Abstract
Psychiatric disorders are a heterogeneous group of mental illnesses associated with a high social and economic burden on patients and society. The complex symptomatology of these disorders, coupled with our limited understanding of the structural and functional abnormalities affecting the brains of neuropsychiatric patients, has made it difficult to develop effective medical treatment strategies. With the advent of reprogramming technologies and recent developments in induced pluripotent stem (iPS) cell-based protocols for differentiation into defined neuronal cultures and 3-dimensional cerebral organoids, a new era of preclinical disease modeling has begun which could revolutionize drug discovery in psychiatry. This review provides an overview of iPS cell-based disease models in psychiatry and how these models contribute to our understanding of pharmacological drug action. We also propose a refined iPSC-based drug discovery pipeline, ranging from cell-based stratification of patients through improved screening and validation steps to more precise psychopharmacology.
Mettre la traduction ES
Mettre la traduction FR
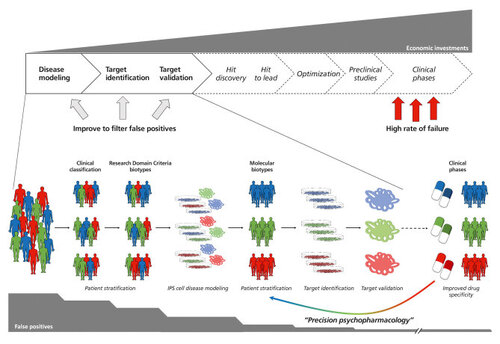
An improved approach for drug discovery including cellular iPS cell-based 2D models and cerebral organoids. The implementation of iPS cell-derived 2D and 3D culture systems could improve several aspects of the drug discovery pipeline in psychopharmacology. Based on prestratified patient cohorts (either by clinical manifestation or by research domain criteria), iPS cell-based disease models should help to further stratify patients according to molecular alterations referred to as “molecular biotypes.“ Starting from those molecular biotypes, iPS cell-based target identification using 2-dimensional cultures followed by target validation applying cerebral organoids should minimize false positives processed via the drug discovery pipeline. Finally, the improved drug specificity could feed back into the advanced patient stratification eventually leading to better medical treatment specific to the molecular biotypes.
Introduction
Psychiatric diseases are devastating disorders of complex and diverse etiology. The highly polygenic disease architecture and the variable genetic, epigenetic, and environmental factors that contribute to the manifestation of mental diseases have made it challenging to understand their development and pathophysiology. Citation1 Genome-wide association studies of large cohorts of individuals with neuropsychiatric disorders have led to a wealth of data on the genetics of these diseases. The proportion of patients in whom candidate causal or contributing genetic variants are identified is, however, still very limited. Even though medication is available to relieve symptoms and disruptive behaviors, there are often no effective pharmacological treatments to improve the core deficits. New drug development has stalled due to the lack of well-defined molecular targets, our restricted understanding of the origin and the biological mechanism of psychiatric disorders, and the limitations of current research models to investigate biological processes within the human brain under healthy and pathologic conditions. Citation2 Thus far, animal models such as mice have been used to provide behavioral and molecular readouts, which allowed specification of the role of specific pathways in psychopathology. However, animal models have several limitations: they cannot fully reproduce the plethora of psychiatric symptoms observed in patients, nor can they recapitulate the complex structure and function of human brains. Citation3 Most human post-mortem specimens represent a late stage or end point of the disease and thus cannot provide information about how these disorders develop. Moreover, the pharmacological treatment and the comorbidities of psychiatric disorders represent confounding factors that make it difficult to obtain a clear and uniform readout from post-mortem studies. Citation4 Lastly, biopsies of brain tissue are an invasive approach associated with several ethical concerns, which—considering the low yield and the impossibility of expanding post-mitotic neurons in vitro—do not represent a powerful option.
