Abstract
Introduction
This case series aims to study the effectiveness of Renasys-GO™ negative pressure wound therapy system in the healing of diabetic lower limb ulcers.
Materials and methods
An electronic vacuum pump (Renasys-GO™, Smith & Nephew GmbH) was used to apply negative pressure wound therapy on wounds, with pressure settings determined according to clinical indication. Changes in wound dimension, infection status and duration of treatment were recorded over the course of Renasys-GO™ therapy in 10 patients with diabetic lower limb ulcers.
Results
Healing was achieved in all wounds, three by secondary closure and seven by split-thickness skin grafting. Eight wounds showed a reduction in wound size. The average duration of treatment with Renasys-GO™ therapy was 15.9 days, and all wounds showed sufficient granulation and were cleared of bacterial infection at the end of therapy.
Conclusions
Renasys-GO™ therapy may be beneficial in the treatment of diabetic lower limb ulcers and wounds. In this study, which included wounds presenting as post-surgery ray amputation, metatarsal excision wounds, post-debridement abscesses and ulcers, the Renasys-GO™ therapy prepared all wounds for closure via split-thickness skin grafting or secondary healing by promoting granulation tissue and reducing bacterial infection in approximately 2 weeks.
Singapore has one of the highest incidences of diabetes mellitus (DM) among developed countries. In 2010, 11.3% of those aged 18–69 years were living with the disease (Citation1) It has been reported that approximately 15% of patients with DM will develop a foot ulcer at least once in their lifetime, with 85% of amputations in diabetic patients preceded by foot ulceration (Citation2).
Diabetic foot ulcers are often chronic and difficult to heal due to a range of diabetes-related pathogenic abnormalities, such as ischemia, defects in angiogenesis and impaired immune response. Developing effective strategies for management of diabetic foot ulcers is essential to improving quality of life and reducing the rates of lower limb amputation in diabetic patients.
One such strategy is negative pressure wound therapy (NPWT). In the last two decades, NPWT has become an accepted treatment choice for a variety of complex wounds including open abdominal wounds, pressure ulcers and diabetic foot ulcers (Citation3, Citation4). NPWT has been shown to facilitate wound closure in diabetic foot ulcers by promoting tissue granulation formation, increasing angiogenesis and perfusion and reducing infectious agents (Citation5, Citation6). For chronic leg ulcers, NPWT has also been shown to be effective in reducing wound healing time and wound bed preparation time as compared to conventional dressings (Citation7). A recent systematic review by Xie et al. have reported sufficient evidence to confirm the efficacy of NPWT in accelerating healing specifically in chronic diabetic leg wounds (Citation8).
A range of NPWT systems is available on the market, with the V.A.C.™ system (KCI Inc., San Antonio, TX, USA) being one of the earliest available and most widely used clinically. A relatively new option for NPWT delivery is the Renasys-GO™ system (Smith & Nephew GmbH). Like V.A.C™, Renasys GO™ is a polyurethane foam-based NPWT system. As Renasys-GO™ is relatively new, the number of studies on its effectiveness for various types of chronic wounds are limited. It has been suggested that Renasys-GO™ achieves comparable effects as the V.A.C.™ system for both chronic and acute wounds (Citation9). This prospective case series aims to evaluate the effectiveness of Renasys-GO™ in the treatment of diabetic lower limb wounds. We hypothesized that Renasys-GO™ therapy is beneficial in the treatment of diabetic lower limb ulcers and wounds.
Materials and methods
A prospective case series of 10 patients was conducted by the National University Hospital, Diabetic Foot Team, in Singapore. These patients were seen from January 2013 to March 2013. The study population was made up of six males and four females with a mean age of 54 (range: 37–66 years).
Indications
In this study, the indications for usage of Renasys GO™ included ray (toe and metatarsal) amputation wounds, post-drainage wounds of abscess and post-debridement wounds for necrotizing fasciitis and ulcers of the heel, dorsum of foot and of the sole. Four ulcers were ray amputation wounds, two were wounds post-drainage of abscess, one was on the dorsum of the foot, one was on the left shin, one was on the left foot and one was on the right calf. Each ulcer was classified as either a Grade 2 ulcer or a Grade 3 ulcer, according to Wagner's Classification (Citation10).
