channel) channels' activity.Neuroscience. doi:10.1016/j.neuroscience. 2004.10.04220051312275282
- Foss-FeigJH.AdkinsonBD.JiJL.et al.Searching for cross-diagnostic convergence: neural mechanisms governing excitation and inhibition balance in schizophrenia and autism spectrum disorders.Biol Psychiatry. doi:10.1016/j.biopsych.2017.03.005.20178110848861
- BrintonRD.YaoJ.YinF.MackWJ.CadenasE.Perimenopause as a neurological transition state.Nat Rev Endocrinol. doi:10.1038/nrendo.2015.82.2015117393405
- SchillerCE.JohnsonSL.AbateAC.SchmidtPJ.RubinowDR.Reproductive steroid regulation of mood and behavior.Compr Physiol. doi:10.1002/cphy.c150014.20166311351160
- RaichleME.SnyderAZ.A default mode of brain function: A brief history of an evolving idea.Neuroimage. doi:10.1016/j.neuroimage.2007.02.041.200737410831090
- OlssonA.OchsnerKN.The role of social cognition in emotion.Trends Cogn Sci. doi:10.1016/j.tics.2007.11.010.20081226571
- KorgaonkarMS.FornitoA.WilliamsLM.GrieveSM.Abnormal structural networks characterize major depressive disorder: A connectome analysis.Biol Psychiatry. doi:10.1016/j.biopsych.2014.02.018.2014767567574
- ChenAC.OathesDJ.ChangC.et al.Causal interactions between fronto-parietal central executive and default-mode networks in humans.Proc Natl Acad Sci U S A.2013110491994419949. doi:10.1073/pnas.131 1772110.24248372
- ShelineYI.PriceJL.YanZ.MintunMA.Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus.Proc Natl Acad Sci U S A. doi:10.1073/pnas.1000446107.2010107241102011025
- BermanKF.SchmidtPJ.RubinowDR.et al.Modulation of cognition-specific cortical activity by gonadal steroids: A positron-emission tomography study in women.Proc Natl Acad Sci U S A. doi:10.1073/pnas.94.16.88361997941688368841
- McHenryJA.OtisJM.RossiMA.et al.Hormonal gain control of a medial preoptic area social reward circuit.Nat Neurosci. doi:10.1038/nn.44872017203449458
- PutnamFW.The Way We Are: How States of Mind influence Our Identities, Personality and Potential for Change. International Psychoanalytic Books.2016
- BunneyWE.DennisL.BorgeGF.The “switch process” in manic-depressive illness - Part 1.Arch Gen Psychiatry.19722732953025051618
- SchmidtPJ.NiemanL.DanaceauMA.et al.Estrogen replacement in perimenopause-related depression: A preliminary report.Am J Obstet Gynecol.2000183241442010942479
- SoaresCN.AlmeidaOP.JoffeH.CohenLS.Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: A double-blind, randomized, placebo-controlled trial.Arch Gen Psychiatry.200158652953411386980
- SchmidtPJ.Ben DorR.MartinezPE.et al.Effects of estradiol withdrawal on mood in women with past perimenopausal depression: A randomized clinical trial.JAMA Psychiatry. doi:10.1001/jamapsychiatry.2015.0111.2015727
- BlochM.SchmidtPJ.DanaceauM.MurphyJ.NiemanL.RubinowDR.Effects of gonadal steroids in women with a history of postpartum depression.Am J Psychiatry. doi:10.1176/appi.ajp.157.6.924.20001576924930
- SchmidtPJ.GroverGN.MullerKL.RubinowDR.NiemanLK.MerriamGR.Lack of effect of induced menses on symptoms in women with premenstrual syndrome.N Engl J Med. doi:10.1056/NEJM199104253241705.19913241711741179
- SchmidtPJ.NiemanLK.DanaceauMA.AdamsLF.RubinowDR.Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome.N Engl J Med. doi:10.1056/NEJM199801223380401.19983384209216
- SchmidtPJ.MartinezPE.NiemanLK.et al.Premenstrual dysphoric disorder symptoms following ovarian suppression: Triggered by change in ovarian steroid levels but not continuous stable levels.Am J Psychiatry. doi:10.1176/appi.ajp.2017.16101113.201717410980989
- DubeyN.HoffmanJF.SchuebelK.et al.The ESC/E(Z) complex, an effector of response to ovarian steroids, manifests an intrinsic difference in cells from women with premenstrual dysphoric disorder.Mol Psychiatry. doi:10.1038/mp.2016.229.201722811721184
- SellersK.RavalP.SrivastavaDP.Molecular signature of rapid estrogen regulation of synaptic connectivity and cognition.Front Neuroendocrinol. doi:10.1016/j.yfme.2014.08.001.2015367289
- ZweifelJE.O'BrienWH.A meta-analysis of the effect of hormone replacement therapy upon depressed mood.Psychoneuroendocrinology.19972231892129203229
- RubinowDR.JohnsonSL.SchmidtPJ.GirdlerSS.GaynesBN.Efficacy of estradiol in perimenopausal depression: so much promise and so few answers.Depress Anxiety. doi:10.1002/da.22391.2015328539549
- SuzukiS.BrownCM.Dela CruzCD.YangE.BridwellDA.WisePM.Timing of estrogen therapy after ovariectomy dictates the efficacy of its neuroprotective and antiinflammatory actions.Proc Natl Acad Sci U S A. doi:10.1073/pnas.0610394104.20071041460136018
- ZhangQG.HanD.WangRM.DongY.YangF.VadlamudiRK.BrannDW.C terminus of Hsc70-interacting protein (CHIP)-mediated degradation of hippocampal estrogen receptor-alpha and the critical period hypothesis of estrogen neuroprotection.Proc Natl Acad Sci U S A. doi: 10.1073/pnas.1104391108.201110835E617E624
- HarmanSM.NaftolinF.BrintonEA.JudelsonDR.Is the estrogen controversy over? Deconstructing the Women's Health Initiative study: A critical evaluation of the evidence.Ann N Y Acad Sci. doi:10.1196/annals.1347.004.200510524356
- Writing Group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial.JAMA. doi:10.1001/jama.288.3.321.20022883321333
- NaftolinF.TaylorHS.KarasR.et al.The Women's Health Initiative could not have detected cardioprotective effects of starting hormone therapy during the menopausal transition.Fertil Steril. doi:10.1016/j.fertnstert.2004.02.095.200481614981501
- HaysJ.OckeneJK.BrunnerRL.et al.Effects of estrogen plus progestin on health-related quality of life.N Engl J Med. doi:10.1056/NEJMoa030311.20033481918391854
- Women's Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial.JAMA. doi:10.1001/jama.291.14.1701.20132911417011712
- RossouwJE.PrenticeRL.MansonJE.et al.Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause.JAMA.2007297131465147817405972
- GordonJL.RubinowDR.Eisenlohr-MoulTA.XiaK.SchmidtPJ.GirdlerSS.Efficacy of transdermal estradiol and micronized progesterone in the prevention of depressive symptoms in the menopause transition: A randomized clinical trial.JAMA Psychiatry. doi:10.1001/jamapsychiatry.2017.3998.2018.752