In this context, the advent of reprogramming technology and the potential to differentiate induced pluripotent stem cells (iPS cells) into almost any desired cellular subtype has revolutionized modern biomedicine and provides a new platform to study human pathologies in vitro. Citation5 Merging iPS cell technology with human genetics offers the great opportunity to generate unique biological information about the molecular mechanisms involved in the pathophysiology of mental illnesses and to explore new drug targets. As reprogrammed cells carry the genetic background of a patient, they represent a valid platform for identifying genetic predictors of drug responses and associating cellular abnormalities with clinical phenotypes in a human context. In recent years, iPS cell-based models derived from psychiatric patients have recapitulated the key molecular features of psychiatric disorders such as schizophrenia (SCZ), autism spectrum disorders (ASDs), and bipolar (BP) disorders Citation2 , Citation6 and have led to the first psychopharmacological screens. Citation7 - Citation9 However, classical iPS cell models based on homogeneous cellular populations grown in the culture dish neglect the fact that the human brain is an integrated 3-dimensional (3D) tissue structure composed of multiple cell types and extracellular matrix. In this context, the dimensional complexity of in vitro models has been increased with the generation of cerebral organoids derived from iPS cells. These organoids allow us for the first time to model the 3D structure, organization, composition, and connectivity of the human brain Citation10 and thus open up the possibility of studying complex human brain development and pathology outside the human body. Cerebral organoids resemble the early developing human brain also with respect to gene expression programs Citation11 ; they exhibit human specific cellular diversity, 3D organization, histological layers, and migration patterns (reviewed in ref 12). Today, several protocols to generate standardized and even region-specific brain organoids are available representing for instance the dorsal and/or ventral telencephalon, the midbrain, the hippocampus, or the cerebellum. Citation13 - Citation17 With the advances in cell culture techniques, human cerebral organoids have become an integral part of cell-based disease modeling of the brain (reviewed in ref 11) and it can be assumed that this pivotal role will even increase in the future. In this review we will summarize the most significant findings obtained with iPS cell-based models of psychopathologies and will discuss how 2D and 3D models will potentially revolutionize psychopharmacology; both form an experimental model point of view as well as in the translational drug development pipeline.
IPS cell-based psychopharmacology
During the last decade, multiple psychiatric diseases have been successfully translated to iPS cell-based in- vitro modeling, investigating alterations associated with these disorders at a cellular or network level. Initially, most researchers concentrated on those few known and well-described copy number variations or mutations with high penetrance. In more recent years an increasing number of studies have started to investigate sporadic, complex, and polygenic cases of mental disease (for an overview of published iPS cell-based models of mental disorders, see Table I ). IPS cell-based neuropharmacology still remains in its infancy, with most neuropharmacological interventions being based on standard clinical pharmacological intervention. Still, interesting observations occur from those studies linking, for instance, clinical drug response to molecular alterations in cellular models. With the increasing number of measurable and quantifiable phenotypes described in cellular disease models the way will be paved for more unbiased pharmacological approaches which may eventually lead to the identification of new classes of psychopharmacological compounds and drugs. In the following paragraphs, we will concentrate on those iPS cell-based models for psychiatric disease in which pharmacological interventions were applied. For a more complete overview of iPS cell-based models of psychiatric disorders we refer to the excellent and comprehensive reviews published on this topic. Citation2 , Citation3 , Citation10
Schizophrenia
With a prevalence of almost 1% of the population, Citation18 schizophrenia (SCZ) is one of the most frequent major psychoses. It is a highly polygenic psychiatric disorder characterized by a complex and variegated symptomatology. The molecular and cellular defects that contribute to disease initiation and/or progression are, however, still largely unknown. The first detailed characterization of iPS cell-derived neurons from schizophrenic patients was largely based on two families with strong familial history indicating an underlying genetic alteration with high penetrance. Indeed, genetic characterization of these patients revealed that one family harbored multiple copy number variations (CNVs) in schizophrenia-associated genes such as a deletion in the NRG3 gene. This led to strong reduction of NRG3 expression which was, very interestingly, also found in the other family even though without a clear genetic link. NRG3 is involved in multiple processes during brain development and synaptic function. Neuronal cultures derived from these patients exhibited some striking defects in the maturity- and activity-independent synaptic transmission of a neurotropic virus, an epiphenomenon which indicates