Study protocol
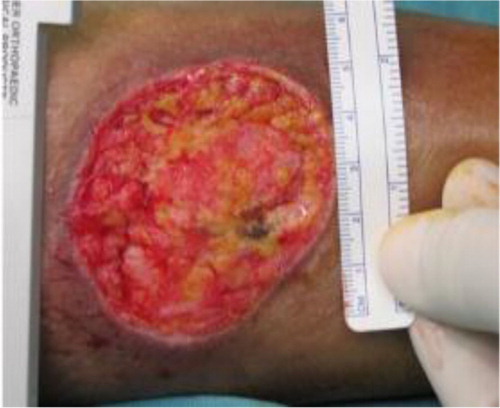
Documentation in the study protocol included the patient's demographics, diabetic history, presence of complications and infection markers. All wounds were monitored and photographed as they progressed and wound dimensions were measured with a ruler (). The location and diagnosis of the ulcer was recorded as well as the presence of any granulation tissue. Clinical investigations performed prior to therapy included markers of infection (leukocyte count, C-reactive protein and erythrocyte sedimentation rate). A swab from the wound was sent for culture and sensitivity tests before application of the NPWT system.
The NPWT settings, mode of application, date of initiation and end of NPWT, number and date of NPWT dressing changes were recorded. The number of formal repeated surgical debridements was also recorded. All patients were followed up until the completion of wound healing.
Application techniques
Prior to Renasys-GO™ NPWT treatment, adequate surgical debridement was performed on all wounds to remove any necrotic and infected tissue. After debridement, the wound was thoroughly cleaned and irrigated by jet lavage.
The NPWT dressing was cut to fit the size and shape of the wound and the number of pieces used recorded to ensure all pieces were removed at each dressing change. The wounds were sealed using semi-permeable film drape and a hole was cut in the center of the film. The soft port was placed directly over the hole in the film and its tubing was connected to the canister tubing. The pressure level and mode of the device was then set by the clinician.
For all patients, the NPWT device was set at continuous or mode with negative pressure of 100 or 120 mmHg. The pressure settings were dependent on the amount of exudate from the wound and patient's skin sensitivity since pressures may need to be lower to reduce pain on the skin.
NPWT dressing changes were performed every 48–72 hours by a trained medical officer or nurse. The number of NPWT dressing changes ranged from two to nine dressings, while the duration of NPWT treatment was 7–26 days.
Results
summarizes the characteristics of the study cohort. The age of patients in this study ranged from 37 to 66 (median: 54). There were six males and four females. Five patients were Malays, three Indians, and two Chinese. Complications of DM seen in the study patients included peripheral neuropathy, vasculopathy, nephropathy and diabetic retinopathy. Comorbidities which affected the study group included hypertension, hyperlipidemia and peripheral vascular disease.
Table 1 Patient characteristics
shows the Renasys-GO™ NPWT settings for the patients. A pressure of 100 mmHg was applied to four of the wounds, while a pressure of 120 mmHg was applied to the remaining six wounds. The frequency of NPWT dressing changes was every 48–72 hours. Wounds were administered NPWT for an average of 15.9 days (range 7–26) while an average of 5.2 NPWT dressings (range 2–9) was used per patient in the study group. The average length of NPWT treatment was 15.9 days. illustrates the changes in wound size and final outcome of wounds for all patients. Overall, there was a reduction in wound size for most of the patients. Eight out of 10 patients showed a reduction in wound size. The remaining two patients demonstrated an increase in size of the wound due to repeated debridements. Seven of the 10 wounds healed by a split-thickness skin graft (STSG) and three wounds healed by secondary closure.
Table 2 Treatment details and machine settings
Table 3 Treatment outcomes
The average size of the wounds at the beginning of the study was 39.95 cm2 (range: 5.00–156.00 cm2), and by the end of the study, the average wound size had reduced to 37.339 cm2 (range: 6.00–149.80 cm2). The mean percentage reduction in wound size was 22.4% (range: 2.8–55%).