defects in synaptic assembly. Citation8 Interestingly, the researchers observed that the antipsychotic drug loxapine was able to alleviate the defects in virus spreading which was associated with an increase in NRG1 expression. Other antipsychotics such as clozapine, olanzapine, risperidone, and thiorizadine had no effect on the observed phenotype indicating a heterogenous and complex action of loxapine besides dopamine (D1, D2, D4) receptor antagonism. The authors also identified alterations in Wnt signaling which was further addressed in a subsequent study using neural precursors generated from the same iPS cell lines. This study also identified an increased susceptibility of the cells to redox challenges as well as a defect in progenitor cell migration. In contrast to the synaptic virus spreading phenotype, loxapine treatment did not result in any improvement of the observed alterations. Citation19 A more recent study focused on STEP61, a brain-specific phosphatase involved in the regulation of synaptic function, which was upregulated in iPS cell-derived telencephalic excitatory neurons from two SCZ patient cohorts. STEP61 overactivation induces an increased internalization of NMDAR receptors, thus altering the inhibitory/excitatory balance at synaptic level. Of note, the antipsychotics loxapine and clozapine both normalized the activity of the phosphatase. Citation20 Considering the impairment of the GABAergic system in the etiology of SCZ, in another study, authors derived a homogeneous culture of cortical interneurons from 14 patients in treatment with clozapine. SCZ interneurons showed decreased levels of different protocadherins, a result in line with studies in animal models and post mortem specimens. The dysregulated protocadherin-pathway resulted in an impairment in dendritic arborization that was corrected by phosphokinase C inhibitor treatment. Citation21
Autism
ASDs are neurodevelopmental disorders with a complex genetic background and characterized by a plethora of symptoms related to sociability and intellectual disabilities. Citation22 Considering the polygenic origin of ASD, increasing scientific attention has recently been focused on iPS cells. Indeed, this approach allows investigation of the complex in vitro dynamics and sheds light on the mechanisms underpinning the different manifestation of ASD. With respect to neuropharmacological intervention, Marchetto and collaborators found an increased cellular proliferation associated with the increased activity of the β-catenin/BRN2 cascade in neural progenitors obtained from a cohort of idiopathic autistic patients with clinical signs of early-age macrencephaly. Neurons derived thereof showed signs of abnormal neurogenesis and synaptogenesis. The neuronal phenotype could be normalized by the treatment with insulin-like growth factor 1 (IGF-1), a drug in clinical trials for ASD. Citation23 The same drug was also beneficial in an iPS cell model harboring a disruption of the cation channel TRPC6 . In this model, impaired neuronal development, morphology, and function were observed, which was partially rescued with IGF-1 and hyperforin, the major active component of St John’s wort. Citation24
Next to the idiopathic variants, several genetically defined syndromes exist, which clinically present with similarities to the idiopathic ADS. These include Rett syndrome (alterations in the MECP2 or CDKL5 gene), Williams-Beuren syndrome (deletion on chromosome 7q11.23), Fragile X syndrome (expansion of a CGG triplet repeat in the FRM1 gene) or Timothy syndrome (mutations in the CACNA1C gene encoding the calcium channel Cav1.2 α subunit). The neuronal phenotypes of Rett syndrome have been characterized in iPS cells derived from patients harboring mutations in the MECP2 and CDKL5 genes. Indeed, neurons derived from Rett patients exhibited a decreased number of excitatory synapses, a reduction in spine densities, a smaller soma size, and electrophysiological defects, Citation7 phenotypes also replicated in subsequent studies. Citation25 , Citation26 Increasing MECP2 by gentamycin, a drug facilitating ribosomal read-through of premature stop codons in mutant MECP2 as well as IGF-1 were able to increase the number of glutamatergic synapses in this model. Citation7
Interestingly, MECP2 mutant astrocytes were also shown to actively contribute to the morphological and functional deficits of wild-type neurons, indicating a non-cell autonomous mode of action in the disease pathogenesis. Again, IGF1 or a peptide containing the first three amino acids of IGF1 were able to partially rescue the observed alterations in that model. Citation27 Mechanistically, pharmacological IGF1 treatment was demonstrated to be linked to an increased expression of the neuron-specific K + -Cl − cotransporter2 (KCC2) , a downstream target of MECP2 and dysregulated in Rett syndrome-specific iPS cell-derived neurons. As a consequence, the developmental shift in function of the neurotransmitter GABA from excitation to inhibition which was delayed in Rett neurons was rescued towards the time course found in control iPS cell-derived neurons. Citation28 In Timothy syndrome iPS cell-derived neurons, Pasca and coworkers found that defects in calcium signaling and activity-dependent gene expression. They also identified abnormal expression of tyrosine hydroxylase, a phenotype which could be reverted by the atypical L-type channel blocker roscovitine. Citation29 With respect to Fragile X syndrome, a straightforward approach is to identify drugs reactivating the expression of the otherwise silenced FMR1 gene. Using iPS cell-derived neurons, Bar Nur and colleagues investigated selected Food and Drug Administration (FDA)-approved drugs including the histone deacetylase inhibitor trichostatin-A (TSA) and the demethylating agents 5-azacytidine (5-azaC). They found that 5-azaC, but not TSA, upregulated FRM1 expression, even though concentrations used were beyond physiological levels. Citation30
Mood disorders
Mood disorders are characterized by recurrent fluctuations in mood state that can lead to a dramatic reduction of the quality of life. Unipolar or major depression (MD) is the most common mood disorder and has been predicted to become the first cause of disability by 2030. Citation31 While several factors contribute to the onset of MD, the serotonergic system seems to play a crucial role in the underpinning molecular alteration. Consequently, the majority of the drugs prescribed to depressed patients modulate the serotonergic system by increasing the synaptic availability of the monoamine or by interfering with the activity of the serotonergic receptors. Citalopram, a selective serotonin reuptake inhibitor (SSRI) has been applied to human iPS cell-derived serotoninergic neurons and serotoninergic neurons generated by direct cell conversion. All studies demonstrated elevated extracellular serotonin levels following SSRI treatment of the neurons. Citation32 - Citation34 Mood swings from mania to depression are characteristic for bipolar disorder (BPD), another disease from the mood disorder spectrum. BPD affects more than 1% of the general population, and is thus among the leading causes of disability in young adults. Citation35 Lithium is currently the best-characterized drug for the treatment of BPD, even though not all patients respond to lithium administration. In line with this, lithium was shown to be effective in rescuing some of the defects identified in iPS cell-derived cultures from BPD patients including calcium transients, Citation36 adhesion, Citation37 progenitor proliferation, Citation38 excitability, Citation39 , Citation40 or altered ratios of active/inactive modulators of dendritic spine formation. Citation41 Interestingly, clinical response of patients to lithium strongly correlated to the in vitro response with respect to the “hyperexcitability phenotype” observed in iPS cell models allowing predictions in both directions—from the patient to the in-vitro model and vice versa. Citation39 , Citation40
Cerebral organoids: 3D models of psychiatric disease
Considering that many of the identified cellular phenotypes associated with psychiatric disorders are connected to defects in progenitor proliferation, cellular migration, or neuronal morphology and synapse formation, cerebral organoids are attractive models to investigate the extent to which these alterations result in architectonical or structural impairments in 3D. In recent years, cerebral organoids have successfully been applied to studying ASD and SCZ-related pathologic phenotypes (for an overview see Table I ). In the first reported study from 2015, the authors investigated the structure and the transcriptomic profile of telencephalic organoids derived from probands with idiopathic ASD, compared with unaffected first-degree family members. They found an imbalance in the production of excitatory and inhibitory neurons caused by an accelerated cell cycle in the GABAergic neuronal lineage. Citation42 Another study related to ASD showed that cerebral organoids generated from iPS cells heterozygous for the chromodomain helicase DNA-binding protein 8 (CHD8) gene have altered transcriptomic signatures mainly related to neurogenesis, neuronal differentiation, forebrain development, Wnt/β-catenin signaling, and axonal guidance. Citation43 The authors found a marked upregulation of TCF4 , a candidate gene potentially involved in other psychiatric disorders such as SCZ and BP. More recently, cortical organoids were derived from a selected cohort of ASD patients with macrocephaly. The organoids showed increased thickness of the cortical plate and aberrant complex neurite outgrowth of newborn neurons, a phenotype in line with the increased brain size of the patients. The authors claimed that the abnormal growth acceleration in ASD organoids is ascribable to the alteration of specific gene modules and the consequent altered trajectory during the early phases of cortical development. This elegant approach confirmed the involvement of neural precursors in ASD-related structural alterations. Citation44 Defects in neurogenesis and neuronal differentiation were also observed in cerebral organoids derived from patients harboring mutations in the MECP2 . Specifically, organoids showed structural abnormalities in ventricular area and radial thickness, together with increased cellular proliferation and decreased neuronal maturation. This study pointed out the role played by two specific miRNAs (miR-199 and miR-214) in the altered phenotype, suggesting a new molecular mechanism downstream of MECP2 mutations. Citation45 A study focusing on mutations of DISC-1 (disrupted-in-schizophrenia 1) and its interaction with Ndel1, a protein involved in cell cycle control, showed that the DISC-1 /Ndel1 interaction is fundamental for the correct regulation of mitosis in radial glial cells both in iPS cell mutant for DISC-1 and SCZ patient-derived telencephalic organoids. Citation17 Together, these are exciting examples demonstrating the power of the organoid technology and pave the way for future studies implementing psychopharmacology.