Case study 1
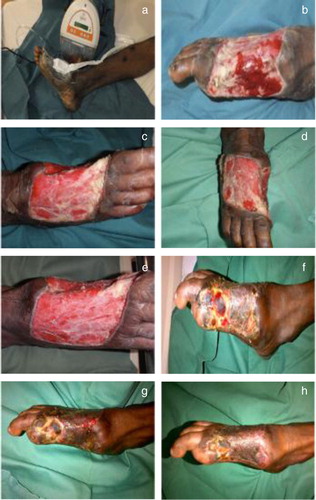
A 42-year-old Indian male presented with a dorsal foot ulcer Wagner Grade 3 and exposed tendons. Risk factors associated with healing consisted of an 8-year history of type 2 DM and hyperlipidemia. The patient had previously undergone three surgical wound debridements and ray amputation of the big toe in January 2013 in a different facility where he was offered a below-the-knee amputation. Patient had refused and presented to our facility for further care.
Patient's dorsalis pedis and posterior tibial pulses were palpable, with ankle brachial index and toe brachial index readings of 1.23 and 0.87, respectively and a capillary refill timing of <2 seconds. The patient's Semmes-Weinstein monofilament test was 5/10 with a 24 V reading on the neurothesiometer. Markers of infection were as follows: white blood cell (WBC) count of 12×109/L, erythrocyte sedimentation rate (ESR) of 141 mm/hr and C-reactive protein (CRP) of 138 mg/L. The markers of wound healing included 14.5% for hemoglobin (Hb) A1C, 11.6 g/dL for Hb and 20 g/L for albumin. Renal function indicated a level of 54 µmol/L for creatinine and 3.7 mmol/L for blood urea nitrogen. A microbiology test for culture and sensitivity indicated that the following microorganisms were present: Proteus mirabilis, Enterococcus faecalis and Coagulase-negative Staphylococcus aureus.
The patient was administered intravenous piperacillin/tazobactam (Tazocin) and surgical wound debridements were performed. This was followed by the application of Renasys-GO™ NPWT dressing with bedside debridements post-operation between dressing changes. By the 17th post-operative day, a microbiology culture test was performed without any growth of microorganisms found. A STSG was carried out and intravenous antibiotic therapy was discontinued after 2 weeks. The STSG wound became slightly infected in the fourth week. This was treated with regular dressings and completely healed in the eighth week and gained his ambulatory status ().
Case study 2
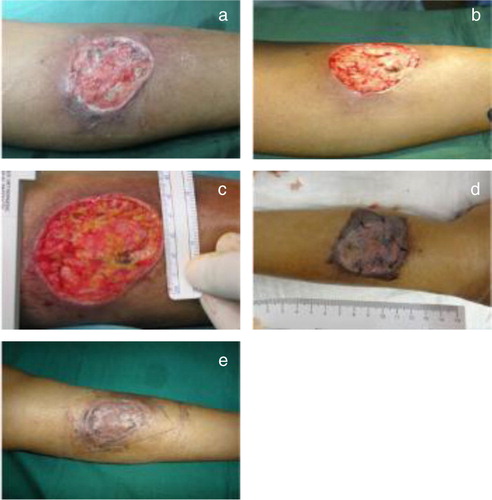
A 65-year-old Malay female presented with an ulcer on the left shin. Risk factors associated with healing consisted of a history of more than 10 years of type 2 DM, hyperlipidemia and hypertension.
Patient's dorsalis pedis and posterior tibial pulses were found to be palpable. Markers of infection were as such: WBC 11.5×109/L, ESR 53 mm/hr and CRP 42 mg/L. The markers of healing were >16.0% for HbA1C, 12.0 g/dL for Hb and 35 g/L for albumin. Renal function indicated a level of 105 µmol/L for creatinine and 6.6 mmol/L for blood urea nitrogen. Klebsiella pneumonia was identified to be present in the patient's wound.
Hydrosurgical debridement of the infected ulcer was carried out before application of the Renasys-GO™ NPWT dressing. A final debridement along with a STSG was then performed, followed by the NPWT application. The wound was inspected 5 days after the STSG and healed completely after 2 months. The wound area was decreased by 31.00% ().
Discussion
The effectiveness of NPWT for diabetic foot wounds is well supported by previous research (Citation5–Citation8). Renasys GO™ is a relatively new NPWT system that Rahmanian-Schwarz et al. has shown to achieve comparable results for both chronic and acute wounds (Citation9). The present study is the first one in Singapore to explore the effectiveness of this newer system for diabetic lower limb ulcers.
Wound size reduction
Compared to conventional dressings, NPWT provides a controlled wound environment that is separate from the external surroundings. This enables wound healing to occur under clean conditions, with moisture level controlled by altering pressure settings. Hence, the healing of chronic wounds such as diabetic foot ulcers is enhanced by the usage of NPWT dressings. This was demonstrated by Eginton et al., showing a 49% compared to 7.7% reduction in wound depth and 59% compared to 0.1% reduction in wound volume when diabetic foot ulcers were treated with VAC therapy as compared to moist gauze dressings (Citation11). Our study showed an average reduction in wound area of 22.4%, with eight out of 10 wounds having a decreased wound area after treatment.
Promotion of granulation tissue
Another effect of NPWT is the stimulation of tissue granulation in the wound bed. A freshly granulating wound bed indicates that the wound has entered the proliferative stage of wound healing and permits either secondary closure or the usage of other wound closure techniques. Morykwas et al. showed that wounds treated with NPWT dressing, either continuous or intermittent, granulated better than those treated using conventional dressing (Citation12). In this study, the time required to achieve wound bed preparation for surgical intervention was taken as the time from initiation of Renasys-GO™ therapy to the achievement of a continuous and fresh bed of granulation in the wound. All wounds were able to achieve adequate granulation tissue before closure by secondary healing (three out of 10 patients) or by a successful STSG (seven out of 10 patients).
Reduction of bacterial infection
The Renasys-GO™ therapy was observed to be beneficial in reducing bacterial infection in the wounds studied. This potential benefit of NPWT was suggested in a swine model study by Morykwas et al. (Citation12). A significant reduction in the bacterial load of inflicted chronic wounds was achieved by the fifth day for those treated with NPWT, but required an additional 6 days to reach the same reduced level in wounds untreated with NPWT. In our study, culture and sensitivity tests found colonization of all 10 wounds at the start of NPWT (). However, after NPWT treatment, all 10 wounds showed clearance of bacterial infection, allowing surgical intervention to be undertaken or secondary closure to occur.
Length of time
In this study, the average duration required to complete the NPWT was 15.9 days, with a range from 7 to 26 days. This is significantly shorter than the average time reported by Armstrong et al. (32.9 days) and Clare et al. (57.4 days) (Citation13, Citation14). However, in Singapore, the cost of Renasys-GO™ therapy is relatively high and adds on to patients’ hospitalization costs. As such, to make this NPWT more affordable for our patients, STSG was performed once the wound bed preparation was achieved with sufficient granulation tissue and wound cultures being negative for bacterial growth. It is also interesting to note that the average length of time taken for our NPWT treatment (15.9 days) was shorter than the average length of time taken for other similar conventional treatments (23.3 days) (Citation15).
Pressure levels
Birke-Sorenson et al. along with the International Expert Panel on Negative Pressure Wound Therapy have recommended a range of NPWT settings between 50 and 150 mmHg (Citation16). Lower negative pressures may be considered for pain reduction, and higher negative pressures may be considered for high volume of wound exudates (Citation16). In our study, NPWT settings were between 100 mmHg (four patients) to 120 mmHg (six patients). Lower negative pressures (100 mmHg) were used for pain reduction and higher negative pressures (120 mmHg) were used for a high volume of wound exudates.
Study limitations
Limitations of this study includes a low patient number, short term follow-up and lack of comparison of the Renasys-GO™ therapy versus other forms of NPWT or conventional dressings. With the positive results seen in our case series, we recommend future studies such as a prospective randomized controlled clinical trial comparing the efficacy of Renasys-GO™ therapy versus other treatment modalities.
Conclusion
In this study, the Renasys-GO™ NPWT system has been shown as beneficial in the treatment of diabetic lower limb ulcers and wounds, which may include wounds with exposed tendons, fascia or bone after surgical debridement. In addition, this NPWT system was able to prepare all wounds for closure via STSG or secondary healing by promoting sufficient granulation tissue and by reducing bacterial infection of the wounds in a reasonable amount of time (average of 15.9 days).