From candidate pharmacology to drug discovery
Even though most studies so far concentrated on few candidate drugs, the feasibility of translating iPS cell models to cellular drug screening has been demonstrated. Kauffmann and coworkers used fragile X iPS cell-derived neural precursors to screen for the reactivation of the silenced FMR1 testing a total of 50 000 compounds. They identified a small set of compounds showing a significant increase in FMR1 expression. Citation46 Another study set up a sensitive fluorescence resonance energy transfer-based assay for the determination of FMR1 levels, testing more than 5000 compounds, among those about 4000 FDA-approved drugs. Six drugs showed an increase in FMR1 expression, even though none of the tested drugs induced FMR1 to clinically relevant levels. Citation47 A more recent publication demonstrates how iPS cells could be used to predict drug response in case of psychiatric disorders. Comparing transcriptional profiles of cancer cell lines, neural progenitors from healthy controls and neural progenitor cell lines from patients with SCZ exposed to a set of 135 drugs, the authors identified several drugs reversing post-mortem SCZ-associated transcriptomic signatures in a cell-type and disease-specific manner. Citation48 Such examples nicely demonstrate how to incorporate patient-derived material with idiosyncratic genetic compositions into larger, OMICS-based screening approaches and thus may help to combine genetics, cell-based molecular profiling, and pharmacology. Citation49 It is important to mention that with modern gene editing approaches, iPS cells are also amenable to large-scale genetic manipulation including iCRISPR—a platform which allows rapid, multiplexable, and inducible genome editing in human PSC raising the possibility to introduce multiple modifications at different loci simultaneously in the same cell. Citation50 For the cerebral organoid cultures, several further optimization steps are required to broadly and effectively include this system in psychopharmacology and drug discovery. One important issue is the question of scalability. Unlike 2D cultures, cerebral organoids still represent heterogeneous cultures with considerable variation within and across batches. The development of improved culture conditions including defined extracellular matrices might facilitate the generation of more reproducible organoid systems (reviewed in ref 51) The issue of scalability is also relevant for the phenotypic analysis of organoids. Due to their complex and heterogeneous 3D nature, time-consuming processing procedures such as sectioning and staining of single slides are still standard. Advanced clearing protocols might help to develop standardized high-throughput imaging analysis protocols and single-cell profiling might help bypass issues with respect to cellular heterogeneity. For functional profiling, organoids with mature neuronal circuits of consistent quality have to be developed. At some point, increasing the complexity by including non-neuroectodermal populations such as microglial cells Citation52 or a vasculature Citation53 , Citation54 might be necessary to model the complex interaction of different cell populations in the response to pharmacological intervention.
Reconstructing the translational drug discovery pipeline in psychopharmacology
Central nervous system (CNS) drug development is considered risky, especially because so many promising CNS drugs failed in late-stage clinical trials, after significant investments had been made. Citation55 Since 1975, only 33 drugs have been registered for psychiatry, Citation56 and the pharmaceutical industry is constantly cutting down research into psychiatric drugs, Citation57 , Citation58 implying that the drug development pipeline for psychiatric drugs is currently running dry. In the classical pipeline, molecular targets for drug discovery are mostly defined on transformed cell lines and/or transgenic mice harboring or overexpressing one of the few CNVs or mutations with high penetrance. Such models insufficiently reflect the majority of psychiatric patients and the specific situation in the human brain leading to a plethora of false-positive results which are processed via the entire drug discovery pipeline before eventually proven ineffective in clinical trials. One reason for the ineffectiveness of drug discovery in psychiatry might be due to the classification of psychiatric spectrum disorders according to clinical symptoms ignoring the recent developments towards a stratification and classification of patients according to specific domain criteria (also called Research Domain Criteria [RDoC]). Citation59 Molecular studies and functional assessment of psychiatric patients strongly suggest breaking with conventional clinical classifications and stratifying patients into specific research domain criteria-defined “biotypes.” Citation60 Considering the high costs of failures in the late stages of drug discovery, it will be important to dramatically reduce the false-positive results in early stages of the pipeline. In this context and as outlined above, iPS cell-based models will develop towards a pivotal role in the patient stratification and classification processes as well as target identification and validation, eventually leading to a more effective drug discovery pipeline. The refined drug discovery pipeline including iPS cell-based 2D models and cerebral organoids is outlined in Figure 1 . In such a scenario, the classification of patients into heterogeneous collectives by clinical manifestation is refined by applying research domain criteria from unbiased clinical testing (such as MRI and functional studies). IPS cell-based models (2D and organoids) investigating molecular and functional signatures on a cellular or network level should lead to a further refinement into “molecular target biotypes.” Performing high content screens directly in iPS cell-derived neural cultures from such biotype-stratified individuals should minimize artefacts due to molecular heterogeneity and an inadequate cellular physiology, and should at the same time provide information about toxicology of a certain compound to predict the insurgence of side effects in authentic human brain cells. Secondary screens based on complex cellular models such as cerebral organoids should further reduce the number of false-positive hits processed into the subsequent steps of drug discovery. Thus, in such a refined pipeline, iPS cell-based systems will streamline drug development by biotype stratification, target identification and validation eventually leading to precise “mechanistic” pharmacological intervention and accordingly to the development of the so-called “precision psychiatry.” Citation4
Conclusion
IPS cell-based in vitro models are promising tools to study previously inaccessible aspects of human brain development and neuropsychiatric diseases. It is, however, important to note that iPSC-derived brain models have their limitations with respect to reproducing the in vivo situation of the human brain. In this context major restrictions are the lack of maturity and limitations in the cellular composition (these models do not contain blood vesicles or immune cells). We should also consider that each iPSC clone represents one epigenetic variant of the given genetic background and thus results need to be reproduced across several clones and individuals. Protocol improvements that enable greater maturation and cellular diversity combined with advanced readout techniques including high-throughput single cell OMICs and whole-organ imaging will enable to investigate previously experimentally inaccessible processes disturbed in neuropsychiatric disease and will by that be of great use for the translational drug discovery pipeline.
00000The authors declare no financial conflicts of interest. The work was supported by the ERA-NET NEURON, JTC 2015 Neurodevelopmental Disorders, STEM-MCD (to JL), by the German Federal Ministry of Education and Research (BMBF) funded project EndoProtect (grant 01GQ1423B to PK). The authors also acknowledge the generous financial support by the Hector Stiftung II.
REFERENCES
- GandalMJ,LeppaV,WonH,ParikshakNN,GeschwindDH.The road to precision psychiatry: translating geanetics into disease mechanisms.Nat Neurosci.201619111397140727786179
- SolimanMA,AboharbF,ZeltnerN,StuderL.Pluripotent stem cells in neuropsychiatric disorders.Mol Psychiatry.20172291241124928322279
- FalkA,HeineVM,HarwoodAJ,et al.Modeling psychiatric disorders: from genomic findings to cellular phenotypes.Mol Psychiatry. 2016219132127324182
- HoffmanGE,SchrodeN,FlahertyE,Brennand KJ.New considerations for hiPSC-based models of neuropsychiatric disorders.Mol Psychiatry.2019241496629483625
- ShiY,InoueH,WuJC,YamanakaS.Induced pluripotent stem cell technology: a decade of progress.Nat Rev Drug Discov.201716211513027980341