Conflict of interest and funding
The authors declare that they have no conflict of interest and have not received any funding or benefits from industry to conduct this study.
References
- Khalik S. Health alert: one in 3 will develop diabetes. The Straits Times 2012: Section B: 1.
- Reiber GE, Boyko EJ, Smith DG, Harris MI, Cowie CC, Stern MP, Boyko EJ, Reiber GE, Bennett PH. Lower extremity foot ulcers and amputations in diabetes. 1995; Washington, DC: U.S. Government Printing Office. 409–28. Diabetes in America. 2nd ed.
- European Wound Management Association (EWMA). Position document: topical negative pressure in wound management. 2007; London: MEP Ltd. Available from: http://www.woundsinternational.com/pdf/content_46.pdf [cited 21 April 2014]..
- National Institute for Health and Care Excellence (NICE). Negative pressure wound therapy for the open abdomen: guidance. 2013; London: National Institute for Health and Care Excellence. Available from: http://guidance.nice.org.uk/IPG467/Guidance/pdf/English [cited 21 April 2014]..
- Eginton MT, Brown KR, Seabrook GR, Towne JB, Cambria RA. A prospective randomized evaluation of negative-pressure wound dressings for diabetic foot wounds. Ann Vasc Surg. 2003; 17: 645–49.
- Blume PA, Walters J, Payne W, Ayala J, Lantis J. Comparison of negative pressure wound therapy using vacuum-assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers: a multicenter randomized controlled trial. Diabetes Care. 2008; 31: 631–6.
- Vuerstaek JD, Vainas T, Wuite J, Nelemans P, Neumann MH, Veraart JC. State-of-the-art treatment of chronic leg ulcers: a randomized controlled trial comparing vacuum-assisted closure (V.A.C.) with modern wound dressings. J Vasc Surg. 2006; 44: 1029–38.
- Xie X, McGregor M, Dendukuri N. The clinical effectiveness of negative pressure wound therapy: a systematic review. J Wound Care. 2010; 19 : 490–5. 2011; 20: 89. Erratum for: J Wound Care.
- Rahmanian-Schwarz A, Willkomm LM, Gonser P, Hirt B, Schaller HE. A novel option in negative pressure wound therapy (NPWT) for chronic and acute wound care. Burns. 2012; 38: 573–77.
- Wagner FW Jr. The diabetic foot. Orthopedics. 1987; 10: 163–72. [PubMed Abstract].
- Eginton MT, Brown KR, Seabrook GR, Towne JB, Cambria RA. A prospective randomised evaluation of negative-pressure wound dressings for diabetic foot wounds. Ann Vasc Surg. 2003; 17: 645–9.
- Morykwas MJ, Argenta LC, Shelton-Brown EI, McGuirt W. Vacuum- assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg. 1997; 38: 553–62.
- Armstrong DG, Lavery LA, Abu-Rumman P, Espensen EH, Vazquez JR, Nixon BP, etal. Outcomes of subatmospheric pressure dressing therapy on wounds of the diabetic foot. Ostomy Wound Manage. 2002; 48: 64–8. [PubMed Abstract].
- Clare MP, Fitzgibbons TC, McMullen ST, Stice RC, Hayes DF, Henkel L. Experience with the vacuum assisted closure negative pressure technique in the treatment of non-healing diabetic and dysvascular wounds. Foot Ankle Int. 2002; 23: 896–901. [PubMed Abstract].
- Nather A, Chionh SB, Han AYY, Chan PP, Nambiar A. Effectiveness of Vacuum-assisted Closure (VAC) therapy in the healing of chronic diabetic foot ulcers. Ann Acad Med Singapore. 2010; 39: 353–8. [PubMed Abstract].
- Birke-Sorensen H, Malmsjo M, Rome P, Hudson D, Krug E, Berg L, etal. International Expert Panel on Negative Pressure Wound Therapy [NPWT-EP], Martin R, Smith J. Evidence-based recommendations for negative pressure wound therapy: treatment variables (pressure levels, wound filler and contact layer) – steps towards an international consensus. J Plast Reconstr Aesthet Surg. 2011; 64: S1–16.