- QuadratoG,BrownJ,ArlottaP.The promises and challenges of human brain organoids as models of neuropsychiatric disease.Nat Med.201622111220122827783065
- MarchettoMC,CarromeuC,AcabA,et al.A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells.Cell.2010143452753921074045
- BrennandKJ,SimoneA,JouJ,et al.Modelling schizophrenia using human induced pluripotent stem cells.Nature.2011473734622122521490598
- WangP,LinM,PedrosaE,et al.CRISPR/Cas9-mediated heterozygous knockout of the autism gene CHD8 and characterization of its transcriptional networks in neurodevelopment.Mol Autism.201565526491539
- ArdhanareeswaranK,MarianiJ,CoppolaG,AbyzovA,VaccarinoFM.Human induced pluripotent stem cells for modelling neurodevelopmental disorders.Nat Rev Neurol.201713526527828418023
- AminND,PaşcaSP.Building models of brain disorders with three-dimensional organoids.Neuron.2018100238940530359604
- Di LulloE,KriegsteinAR.The use of brain organoids to investigate neural development and disease.Nat Rev Neurosci.2017181057358428878372
- MugurumaK,NishiyamaA,KawakamiH,HashimotoK,SasaiY.Self-organization of polarized cerebellar tissue in 3D culture of human pluripotent stem cells.Cell Rep.201510453755025640179
- IefremovaV,ManikakisG,KrefftO,et al.An organoid-based model of cortical development identifies non-cell-autonomous defects in wnt signaling contributing to Miller-Dieker syndrome.Cell Rep.2017191505928380362
- JoJ,XiaoY,SunAX,et al.Midbrain-like organoids from human pluripotent stem cells contain functional dopaminergic and neuromelanin-producing neurons.Cell Stem Cell.201619224825727476966
- QianX,JacobF,SongMM,NguyenHN,SongH,MingGL.Generation of human brain region-specific organoids using a miniaturized spinning bioreactor.Nat Protoc.201813356558029470464
- YeF,KangE,YuC,et al.DISC1 Regulates neurogenesis via modulating kinetochore attachment of Ndel1/Nde1 during mitosis.Neuron.201796510411054.e104529103808
- OwenMJ,SawaA,MortensenPB.Schizophrenia.Lancet.201638810039869726777917
- BrennandK,SavasJN,KimY,et al.Phenotypic differences in hiPSC NPCs derived from patients with schizophrenia.Mol Psychiatry.201520336136824686136
- XuJ,HartleyBJ,KurupP,et al.Inhibition of STEP.Mol Psychiatry.201823227128127752082
- ShaoZ,NohH,Bin KimW,et al.Dysregulated protocadherin-pathway activity as an intrinsic defect in induced pluripotent stem cell-derived cortical interneurons from subjects with schizophrenia.Nat Neurosci.201922222924230664768
- IlievaM,Fex SvenningsenÅ,ThorsenM,MichelTM.Psychiatry in a dish: stem cells and brain organoids modeling autism spectrum disorders.Biol Psychiatry.201883755856829295738
- MarchettoMC,BelinsonH,TianY,et al.Altered proliferation and networks in neural cells derived from idiopathic autistic individuals.Mol Psychiatry.201722682083527378147
- Griesi-OliveiraK,AcabA,GuptaAR,et al.Modeling non-syndromic autism and the impact of TRPC6 disruption in human neurons.Mol Psychiatry.201520111350136525385366
- RicciardiS,UngaroF,HambrockM,et al.CDKL5 ensures excitatory synapse stability by reinforcing NGL-1-PSD95 interaction in the postsynaptic compartment and is impaired in patient iPSC-derived neurons.Nat Cell Biol.201214991192322922712
- DjuricU,CheungAYL,ZhangW,et al.MECP2e1 isoform mutation affects the form and function of neurons derived from Rett syndrome patient iPS cells.Neurobiol Dis.201576374525644311
- WilliamsEC,ZhongX,MohamedA,et al.Mutant astrocytes differentiated from Rett syndrome patients-specific iPSCs have adverse effects on wild-type neurons.Hum Mol Genet.201423112968298024419315
- TangX,KimJ,ZhouL,et al.KCC2 rescues functional deficits in human neurons derived from patients with Rett syndrome.Proc Natl Acad Sci U S A.2016113375175626733678
- PaşcaSP,PortmannT,VoineaguI,et al.Using iPSC-derived neurons to uncover cellular phenotypes associated with Timothy syndrome.Nat Med.201117121657166222120178
- Bar-NurO,CaspiI,BenvenistyN.Molecular analysis of FMR1 reactivation in fragile-X induced pluripotent stem cells and their neuronal derivatives.J Mol Cell Biol.20124318018322430918
- MalhiGS,MannJJ.Depression.Lancet. 2018392101612299231230396512
- VadodariaKC,MertensJ,PaquolaA,et al.Generation of functional human serotonergic neurons from fibroblasts.Mol Psychiatry.2016211496126503761
- XuZ,JiangH,ZhongP,YanZ,ChenS,FengJ.Direct conversion of human fibroblasts to induced serotonergic neurons.Mol Psychiatry.2016211627026216300
- LuJ,ZhongX,LiuH,et al.Generation of serotonin neurons from human pluripotent stem cells.Nat Biotechnol.2016341899426655496
- GrandeI,BerkM,BirmaherB,VietaE.Bipolar disorder.Lancet.2016387100271561157226388529
- ChenHM,DeLongCJ,BameM,et al.Transcripts involved in calcium signaling and telencephalic neuronal fate are altered in induced pluripotent stem cells from bipolar disorder patients.Transl Psychiatry.20144e37525116795
- WangJL,ShamahSM,SunAX,WaldmanID,HaggartySJ,PerlisRH.Label-free, live optical imaging of reprogrammed bipolar disorder patient-derived cells reveals a functional correlate of lithium responsiveness.Transl Psychiatry. 20144e42825158003
- MadisonJM,ZhouF,NigamA,et al.Characterization of bipolar disorder patient-specific induced pluripotent stem cells from a family reveals neurodevelopmental and mRNA expression abnormalities.Mol Psychiatry.201520670371725733313
- MertensJ,WangQW,KimY,et al.Differential responses to lithium in hyperexcitable neurons from patients with bipolar disorder.Nature.20155277576959926524527
- SternS,SantosR,MarchettoMC,et al.Neurons derived from patients with bipolar disorder divide into intrinsically different sub-populations of neurons, predicting the patients‘ responsiveness to lithium.Mol Psychiatry.20182361453146528242870
- TobeBTD,CrainAM,WinquistAM,et al.Probing the lithium-response pathway in hiPSCs implicates the phosphoregulatory set-point for a cytoskeletal modulator in bipolar pathogenesis.Proc Natl Acad Sci U S A.201711422E4462E447128500272
- MarianiJ,CoppolaG,ZhangP,et al.FOXG1-dependent dysregulation of GABA/glutamate neuron differentiation in autism spectrum disorders.Cell.2015162237539026186191
- WangP,MokhtariR,PedrosaE,et al.CRISPR/Cas9-mediated heterozygous knockout of the autism gene CHD8 and characterization of its transcriptional networks in cerebral organoids derived from iPS cells.Mol Autism.201781128321286
- SchaferST,PaquolaACM,SternS,et al.Pathological priming causes developmental gene network heterochronicity in autistic subject-derived neurons.Nat Neurosci.201922224325530617258
- MelliosN,FeldmanDA,SheridanSD,et al.MeCP2-regulated miRNAs control early human neurogenesis through differential effects on ERK and AKT signaling.Mol Psychiatry.20182341051106528439102
- KaufmannM,SchuffenhauerA,FruhI,et al.High-throughput screening using iPSC-derived neuronal progenitors to identify compounds counteracting epigenetic gene silencing in Fragile X Syndrome.J Biomol Screen.20152091101111126024946
- KumariD,SwaroopM,SouthallN,HuangW,ZhengW,UsdinK.High-throughput screening to identify compounds that increase Fragile X mental retardation protein expression in neural stem cells differentiated from Fragile X syndrome patient-derived induced pluripotent stem cells.Stem Cells Transl Med.20154780080825999519
- ReadheadB,HartleyBJ,EastwoodBJ,et al.Expression-based drug screening of neural progenitor cells from individuals with schizophrenia.Nat Commun.201891441230356048
- ZhangJ,LiH,TrounsonA,WuJC,NioiP.Combining hiPSCs and human genetics: major applications in drug development.Cell Stem Cell.201721216116528777942
- GonzálezF,ZhuZ,ShiZD,et al.An iCRISPR platform for rapid, multiplexable, and inducible genome editing in human pluripotent stem cells.Cell Stem Cell.201415221522624931489
- YinX,MeadBE,SafaeeH,LangerR,Karp JM,LevyO.Engineering stem cell organoids.Cell Stem Cell.2016181253826748754
- AbudEM,RamirezRN,MartinezES,et al.iPSC-Derived human microglia-like cells to study neurological diseases.Neuron.201794227829328426964
- MansourAA,GonçalvesJT,BloydCW,et al.An in vivo model of functional and vascularized human brain organoids.Nat Biotechnol.201836543244129658944
- PhamMT,PollockKM,RoseMD,et al.Generation of human vascularized brain organoids.Neuroreport.201829758859329570159
- HymanSE.Back to basics: luring industry back into neuroscience.Nat Neurosci.201619111383138427786185
- van der DoefTF,Zaragoza DomingoS,JacobsGE,et al.New approaches in psychiatric drug development.Eur Neuropsychopharmacol.201828998399330056086
- AbbottA.Novartis to shut brain research facility.Nature.2011480737616116222158218
- HymanSE.Revolution stalled.Sci Transl Med.20124155155cm111
- InselT,CuthbertB,GarveyM,et al.Research domain criteria (RDoC): toward a new classification framework for research on mental disorders.Am J Psychiatry.2010167774875120595427
- GandalMJ,HaneyJR,ParikshakNN,et al.Shared molecular neuropathology across major psychiatric disorders parallels polygenic overlap.Science.2018359637669369729439242
